Spontaneous Polycystic Kidneys with Chronic Renal Failure in an Aged House Musk Shrew (Suncus murinus)
Abstract
:1. Introduction
2. Materials and Methods
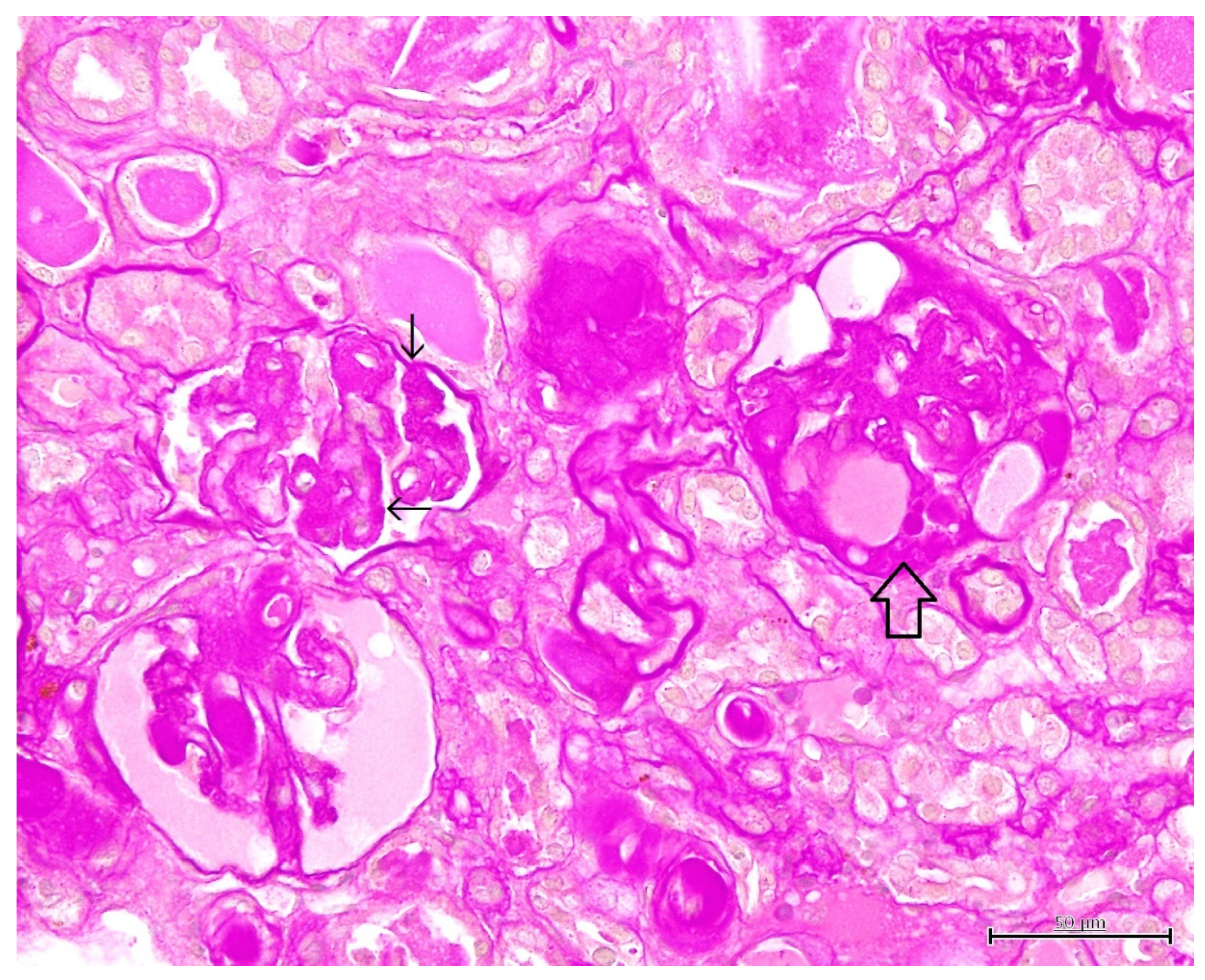
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foo, J.N.; Xia, Y. Polycystic kidney disease: New knowledge and future promises. Curr. Opin. Genet. Dev. 2019, 56, 69–75. [Google Scholar]
- Raptis, V.; Loutradis, C.; Sarafidis, P.A. Renal injury progression in autosomal dominant polycystic kidney disease: A look beyond the cysts. Nephrol. Dial. Transplant. 2018, 33, 1887–1895. [Google Scholar] [CrossRef]
- Colbert, G.B.; Elrggal, M.; Gaur, L.; Lerma, E.V. Update and review of adult polycystic kidney disease. Disease-a-Month 2019, 66, 100887. [Google Scholar] [CrossRef]
- Gómez, B.I.; Little, J.S.; Leon, A.J.; Stewart, I.J.; Burmaeister, D.M. A 30% incidence of renal cysts with varying sizes and den-sities in biomedical research swine is not associated with renal dysfunction. Anim. Model Exp. Med. 2020, 3, 273–281. [Google Scholar] [CrossRef]
- Paepe, D.; Saunders, J.H.; Bavegems, V.; Paës, G.; Peelman, L.J.; Makay, C.; Daminet, S. Screening of ragdoll cats for kidney -disease: A retrospective evaluation. J. Small Anim. Pract. 2012, 53, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Guerra, J.M.; Daniel, A.G.T.; Cardoso, N.C.; Grandi, F.; Queiroga, F.; Cogliati, B. Congenital hepatic fibrosis and polycystic kidney disease not linked to C >A mutation in exon 29 of PKD1 in a Persian cat. J. Feline Med. Surg. Open Rep. 2015, 1, 2055116915619191. [Google Scholar] [CrossRef] [Green Version]
- Stebbins, K. Polycystic disease of the kidney and liver in an adult Persian cat. J. Comp. Pathol. 1989, 100, 327–330. [Google Scholar] [CrossRef]
- Northington, J.W.; Juliana, M.M. Polycystic kidney disease in a cat. J. Small Anim. Pract. 1977, 18, 663–666. [Google Scholar] [CrossRef]
- Rendano, V.T.; Parker, R.B. Polycystic kidneys and peritoneopericardial diaphragmatic hernia in the cat: A case report. J. Small Anim. Pract. 1976, 17, 479–485. [Google Scholar] [CrossRef]
- Jones, T.C.; Hunt, R.D.; King, N.W. Cyst in the kidney. In Veterinary Pathology, 6th ed.; Jones, T.C., Hunt, R.D., King, N.W., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1997; pp. 1114–1116. [Google Scholar]
- Cianciolo, R.E.; Williams, K.J. Renal cysts. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 394–397. [Google Scholar]
