Abstract
Infectious coryza is an acute infectious respiratory disease in chickens that is caused by Avibacterium paragallinarum (A. paragallinarum). Infectious coryza has major economic effects due to decreased egg production in growing birds and slowed growth in broilers. In this study, we isolated and identified 40 strains of A. paragallinarum from chickens that showed typical clinical signs of coryza in part of China from 2019 to 2020. Using a hemagglutination-inhibition test, 11 isolates were identified as serovar A, 10 isolates were identified as serovar B, and 19 isolates were identified as serovar C. Antimicrobial sensitivity tests showed that high minimum inhibitory concentration (MIC) values were encountered for compounds sulfamethoxine sodium and oxytetracycline hydrochloride. Especially, of the 40 A. paragallinarum isolates, 30% had an MIC value of compound sulfamethoxine sodium of 64 μg/mL, 10% of 128 μg/mL, and 15% of 256 μg/mL. For oxytetracycline hydrochloride, 85% of isolates showed MIC values of 64 μg/mL or more. Excitingly, the MIC values of β-lactamase (amoxicillin, ampicillin, and ceftiofur) were low, with 77.5%, 70%, and 92.5% of isolates having an MIC value of ≤1 μg/mL, respectively. Our results may provide a reference for the treatment of infectious coryza.
1. Introduction
Infectious coryza (IC) is an acute infectious respiratory disease in chickens caused by a bacterium of the Pasteurellaceae family, Avibacterium paragallinarum (A. paragallinarum) []. The most prominent character of IC is acute inflammation of the upper respiratory tract, with facial swelling, nasal discharge, and conjunctivitis. IC occurs worldwide and causes significant economic losses due to growth retardation in growing birds and marked drops in egg production in layers. In addition, the control of IC requires intense vaccinations []. Stress is an important factor to be considered in the IC occurrence. In field scenarios and experimental infections, coinfection of A. paragallinarum with other bacteria and viruses increases clinical signs and pathologic lesions [,,]. In brief, high mortality and airsacculitis are the important issues of IC in broilers []. In the past decade, IC has been relatively well controlled in poultry. However, it occurred in many countries, such as China, USA, Indonesia, Great Britain and India, in recent years [,,,,]. In China, IC outbreaks have happened in Beijing, Shandong, and Anhui province since 2012. Therefore, the disease prevention measures to control IC need to be improved.
A. paragallinarum was serotyped into three serovars (A, B, and C) by hemagglutination inhibition (HI) tests according to the Page scheme []. The Kume scheme recognizes the same serogroups as the Page scheme (A1–A4, B–1, and C1–C4) [,]. The Kume scheme is also based on HI tests, but it is only used in a few laboratories because of its high technical requirements. The three Page serovars can be isolated from chickens all over the world and the prevalence of serotypes varies from country to country. In China, all three serovars have been reported. Serovar A was first reported in 1987, serovar C in 1995, and serovar B in 2003 [,]. Page or Kume serogroups are generally considered to represent three different immunovars []. There is no cross-protection among different serovars and the cross-protection within Page serovar B is not universal []. There is generally good cross-protection between the four serotypes A. Some of the four serotypes C have partial cross-protection []. Therefore, the identification of epidemic Page serovars provides the theoretical support for the selection of an appropriate vaccine.
Strict biosecurity and vaccination are the most important measures to prevent and control IC. In addition to vaccination, it is important to choose appropriate antimicrobial agents to treat and control IC. Many antimicrobial agents have been used, but many of them can only reduce the severity of the disease and cannot completely eliminate A. paragallinarum in chickens []. Once chickens experience adverse factors, IC easily recurs. Furthermore, if repeated treatment is used, there is a higher risk of the development of antimicrobial resistance to some antimicrobial agents. An increase in the resistance to antimicrobial drugs by A. paragallinarum has been reported [,,,]. However, the information of antimicrobial susceptibility of A. paragallinarum in China is scarce. This study aims to isolate and identify A. paragallinarum from chickens in several modern, intensive, large chicken farms with typical symptoms of facial swelling and nasal discharge from 2019 to 2020. The commonly used antimicrobial agents erythromycin, tetracycline derivatives, and sulfonamides were not effective in these farms. To provide appropriate treatment for IC, the antimicrobial sensitivity tests for A. paragallinarum are of great importance.
