The Seroprevalence of Chlamydia Infection in Sheep in Shanxi Province, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Investigation Sites
2.3. The Collection of Serum Samples
2.4. Serological Tests
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Pagliarani, S.; Johnston, S.D.; Beagley, K.W.; Dief, H.; Palmieri, C. The occurrence and pathology of chlamydiosis in the male reproductive tract of non-human mammals: A review. Theriogenology 2020, 154, 152–160. [Google Scholar] [CrossRef]
- Chaiwattanarungruengpaisan, S.; Thongdee, M.; Anuntakarun, S.; Payungporn, S.; Arya, N.; Punchukrang, A.; Ramasoota, P.; Singhakaew, S.; Atithep, T.; Sariya, L.A. A new species of Chlamydia isolated from Siamese crocodiles (Crocodylus siamensis). PLoS ONE 2021, 16, e0252081. [Google Scholar] [CrossRef] [PubMed]
- Borel, N.; Polkinghorne, A.; Pospischil, A. A review on chlamydial diseases in animals: Still a challenge for pathologists? Vet. Pathol. 2018, 55, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Knittler, M.R.; Sachse, K. Chlamydia psittaci: Update on an underestimated zoonotic agent. Pathog. Dis. 2015, 73, 1–15. [Google Scholar] [CrossRef]
- Arif, E.D.; Saeed, N.M.; Rachid, S.K. Isolation and identification of Chlamydia abortus from aborted ewes in Sulaimani Province, Northern Iraq. Pol. J. Microbiol. 2020, 69, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, X.; Cui, B.; Shao, Z.; Zhao, X.; Yang, Q.; Song, S.; Wang, Z.; Wang, Y.; Wang, Y.; et al. Abortion and various associated risk factors in dairy cow and sheep in Ili, China. PLoS ONE 2020, 15, e0232568. [Google Scholar] [CrossRef]
- Qin, S.Y.; Yin, M.Y.; Cong, W.; Zhou, D.H.; Zhang, X.X.; Zhao, Q.; Zhu, X.Q.; Zhou, J.Z.; Qian, A.D. Seroprevalence and risk factors of Chlamydia abortus infection in Tibetan sheep in Gansu province, northwest China. Sci. World J. 2014, 2014, 193464. [Google Scholar] [CrossRef]
- Caspe, S.G.; Palarea-Albaladejo, J.; Underwood, C.; Livingstone, M.; Wattegedera, S.R.; Milne, E.; Sargison, N.D.; Chianini, F.; Longbottom, D. Distribution and severity of placental lesions caused by the Chlamydia abortus 1B vaccine strain in vaccinated ewes. Pathogens 2021, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Pichon, N.; Guindre, L.; Laroucau, K.; Cantaloube, M.; Nallatamby, A.; Parreau, S. Chlamydia abortus in pregnant woman with acute respiratory distress syndrome. Emerg. Infect. Dis. 2020, 26, 628–629. [Google Scholar] [CrossRef]