- Confer, A.W.; Panciera, R.J. Renal cysts. In Thomson’s Special Veterinary Pathology, 3rd ed.; McGavin, M.D., Carlton, W.W., Zachary, J.F., Eds.; Mosby: St. Louis, MO, USA, 2001; pp. 240–241. [Google Scholar]
- Newman, S.J. Renal cysts. In Pathologic Basis of Veterinary Disease, 5th ed.; Zachary, J.F., McGavin, M.D., Eds.; Elsevier: St. Louis, MO, USA, 2012; pp. 618–620. [Google Scholar]
- Koslowski, S.; Latapy, C.; Auvray, P.; Blondel, M.; Meijer, L. An Overview of In Vivo and In Vitro Models for Autosomal Dominant Polycystic Kidney Disease: A Journey from 3D-Cysts to Mini-Pigs. Int. J. Mol. Sci. 2020, 21, 4537. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Appendix Ⅸ Blood Analyte Reference Values in Small and some Laboratory Ani-mals. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Elsevier: Burlington, VT, USA, 2008; pp. 889–895. [Google Scholar]
- Yasuhara, M.; Ohama, T.; Matsuki, N.; Saito, H.; Shiga, J.; Inoue, K.; Kurokawa, K.; Teramoto, T. Induction of fatty liver by fasting in suncus. J. Lipid Res. 1991, 32, 887–891. [Google Scholar] [CrossRef]
- Yasuhara, M.; Ohama, T.; Teramoto, T.; Matsuki, N.; Saito, H.; Matsushima, T.; Kurokawa, K. Deficiency of Apolipoprotein B Synthesis in Suncus murinus. J. Biochem. 1991, 110, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Ohama, T.; Mateuki, N.; Wang, C.-H.; Saito, H.; Kinoshita, M.; Tsukamoto, K.; Kurokawa, K.; Katsuragawa, K.; Yamanaka, M.; Teramoto, T. Characterization of Serum Lipoproteins from Suncus: A Candidate Animal Model for Abetalipoproteinemia. J. Biochem. 1993, 113, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, A.; Matsuki, N.; Saito, H.; Tsukamoto, K.; Wakashima, M.; Kinoshita, M.; Yamanaka, M.; Teramoto, T. Deficiency of Acyl CoA Cholesterol Acyl Transferase Activity in Suncus Liver. J. Biochem. 1994, 115, 858–861. [Google Scholar] [CrossRef]
- Ohama, T.; Matsuki, N.; Saito, H.; Tsukamoto, K.; Kinoshita, M.; Katsuragawa, K.; Okazaki, S.; Yamanaka, M.; Teramoto, T. Effect of Starving and Refeeding on Lipid Metabolism in Suncus. J. Biochem. 1994, 115, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Nagayoshi, A.; Matsuki, N.; Saito, H.; Tsukamoto, K.; Kaneko, K.; Wakashima, M.; Kinoshita, M.; Yamanaka, M.; Teramoto, T. Defect in Assembly Process of Very-Low-Density Lipoprotein in Suncus Liver: An Animal Model of Fatty Liver. J. Biochem. 1995, 117, 787–793. [Google Scholar] [CrossRef]