2. Materials and Methods
2.1. Sampling
The typical clinical signs of IC appeared in different chicken farms located in Jiangsu, Hebei, and Ningxia Hui Autonomous Region of China from 2019 to 2020. The chickens showed respiratory symptoms, tears, facial edema, and decreased egg production by more than 50%. In this study, the clinical samples were obtained from 12 modern, intensive, large chicken farms with IC outbreaks, which were submitted to our laboratory for post mortem examination. One hundred and eighty-two samples were collected from the nasal and infraorbital sinuses of chickens with clinical facial edema and discharge. Most isolates were from commercial chicken production systems. Farms located in Hebei and Ningxia Hui Autonomous Region raised layer parent stock. The breed of chickens included Hy-line brown, Jing Brown No. 1, and Nongda 3. We did not collect samples of chicken flocks regularly throughout the year to conduct a systematic epidemiological survey. However, as long as IC occurred in these chicken farms, we collected samples for A. paragallinarum isolation and identification. Because the occurrence of IC was mainly concentrated in the change of season (from April to July and from October to November), the samples also concentrated in these times. In addition, due to the influence of Corona Virus Disease 2019, only samples for the second half of the year were collected in 2020. The origin of the samples and isolates is shown in Table 1.

Table 1.
Origin of Avibacterium paragallinarum isolates.
2.2. A. paragallinarum Isolation
The samples were cultured on chocolate agar or trypticase soy agar supplemented with 10% fetal bovine serum and 0.0025% reduced nicotinamide adenine dinucleotide at 37 °C under 5% CO2 for 24–48 h. One suspected colony with typical A. paragallinarum morphology was selected and further streaked on chocolate agar for purification. Afterward, the plates were incubated at 37 °C in a 5% CO2 incubator for 24–48 h. The suspected colonies were grown in trypticase soy broth supplemented with 10% fetal bovine serum and 0.0025% reduced nicotinamide adenine dinucleotide at 37 °C for 16 h. The pure culture was identified by Gram staining and classical biochemical methods. Bacterial DNA was extracted using a TIANamp Bacteria DNA kit (Transgen Biotech Co., Ltd., Beijing, China), according to the manufacturer’s instructions. To determine if the isolates were A. paragallinarum, all of them were identified using primers of HPG-2 F (TGAGGGTAGTCTTGCACGCGAAT) and HPG-2 R (CAAGGTATCGATCGTCTCTCTACT), which are specific to A. paragallinarum []. All the A. paragallinarum isolates were kept at −70 °C until they were used.
2.3. Serotyping
All A. paragallinarum isolates were serotyped with specific antisera according to the Page scheme. The reference strains of A. paragallinarum 221 (serovar A), Spross (serovar B), and H-18 (serovar C) were purchased from China Veterinary Culture Collection Center (Beijing, China) and were used as control. Antisera for the hemagglutination-inhibition (HI) test were obtained as reported in a previous study []. The isolates were grown in trypticase soy broth supplemented with 10% fetal bovine serum and 0.0025% reduced nicotinamide adenine dinucleotide at 37 °C for 16 h. After incubation, the isolates were centrifuged, washed with phosphate-buffered saline, and treated with potassium thiocyanate for 2 h at 4 °C. The bacteria were sonicated, centrifuged, washed, and resuspended in phosphate-buffered saline as antigens. The glutaraldehyde-fixed chicken erythrocytes were used for the HI test. The serotypes of the isolates corresponded to the antiserum with the highest HI titer.