- Baud, D.; Greub, G. Intracellular bacteria and adverse pregnancy outcomes. Clin. Microbiol. Infect. 2011, 17, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Lou, Z.; Fei, Y. Prevalence, diagnosis, and vaccination situation of animal chlamydiosis in China. Front. Vet. Sci. 2018, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.T.; Liang, Q.L.; Li, R.L.; Zheng, F.G.; Liu, Q. Serological evidence of Toxoplasma gondii and Chlamydia infection in alpacas (Vicugna pacos) in Shanxi Province, northern China. Microb. Pathog. 2020, 149, 104399. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Yang, B.T.; Yin, Z.W.; Wang, W.; Zhao, Q.; Jiang, J. A seroepidemiological survey of Toxoplasma gondii and Chlamydia infection in chickens, ducks, and geese in Jilin Province, northeastern China. Vector Borne Zoonotic Dis. 2020, 20, 825–830. [Google Scholar] [CrossRef]
- Ni, X.; Qin, S.; Lou, Z.; Ning, H.; Sun, X. Seroprevalence and risk factors of Chlamydia infection in domestic rabbits (Oryctolagus cuniculus) in China. Biomed. Res. Int. 2015, 2015, 460473. [Google Scholar] [CrossRef]
- Sun, L.X.; Liang, Q.L.; Hu, X.H.; Li, Z.; Yang, J.F.; Zou, F.C. First report of Chlamydia seroprevalence and risk factors in domestic black-boned sheep and goats in China. Front. Vet. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Anderson, I.E.; Herring, A.J.; Jones, G.E.; Low, J.C.; Greig, A. Development and evaluation of an indirect ELISA to detect antibodies to abortion strains of Chlamydia psittaci in sheep sera. Vet. Microbiol. 1995, 43, 1–12. [Google Scholar] [CrossRef]
- Liu, S.S.; Chu, J.; Zhang, Q.; Sun, W.; Zhang, T.Y.; He, C. Development of a novel pmpD-N ELISA for Chlamydia psittaci infection. Biomed. Environ. Sci. 2016, 29, 315–322. [Google Scholar]
- Chen, W.; Liu, Y.; Chen, J.; Ma, Y.; Song, Y.; Cen, Y.; You, M.; Yang, G. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int. Immunopharmacol. 2021, 99, 107938. [Google Scholar] [CrossRef]
- Merdja, S.E.; Khaled, H.; Aaziz, R.; Vorimore, F.; Bertin, C.; Dahmani, A.; Bouyoucef, A.; Laroucau, K. Detection and genotyping of Chlamydia species responsible for reproductive disorders in Algerian small ruminants. Trop. Anim. Health Prod. 2015, 47, 437–443. [Google Scholar] [CrossRef]
- Runge, M.; Binder, A.; Schotte, U.; Ganter, M. Investigations concerning the prevalence of Coxiella burnetii and Chlamydia abortus in sheep in correlation with management systems and abortion rate in Lower Saxony in 2004. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 138–143. [Google Scholar] [PubMed]