- Nagayoshi, A.; Saito, H.; Kaneko, K.; Shimazu, N.; Suga, S.; Wakashima, M.; Kinoshita, M.; Yamanaka, M.; Teramoto, T. Role of Acyl Coenzyme A Cholesterol Acyltransferase in Intrahepatic Processing of apo B-Lipoprotein in Suncus. J. Biochem. 1995, 118, 259–264. [Google Scholar] [CrossRef]
- Bush, B.M. Plasma triglycerides. Plasma cholesterol. In Interpretation of Laboratory Results for Small Animal Clinicians; Blackwell Scientific Publications: Hoboken, NJ, USA, 1991; pp. 267–277. [Google Scholar]
- Pedersen, K.M.; Pedersen, H.D.; Häggström, J.; Koch, J.; Elsbøll. Increased mean arterial pressure and aldosterone-to-renin ratio in Persian cats with polycystic kidney disease. J. Vet. Intern. Med. 2003, 17, 21–27. [Google Scholar]
- Wilson, P.D.; Norman, J.T.; Kuo, N.T.; Burrow, C.R. Abnormalities in extracellular matrix regulation in autosomal domi-nant polycystic kidney disease. Contrib. Nephrol. 1996, 118, 126–134. [Google Scholar]
- Grantham, J.J.; Mulamalla, S.; Swenson-Fields, K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2011, 7, 556–566. [Google Scholar] [CrossRef]
- Xue, C.; Mei, C.-L. Polycystic Kidney Disease and Renal Fibrosis. Ren. Fibros. Mech. Ther. 2019, 1165, 81–100. [Google Scholar] [CrossRef]
- Whary, M.T.; Baumagarth, N.; Fox, J.G.; Barthold, S.W. Urinary tract. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: London, UK, 2015; pp. 133–134. [Google Scholar]
- Jang, H.-S.; Kim, J.I.; Jung, K.-J.; Kim, J.; Han, K.-H.; Park, K.M. Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; Ruedl, C.; Karjalainen, K. Most tissue-resident macrophages except microglia are derived from fetal hematopoiet-ic stem cells. Immunity 2015, 43, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, D.A.D.; Hughes, J. The Origins and Functions of Tissue-Resident Macrophages in Kidney Development. Front. Physiol. 2017, 8, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, K.A.; Yang, Z.; Lever, J.M.; Li, Z.; Croyle, M.J.; Agarwal, A.; Yoder, B.K.; George, J.F. Kidney resident macrophages in the rat have minimal turnover and replacement by blood monocytes. Am. J. Physiol. Physiol. 2021, 321, F162–F169. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.A.; Song, C.J.; Li, Z.; Lever, J.M.; Crossman, D.K.; Rains, A.; Aloria, E.J.; Gonzalez, N.C.; Bassler, J.R.; Zhou, J.; et al. Tissue-resident macro-phages promote renal cystic disease. J. Am. Soc. Nephrol. 2019, 30, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zimmerman, K.A.; Yoder, B.K. Resident Macrophages in Cystic Kidney Disease. Kidney360 2020, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]