2.4. Antimicrobial Sensitivity Test
All isolates and reference strains (221, Spross, and H-18) were tested by the minimum inhibitory concentration (MIC) with the most commonly used antimicrobial agents. The modified broth microdilution method referred to the performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals developed in the Clinical and Laboratory Standards Institute (CLSI, 2013). Isolates and reference strains were grown in trypticase soy broth supplemented with 10% fetal bovine serum and 0.0025% reduced nicotinamide adenine dinucleotide at 37 °C for 16 h. The A. paragallinarum culture was diluted in trypticase soy broth medium to 107 cfu/mL. The 96-well microdilution plates were used for the MIC test. The antimicrobial agents were double-diluted to 0.0625–256 μg/mL with 100 μL trypticase soy broth medium. Then, the 100 μL diluted A. paragallinarum culture was inoculated into each well. The A. paragallinarum culture and antimicrobial agents were used as negative control and antibiotic control, respectively. Then, the plates were incubated at 37 °C for 20 h. The MIC values were determined by a photometer. Valnemulin hydrochloride (VA), compound sulfamethoxine sodium (COSMMS), doxycycline hydrochloride (DO), oxytetracycline hydrochloride (OT), amoxicillin (AMX), florfenicol (FFC), lincomycin/spectinomycin (LS), gentamycin sulfate (CN), enrofloxacin (ENR), ampicillin (AMP), tylvalosin tartrate (TAT), and ceftiofur (CTF) were used for this study. Three independent experiments were conducted.
3. Results
3.1. Isolation and Serotyping of A. paragallinarum
Dew-like colonies were identified on chocolate agar or trypticase soy agar supplemented with 10% fetal bovine serum and 0.0025% reduced nicotinamide adenine dinucleotide. After biochemical and PCR testing, a total of 40 A. paragallinarum strains were isolated and identified from clinical samples of suspected IC disease. The isolation rate of A. paragallinarum was 22%. Combined with the incidence information provided by the chicken farms, IC occurs frequently in the change of season (from April to July and from October to November). Chickens were infected mainly in the laying period, as well as in the brooding period, at 30–40 days old. The results of the HI test showed that 11 isolates were identified as serovar A, 10 isolates were identified as serovar B, and 19 isolates were identified as serovar C. In most chicken farms, only one serotype was prevalent in the process of an outbreak. However, two serotypes were prevalent during an outbreak in farms G, I, and J. Three serotypes were prevalent during an outbreak in farm F. Serovar A was prevalent in April 2019, but serovar C was prevalent in October 2020 in farm B.
3.2. Antimicrobial Susceptibility Testing
Table 2 shows the MICs of 12 antimicrobial agents against 40 A. paragallinarum isolates and reference strains (221, Spross, and H-18). The MIC values ranged from ≤0.0625 to 64 μg/mL for VA. As shown in Figure 1A, 10% of isolates showed MIC values of 16 μg/mL or more for VA. The MIC values of other β-lactamase (AMX, AMP, and CTF) were low (Figure 1B–D), while high MIC values were encountered for COSMMS and OT. Especially, the MIC values ranged from ≤0.0625 to 4 μg/mL for CTF and 92.5% of isolates showed MIC ≤ 1 μg/mL. Of the 40 A. paragallinarum isolates, 2.5% had an MIC value of COSMMS of 8 μg/mL, 22.5% of 16 μg/mL, 20% of 32 μg/mL, 30% of 64 μg/mL, 10% of 128 μg/mL, and 15% of 256 μg/mL (Figure 1E). MIC values ranged from ≤0.125 to 32 μg/mL for DO, with 12.5% of isolates showing MIC values of 16 μg/mL or more (Figure 1F), while 85% of isolates showed MIC values of 64 μg/mL or more for OT (Figure 1G). About 20% of isolates showed MIC values of 16 μg/mL or more for FFC, CN, and ENR (Figure 1H–J). The A. paragallinarum isolates displayed a wide variance in MIC values for LS with a range from ≤0.0625 to 256 µg/mL and the same was observed for TAT, a macrolide antibiotic. Of the 40 isolates, 32.5% and 30% of isolates showed MIC values of 16 μg/mL or more for LS and TAT, respectively (Figure 1K,L).

Table 2.
Minimum inhibitory concentration of A. paragallinarum isolates.