- Chahota, R.; Gupta, S.; Bhardwaj, B.; Malik, P.; Verma, S.; Sharma, A.M. Seroprevalence studies on animal chlamydiosis amongst ruminants in five states of India. Vet. World 2015, 8, 72–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, S.F.; Li, F.; Zheng, W.B.; Liu, G.H. Seroprevalence and risk factors of Chlamydia abortus infection in goats in Hunan Province, subtropical China. Vector Borne Zoonotic Dis. 2018, 18, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Sahele, M.; Sori, T.; Guyassa, C.; Garoma, A. Seroprevalence and associated risk factors for chlamydiosis, coxiellosis and brucellosis in sheep and goats in Borana pastoral area, southern Ethiopia. BMC Vet. Res. 2020, 16, 145. [Google Scholar] [CrossRef]
- Bommana, S.; Jelocnik, M.; Borel, N.; Marsh, I.; Carver, S.; Polkinghorne, A. The limitations of commercial serological assays for detection of chlamydial infections in Australian livestock. J. Med. Microbiol. 2019, 68, 627–632. [Google Scholar] [CrossRef]
- Rodolakis, A.; Laroucau, K. Chlamydiaceae and chlamydial infections in sheep or goats. Vet. Microbiol. 2015, 181, 107–118. [Google Scholar] [CrossRef]
- Sachse, K.; Bavoil, P.M.; Kaltenboeck, B.; Stephens, R.S.; Kuo, C.C.; Rosselló-Móra, R.; Horn, M. Emendation of the family chlamydiaceae: Proposal of a single genus, Chlamydia, to include all currently recognized species. Syst. Appl. Microbiol. 2015, 38, 99–103. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.Z.; Ma, C.F.; Zhang, X.X.; Zhao, Q.; Ni, H.B. Epidemiological investigation and genotype of Chlamydia exposure in pigeons in three provinces in northern China. Vector Borne Zoonotic Dis. 2018, 18, 181–184. [Google Scholar] [CrossRef]
- Sun, W.W.; Meng, Q.F.; Cong, W.; Shan, X.F.; Wang, C.F.; Qian, A.D. Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhoea virus in commercial dairy and beef cattle in eastern, northern and northeastern China. Parasitol. Res. 2015, 114, 4211–4218. [Google Scholar] [CrossRef]
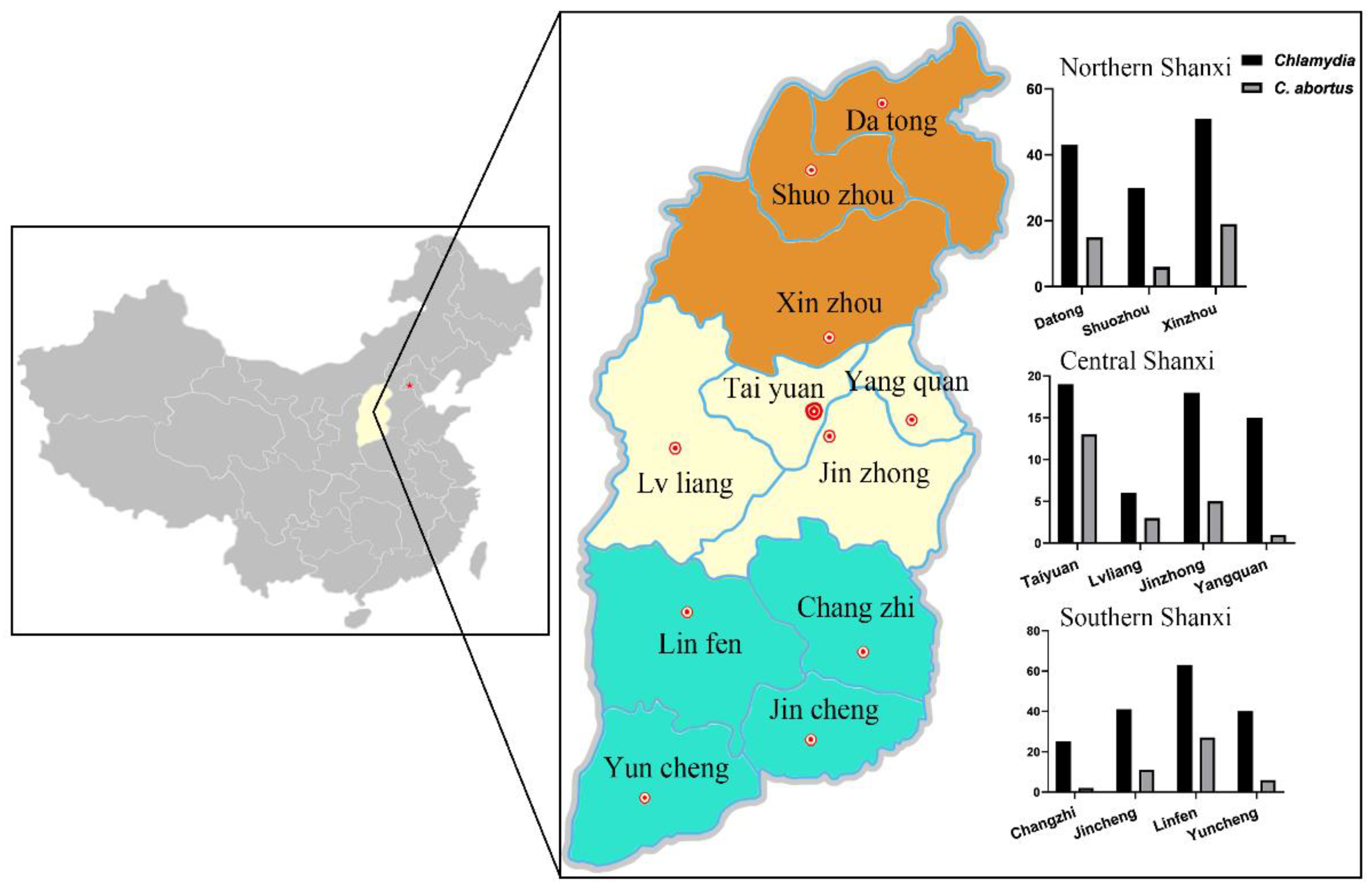
| Geographical Location | City | No. Examined | No. Positive | Prevalence (%) |
|---|---|---|---|---|
| Northern Shanxi | Datong Shuozhou Xinzhou | 90 90 90 | 43 30 51 | 47.78 33.33 56.67 |
| Central Shanxi | Taiyuan Lvliang Jinzhong Yangquan | 85 90 90 90 | 19 6 18 15 | 22.35 6.67 20.00 16.67 |
| Southern Shanxi | Changzhi Jincheng Linfen Yuncheng | 90 90 89 90 | 25 41 63 40 | 27.78 45.56 70.79 44.44 |
| Total | 984 | 351 | 35.67 |
| Variable | Category | No. Examined | No. Positive | Prevalence (%) (95% CI) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Geographical location | Northern Shanxi Central Shanxi Southern Shanxi | 270 355 359 | 124 58 169 | 45.93 (39.98–51.87) 16.34 (12.49–20.18) 47.08 (41.91–52.24) | <0.01 | 4.55 (3.21–6.46) Reference 3.86 (2.66–5.59) |