| Items | Data |
|---|---|
| Chromosome number | 40 |
| Body weight at sexual maturity | Male: 50–70 g Female: 30–50 g |
| The average of life span | 1–1.5 years |
| Sexual cycle | Copulatory ovulator Persistent estrus |
| The average gestation period | 30 days |
| Range number of offspring (average) | 4 to 8 (3) |
| The range weaning age | 20 to 21 days |
| The average basal metabolic rate | 0.403 W |
| Parameters | Measurements | Reference Values (Mean ± SD) * |
|---|---|---|
| TP (g/L) | 70 | 54.9 ± 4.2 |
| Alb (g/L) | 21 | 23.6 ± 3.0 |
| A/G ratio | 0.43 | 0.74 ± 0.09 |
| T-BIL (μmol/L) | 1.71 | 3.08 ± 2.05 |
| BUN (mmol/L) | >49.98 | 23.75 ± 4.36 |
| CRE (μmol/L) | 114.39 | 41.18 ± 25.93 |
| UA (μmol/L) | 47.58 | 111.23 ± 63.64 |
| GLU (mmol/L) | 2.94 | 12.37 ± 5.19 |
| T-CHO (mmol/L) | 3.99 | 0.96 ± 0.19 |
| TG (mmol/L) | 0.53 | 0.28 ± 0.13 |
| AST (U/L) | 285 | 590.87 ± 222.99 |
| ALT (U/L) | 78 | 255.33 ± 146.51 |
| GGT (U/L) | 17 | 24.26 ± 14.87 |
| LDH (U/L) | 186 | 606.87 ± 284.13 |
| ALP (U/L) | 1 | 61.07 ± 21.17 |
| ChE (U/L) | 1 | 1.20 ± 0.78 |
| LAP (U/L) | 26 | 34.93 ± 7.10 |
| AMY (U/L) | 2234 | 1360.87 ± 213.43 |
| CK(U/L) | >2000 | 1288.73 ± 684.33 |
| Na (mmol/L) | 169 | 161.87 ± 4.76 |
| K (mmol/L) | 6 | 4.43 ± 0.73 |
| Cl (mmol/L) | 143 | 121.60 ± 3.66 |
| Ca (mmol/L) | 4.15 | 5.22 ± 0.33 |
| IP (mmol/L) | 1.823 | 2.94 ± 0.53 |
| Mg (mmol/L) | 2.05 | 1.70 ± 0.21 |
| SAA (μg/mL) | 16 | 1.90 ± 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, T. Spontaneous Polycystic Kidneys with Chronic Renal Failure in an Aged House Musk Shrew (Suncus murinus). Vet. Sci. 2022, 9, 123. https://doi.org/10.3390/vetsci9030123
Kimura T. Spontaneous Polycystic Kidneys with Chronic Renal Failure in an Aged House Musk Shrew (Suncus murinus). Veterinary Sciences. 2022; 9(3):123. https://doi.org/10.3390/vetsci9030123
Chicago/Turabian StyleKimura, Tohru. 2022. "Spontaneous Polycystic Kidneys with Chronic Renal Failure in an Aged House Musk Shrew (Suncus murinus)" Veterinary Sciences 9, no. 3: 123. https://doi.org/10.3390/vetsci9030123
APA StyleKimura, T. (2022). Spontaneous Polycystic Kidneys with Chronic Renal Failure in an Aged House Musk Shrew (Suncus murinus). Veterinary Sciences, 9(3), 123. https://doi.org/10.3390/vetsci9030123