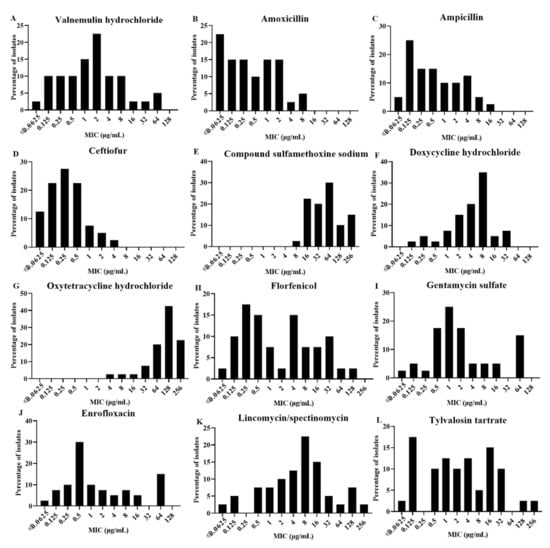
Figure 1.
Distribution of minimum inhibitory concentration values of antimicrobial agents tested: (A) alnemulin hydrochloride, (B) amoxicillin, (C) ampicillin, (D) ceftiofur, (E) compound sulfamethoxine sodium, (F) doxycycline hydrochloride, (G) oxytetracycline hydrochloride, (H) florfenicol, (I) gentamycin sulfate, (J) enrofloxacin, (K) lincomycin/spectinomycin, and (L) tylvalosin tartrate.
4. Discussion
IC has worldwide economic significance and leads to poor growth performance of broilers, as well as decreased egg production among layers. IC can be found all over the world, but its pathogen, A. paragallinarum, is difficult to isolate due to the use of antibiotics in feed []. Moreover, A. paragallinarum must be isolated in the acute infection stage. It is a slow-growing and fastidious bacterium and most strains need V-(nicotinamide adenine dinucleotide) factor for growth in vitro. In addition, A. paragallinarum is easily covered up and overgrown by other Pasteurellaceae bacteria in the process of isolation and culture []. Conventionally, IC can be preliminarily diagnosed according to the rapid spread of the disease and coryza symptoms. The diagnosis is confirmed by isolates with satellite growth in blood agar plates [].
Since 2020, all forms of growth-promoting antibiotics except traditional Chinese medicines have been forbidden to be used as feed additives in China. Although they can be used for treatment, many antibiotics are banned during the laying period or have a strict rest period. With the restricted use of antibiotics, the morbidity and isolation rate of IC has gradually increased in China. In China, A. paragallinarum serovars A, B and C have been reported. Serovars A and C were the major serotypes causing outbreaks of IC, until serovar B appeared in 2003. After that, A. paragallinarum serovar B was detected in Beijing and Tianjin. Over the years, the incidence of Page serovar B infection has significantly increased in the world, including China, Brazil, Egypt, Mexico, South Africa, Germany, and the United States [,,]. In recent years, 28 A. paragallinarum serovar B isolates were isolated from chickens in the Shandong, Liaoning, Hebei, Beijing, Anhui, Sichuan, Jiangsu, and Guangdong provinces []. It has been reported that A. paragallinarum serovar B isolates are as pathogenic as serovars A and C isolates []. In this study, 40 isolates were obtained from 12 modern, intensive, large chicken farms with IC outbreaks and confirmed by HPG-2 PCR. Page serovars A, B, and C were all isolated and 10 isolates were identified as serovar B. In most chicken farms, only one serotype was prevalent during an outbreak. However, two serotypes occurred during an outbreak in farms G, I, and J. Serovars A, B, and C broke out in farm F at the same time. Our results suggested that the immunization of a trivalent vaccine is necessary. A previous study has demonstrated that only partial cross-protection has been seen among serovar B isolates, although there is only one Kume serovar B (B-1). IC broke out even in chickens immunized with bivalent (A + C) or trivalent (A + B + C) inactivated vaccines, which indicated that some cases were possibly related to vaccine failure.
Hmtp210 of A. paragallinarum encodes a 210 kDa outer membrane protein []. Previous research proved that Hmtp210 plays a key role in the pathogenicity of A. paragallinarum, which has the function of hemagglutination, cell adhesion, and biofilm formation activity []. The hypervariable region of Hmtp210 located at about 1100–1600 aa is considered to be the most antigenic region of Hmtp210. The recombinant vaccines for the hypervariable region of Hmtp210 protect chickens against challenge with A. paragallinarum [,]. It has been reported that Hmtp210 is an important protective antigen and a candidate for serotyping []. A multiplex PCR and PCR-RFLP method using the hypervariable region of Hmtp210 was developed to identify the serovar of A. paragallinarum []. However, we also performed this multiplex PCR and demonstrated that this method cannot be used for serotyping. Our result is consisted with Wang et al.’s []. The HI test is one of the most widely used serological tests. It is usually used to detect antibody titers and serotype, followed by epidemiological studies to assess the prevalence of IC. The Page scheme is still the most commonly used and effective serotype method. Therefore, the HI test was used to serotype the A. paragallinarum isolates in this study.