| Management mode | Household animal farms Animal farming cooperatives Large-scale animal farming companies | 684 120 180 | 258 29 64 | 37.72 (34.09–41.35) 24.17 (16.51–31.83) 35.56 (28.56–42.55) | <0.05 | 2.48 (1.59–3.89) Reference 1.73 (1.03–2.91) |
| Total | 984 | 351 | 35.67 (32.68–38.66) |
| Geographical Location | City | No. Examined | No. Positive | Prevalence (%) |
|---|---|---|---|---|
| Northern Shanxi | Datong Shuozhou Xinzhou | 90 90 90 | 15 6 19 | 16.67 6.67 21.11 |
| Central Shanxi | Taiyuan Lvliang Jinzhong Yangquan | 85 90 90 90 | 13 3 5 1 | 15.29 3.33 5.56 1.11 |
| Southern Shanxi | Changzhi Jincheng Linfen Yuncheng | 90 90 89 90 | 1 10 4 1 | 1.11 11.11 4.49 1.11 |
| Total | 984 | 78 | 7.93 |
| Variable | Category | No. Examined | No. Positive | Prevalence (%) (95% CI) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Geographical location | Northern Shanxi Central Shanxi Southern Shanxi | 270 355 359 | 40 22 16 | 14.80 (10.58–19.52) 6.20 (3.69–8.71) 5.93 (3.11–8.74) | <0.01 | 3.72 (2.04–6.82) 1.42 (0.73–2.74) Reference |
| Management mode | Household animal farms Animal farming cooperatives Large-scale animal farming companies | 684 120 180 | 48 10 20 | 7.02 (5.10–8.93) 8.33 (3.39–13.28) 11.11 (6.52–15.70) | >0.05 | Reference 1.21 (0.59–2.45) 1.66 (0.96–2.87) |
| Total | 984 | 78 | 7.93 (6.24–9.61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-X.; Gao, J.; Shi, S.-R.; Gao, W.-W.; Zhu, X.-Q.; Lei, Y.-P.; Zhang, Y.; Zheng, W.-B. The Seroprevalence of Chlamydia Infection in Sheep in Shanxi Province, China. Vet. Sci. 2022, 9, 656. https://doi.org/10.3390/vetsci9120656
Li C-X, Gao J, Shi S-R, Gao W-W, Zhu X-Q, Lei Y-P, Zhang Y, Zheng W-B. The Seroprevalence of Chlamydia Infection in Sheep in Shanxi Province, China. Veterinary Sciences. 2022; 9(12):656. https://doi.org/10.3390/vetsci9120656
Chicago/Turabian StyleLi, Chen-Xu, Jin Gao, Sheng-Rong Shi, Wen-Wei Gao, Xing-Quan Zhu, Yu-Ping Lei, Yu Zhang, and Wen-Bin Zheng. 2022. "The Seroprevalence of Chlamydia Infection in Sheep in Shanxi Province, China" Veterinary Sciences 9, no. 12: 656. https://doi.org/10.3390/vetsci9120656
APA StyleLi, C.-X., Gao, J., Shi, S.-R., Gao, W.-W., Zhu, X.-Q., Lei, Y.-P., Zhang, Y., & Zheng, W.-B. (2022). The Seroprevalence of Chlamydia Infection in Sheep in Shanxi Province, China. Veterinary Sciences, 9(12), 656. https://doi.org/10.3390/vetsci9120656