The treatment of IC has not been widely studied. However, the use of some antimicrobial agents has been reported, especially sulfonamides [,]. Recently, A. paragallinarum isolates have shown resistance to many antimicrobial agents, such as streptomycin, sulfonamides, and OT [,]. In present study, high MIC values were encountered for COSMMS and OT. Of the 40 A. paragallinarum isolates, 30% had an MIC value of COSMMS of 64 μg/mL, 10% of 128 μg/mL, and 15% of 256 μg/mL. Our results showed that 95% of isolates were characterized by high MIC values of OT (≥16 μg/mL), while 12.5% of isolates showed MIC values of ≥16 μg/mL for DO, the other tetracycline. In previous research, 72.2% of A. paragallinarum isolates had an MIC value of ≥16 μg/mL for OT. For DO, MIC values of ≥16 μg/mL were detected in 66.7% of Thailand isolates []. Low MICs of AMP and penicillin were found in Australian field isolates, having MIC values of ≤0.5 and ≤1 μg/mL []. The MIC values of β-lactamase (VA, AMX, AMP, and CTF) were tested in this study; in total, 47.5%, 77.5%, 70%, and 92.5% of isolates had an MIC value of ≤1 μg/mL, respectively. Similar to our results, 83.3% of Thailand isolates were characterized by an MIC ≤ 1 μg/mL for AMP. For all 44 isolates in Dutch isolates, the MIC values of AMP were ≤ 1 μg/mL []. Taiwan isolates differed from ours, with only 27.8% isolates having MIC values of AMP ≤ 1 μg/mL []. For ENR, 50.0% Thailand isolates’ MIC values were ≥ 4 μg/mL [], while all Dutch isolates’ MIC values were ≤ 2 μg/mL []. In this study, 32.5% isolates’ MIC values were ≥ 4 μg/mL. The high MIC values of CN, LS, OT, and TAT, matches the results of previous research studies using agar diffusion [,,].
High MIC values of COSMMS and OT were observed in this study. Since these antimicrobial agents are commonly used in the treatment of IC, the results also suggest that they may not be effective in future treatments in China. Thus, antimicrobial sensitivity tests need be carried out for the selection of effective antimicrobial agents. However, excitingly, we found that the MIC values of β-lactamase were low, especially CTF and AMX. In the following treatment, we recommended AMX and CTF for IC treatment and obtained a good effect in chicken farms.
5. Conclusions
In this study, 182 samples were collected from the nasal and infraorbital sinuses of chickens with clinical facial edema and discharge in 12 modern, intensive, large chicken farms from 2019 to 2020. In total, 40 A. paragallinarum strains were isolated and identified; specifically, 11 isolates were identified as serovar A, 10 isolates were identified as serovar B, and 19 isolates were identified as serovar C. In most chicken farms, only one serotype was prevalent during an outbreak. However, serovars A, B, and C broke out in farm F at the same time. Our results suggest that the immunization of a trivalent vaccine is necessary. The antimicrobial susceptibility was investigated using an MIC test. The A. paragallinarum isolates displayed a wide variance in MICs for LS, with a range from ≤0.0625 to 256 µg/mL, and the same was observed for TAT. The MIC values ranged from ≤0.0625 to 64 μg/mL for VA. The MIC values of β-lactamase (AMX, AMP, and CTF) were low, while high MIC values were observed for COSMMS and OT. Especially, the MIC values ranged from ≤0.0625 to 4 μg/mL for CTF, with 92.5% of isolates showing an MIC ≤ 1 μg/mL. For COSMMS, 10% isolates had an MIC value of 128 μg/mL and 15% of 256 μg/mL. β-lactamases AMX and CTF were effective in the treatment of IC and could be used as a reference treatment strategy for the disease. The information provided by the isolation, serovar identification, and antimicrobial susceptibility of A. paragallinarum will allow researchers to design a more effective use of antimicrobial agents or other methods of controlling IC.
Author Contributions
Conceptualization, X.Z. and M.G.; methodology, X.C.; software, H.Z.; validation, D.L.; formal analysis, X.C.; investigation, H.Z.; resources, X.C.; data curation, M.G.; writing—original draft preparation, M.G.; writing—review and editing, X.Z and Y.W.; visualization, M.G.; supervision, M.G.; project administration, X.Z.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA (CARS-40), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
All samples used in the study were collected from clinical samples provided by chicken farms. Ethical approval was not required.
Informed Consent Statement
Informed consent was obtained from the owners of the chicken farms involved in the study.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors are grateful to all the participating farms for collecting the samples.
Conflicts of Interest
The authors declare that the research study was conducted in the bsence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Blackall, P.J.; Soriano-Vargas, E. Infectious coryza and related bacterial infections. Dis. Poult. 2020, 890–906. [Google Scholar] [CrossRef]
- Blackall, P.J.; Christensen, H.; Beckenham, T.; Blackall, L.L.; Bisgaard, M. Reclassification of Pasteurella gallinarum, [Haemophilus] paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 353–362. [Google Scholar]
- Morales-Erasto, V.; Falconi-Agapito, F.; Luna-Galaz, G.A.; Saravia, L.E.; Montalvan-Avalos, A.; Soriano-Vargas, E.E.; Fernández-Díaz, M. Coinfection of Avibacterium paragallinarum and Ornithobacterium rhinotracheale in Chickens from Peru. Avian Dis. 2016, 60, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.E.; Terzolo, H.R.; Blackall, P.J. Complicated infectious coryza outbreaks in Argentina. Avian Dis. 1994, 38, 672–678. [Google Scholar] [CrossRef]
- Paudel, S.; Hess, M.; Hess, C. Coinfection of Avibacterium paragallinarum and Gallibacterium anatis in Specific-Pathogen-Free Chickens Complicates Clinical Signs of Infectious Coryza, Which Can Be Prevented by Vaccination. Avian Dis. 2017, 61, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, R.A.; Da Silva, A.P.; Egaña-Labrin, S.; Stoute, S.; Kern, C.; Zhou, H.; Cutler, G.; Corsiglia, C. Infectious Coryza: Persistence, Genotyping, and Vaccine Testing. Avian Dis. 2020, 64, 157–165. [Google Scholar] [CrossRef]
- Zhang, P.; Miao, M.; Gong, Y.; Sun, H.; Blackall, P. Infectious coryza due to Haemophilus paragallinarum serovar B in China. Aust. Vet. J. 2003, 81, 96–97. [Google Scholar] [CrossRef] [Green Version]
- Welchman Dde, B.; King, S.A.; Wragg, P.; Wood, A.M.; Irvine, R.M.; Pepper, W.J.; Dijkman, R.; de Wit, J.J. Infectious coryza in chickens in Great Britain. Vet. Rec. 2010, 167, 912–913. [Google Scholar] [CrossRef]
- Patil, V.V.; Mishra, D.; Mane, D.V. 16S ribosomal RNA sequencing and molecular serotyping of Avibacterium paragallinarum isolated from Indian field conditions. Vet. World 2017, 10, 1004–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispo, M.; Sentíes-Cué, C.G.; Cooper, G.L.; Mountainspring, G.; Corsiglia, C.; Bickford, A.A.; Stoute, S. Otitis and meningoencephalitis associated with infectious coryza (Avibacterium paragallinarum) in commercial broiler chickens. J. Vet. Diagn. Investig. 2018, 30, 784–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahyuni, A.E.T.H.; Tabbu, C.R.; Artanto, S.; Setiawan, D.C.B.; Rajaguguk, S.I. Isolation, identification, and serotyping of Avibacterium paragallinarum from quails in Indonesia with typical infectious coryza disease symptoms. Vet. World 2018, 11, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Page, L. Haemophilus infections in chickens. I. Characteristics of 12 Haemophilus isolates recovered from diseased chickens. Am. J. Vet. Res. 1962, 23, 85. [Google Scholar]
- Blackall, P.J.; Eaves, L.E.; Rogers, D.G. Proposal of a new serovar and altered nomenclature for Haemophilus paragallinarum in the Kume hemagglutinin scheme. J. Clin. Microbiol. 1990, 28, 1185–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kume, K.; Sawata, A.; Nakai, T.; Matsumoto, M. Serological classification of Haemophilus paragallinarum with a hemagglutinin system. J. Clin. Microbiol. 1983, 17, 958–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Cheng, J.; Huang, X.; Xu, M.; Feng, J.; Liu, C.; Zhang, G. Characterization of emergent Avibacterium paragallinarum strains and the protection conferred by infectious coryza vaccines against them in China. Poult. Sci. 2019, 98, 6463–6471. [Google Scholar] [CrossRef]
- Blackall, P.J. Infectious coryza: Overview of the disease and new diagnostic options. Clin. Microbiol. Rev. 1999, 12, 627–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Blackall, P.J.; Takigami, S.; Iritani, Y.; Hayashi, Y. Immunogenicity of Haemophilus paragallinarum serovar B strains. Avian Dis. 1991, 965–968. [Google Scholar] [CrossRef]
- Soriano, E.V.; Garduño, M.L.; Téllez, G.; Rosas, P.F.; Suárez-Güemes, F.; Blackall, P.J. Cross-protection study of the nine serovars of Haemophilus paragallinarum in the Kume haemagglutinin scheme. Avian Pathol. 2004, 33, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Heuvelink, A.; Wiegel, J.; Kehrenberg, C.; Dijkman, R.; Soriano-Vargas, E.; Feberwee, A. Antimicrobial susceptibility of Avibacterium paragallinarum isolates from outbreaks of infectious coryza in Dutch commercial poultry flocks, 2008–2017. Vet. Microbiol. 2018, 217, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chukiatsiri, K.; Sasipreeyajan, J.; Blackall, P.J.; Yuwatanichsampan, S.; Chansiripornchai, N. Serovar identification, antimicrobial sensitivity, and virulence of Avibacterium paragallinarum isolated from chickens in Thailand. Avian Dis. 2012, 56, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Jeong, O.-M.; Kang, M.-S.; Jeon, B.-W.; Choi, B.-K.; Kwon, Y.-K.; Yoon, S.-Y.; Blackall, P.J.; Lee, H.-S.; Jung, S.-C.; Kim, J.-H. Isolation and characterization of Avibacterium paragallinarum with different nicotinamide adenine dinucleotide requirements. Vet. Microbiol. 2017, 205, 62–65. [Google Scholar] [CrossRef]
- Luna-Galaz, G.A.; Morales-Erasto, V.; Peñuelas-Rivas, C.G.; Soriano-Vargas, E.; Blackall, P.J. Antimicrobial sensitivity of Avibacterium paragallinarum isolates from four Latin American countries. Avian Dis. 2016, 60, 673–676. [Google Scholar] [CrossRef]
- Chen, X.; Miflin, J.K.; Zhang, P.; Blackall, P. Development and application of DNA probes and PCR tests for Haemophilus paragallinarum. Avian Dis. 1996, 40, 398–407. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blackall, P.J.; Takigami, S.; Iritani, Y.; Hayashi, Y. Pathogenicity and serovar-specific hemagglutinating antigens of Haemophilus paragallinarum serovar B strains. Avian Dis. 1990, 34, 964–968. [Google Scholar] [CrossRef]
- Han, M.-S.; Kim, J.-N.; Jeon, E.-O.; Lee, H.-R.; Koo, B.-S.; Min, K.-C.; Lee, S.-B.; Bae, Y.-J.; Mo, J.-S.; Cho, S.-H.; et al. The current epidemiological status of infectious coryza and efficacy of PoulShot Coryza in specific pathogen-free chickens. J. Vet. Sci. 2016, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Soriano, V.E.; Blackall, P.J.; Dabo, S.M.; Tellez, G.; Garcia-Delgado, G.A.; Fernandez, R.P. Serotyping of Haemophilus paragallinarum isolates from Mexico by the Kume hemagglutinin scheme. Avian Dis. 2001, 45, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.A.; van den Berg, K.; Malo, A. Efficacy of a new tetravalent coryza vaccine against emerging variant type B strains. Avian Pathol. 2003, 32, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Xie, S.; Li, X.; Xu, F.; Li, Y.; Boucher, C.E.; Chen, X. Selection of Avibacterium paragallinarum Page serovar B strains for an infectious coryza vaccine. Vet. Immunol. Immunopathol. 2018, 199, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, E.; Sakaguchi, M.; Matsuo, K.; Hamada, F.; Tokiyoshi, S. Polypeptide for Haemophilus paragallinarum and Process for Preparing the Same; US6919080B2; Juridical Foundation The Chemo-Sero-Therapeutic Research Institute: Kumamoto-Ken, Japan, 2002. [Google Scholar]
- Wang, Y.-P.; Hsieh, M.-K.; Tan, D.-H.; Shien, J.-H.; Ou, S.-C.; Chen, C.-F.; Chang, P.-C. The haemagglutinin of Avibacterium paragallinarum is a trimeric autotransporter adhesin that confers haemagglutination, cell adherence and biofilm formation activities. Vet. Microbiol. 2014, 174, 474–482. [Google Scholar] [CrossRef]
- Sakamoto, R.; Baba, S.; Ushijima, T.; Kino, Y.; Honda, T.; Mizokami, H.; Sakaguchi, M. Development of a recombinant vaccine against infectious coryza in chickens. Res. Vet. Sci. 2013, 94, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-R.; Wu, Y.-R.; Shien, J.-H.; Hsu, Y.-M.; Chen, C.-F.; Shieh, H.K.; Chang, P.-C. Recombinant proteins containing the hypervariable region of the haemagglutinin protect chickens against challenge with Avibacterium paragallinarum. Vaccine 2011, 29, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Yaguchi, K.; Amimoto, K.; Oishi, E. Identification and expression of a gene encoding an epitope that induces hemagglutination inhibition antibody to Avibacterium paragallinarum serovar A. Avian Dis. 2007, 51, 84–89. [Google Scholar] [CrossRef]
- Sakamoto, R.; Kino, Y.; Sakaguchi, M. Development of a multiplex PCR and PCR-RFLP method for serotyping of Avibacterium paragallinarum. J. Vet. Med. Sci. 2012, 74, 271–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sun, H.; Blackall, P.J.; Zhang, Z.; Zhou, H.; Xu, F.; Chen, X. Evaluation of a proposed molecular methodology for the serotyping of Avibacterium paragallinarum. J. Vet. Diagn. Investig. 2016, 28, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lublin, A.; Mechani, S.; Malkinson, M.; Weisman, Y. Efficacy of norfloxacin nicotinate treatment of broiler breeders against Haemophilus paragallinarum. Avian Dis. 1993, 37, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.E.; Davis, R.B.; Sunka, E.M. An evaluation and comparison of spectinomycin and spectinomycin-erythromycin combinations for infectious coryza. Avian Dis. 1968, 12, 1–3. [Google Scholar] [CrossRef]
- Noonkhokhetkong, T.; Chukiatsiri, K.; Sasipreeyajan, J.; Chansiripornchai, N. Determination of Antimicrobial Susceptibility, Antimicrobial Resistance Genes and in vivo Testing of Antimicrobial Susceptibility of Avibacterium paragallinarum. Thai J. Vet. Med. 2013, 43, 525–531. [Google Scholar]
- Blackall, P.J.; Eaves, L.E.; Rogers, D.G. Biotyping of Haemophilus paragallinarum isolates using hemagglutinin serotyping, carbohydrate fermentation patterns, and antimicrobial drug resistance patterns. Avian Dis. 1989, 33, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-M.; Shieh, H.K.; Chen, W.-H.; Sun, T.-Y.; Shiang, J.-H. Antimicrobial susceptibility, plasmid profiles and haemocin activities of Avibacterium paragallinarum strains. Vet. Microbiol. 2007, 124, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Poernomo, S.; Sutarma, S.; Rafiee, M.; Blackall, P. Characterisation of isolates of Haemophilus paragallinarum from Indonesia. Aust. Vet. J. 2000, 78, 759–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).