Prevalence and Molecular Characterization of Echinococcus granulosus Sensu Lato Eggs among Stray Dogs in Sulaimani Province—Kurdistan, Iraq
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection of Dog Faeces Samples
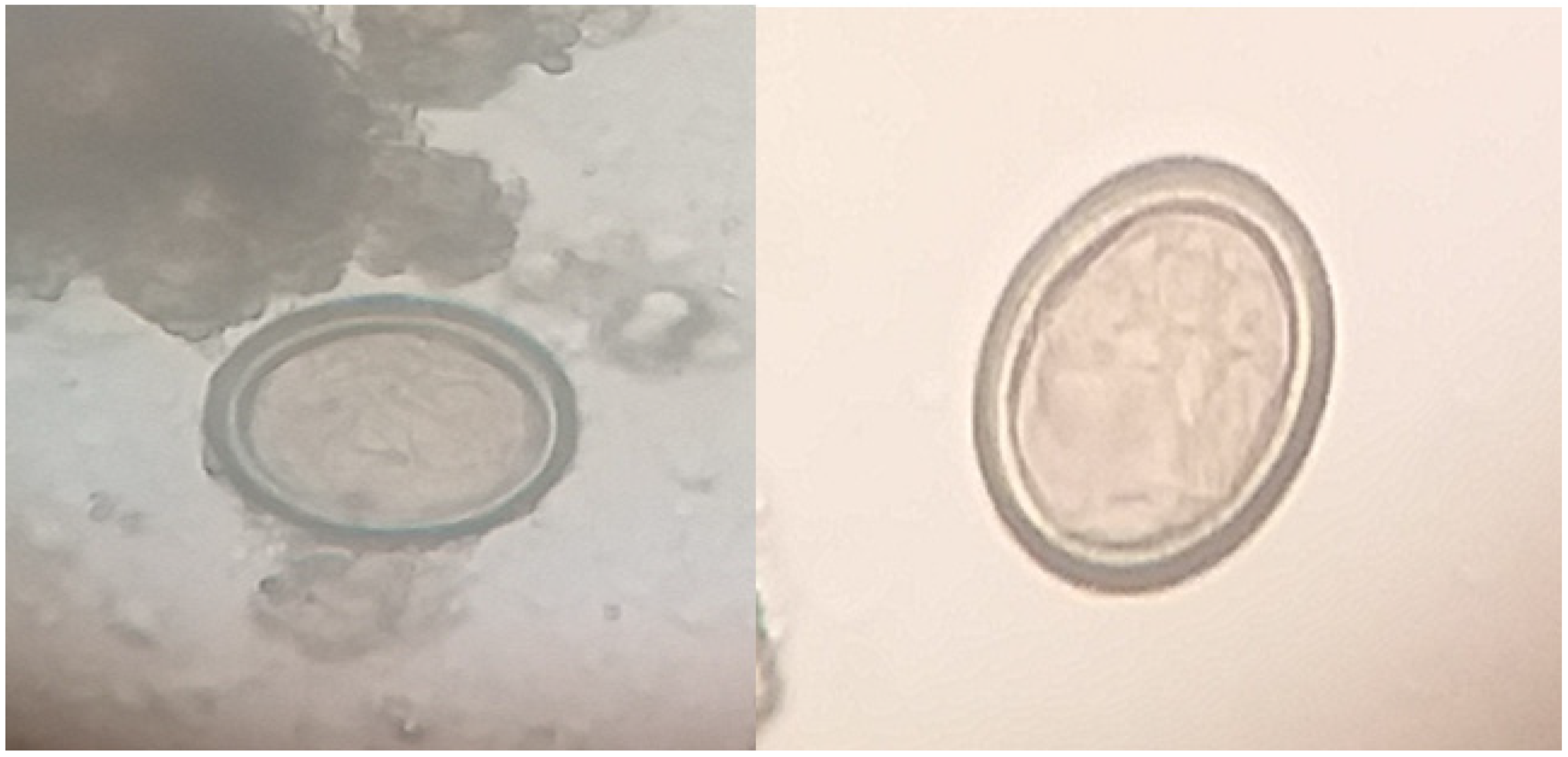
2.3. Isolation of Eggs
2.4. DNA Extraction
2.5. PCR Amplification and Sequencing
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinquepalmi, V.; Monno, R.; Fumarola, L.; Ventrella, G.; Calia, C.; Greco, M.F.; Vito, D.D.; Soleo, L. Environmental Contamination by Dog’s Faeces: A Public Health Problem. J. Environ. Res. Public Health 2013, 10, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Mwambete, K.D.; Ponce-Gordo, F.; Cuesta-Bandera, C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 2004, 91, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Gemmell, M.A.; Meslin, F.X.; Pawlowski, Z.S. (Eds.) Humans and safety precautions. In Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; OIE and WHO: Paris, France, 2001; pp. 238–248. [Google Scholar]
- Torgerson, P.R.; Macpherson, C.N. The socioeconomic burden of parasitic zoonoses: Global trends. Vet. Parasitol. 2011, 182, 79–95. [Google Scholar] [CrossRef]
- Candela, M.G.; Fanelli, A.; Carvalho, J.; Serrano, E.; Domenech, G.; Alonso, F.; Martínez-Carrasco, C. Urban landscape and infection risk in free-roaming cats. Zoonoses Public Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Armua-Fernandez, M.T.; Castro, O.F.; Crampet, A.; Bartzabal, Á.; Hofmann-Lehmann, R.; Grimm, F.; Deplazes, P. First case of peritoneal cystic echinococcosis in a domestic cat caused by Echinococcus granulosus sensu stricto (genotype 1) associated to feline immunodeficiency virus infection. Parasitol. Int. 2014, 63, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Oguz, B.; Selcin, O.; Deger, M.S.; Bicek, K.; Ozdal, N. A Case Report of Echinococcus granulosus sensu stricto (G1) in a Domestic Cat in Turkey. J. Hell. Vet. Medical Soc. 2021, 72, 3529–3534. [Google Scholar] [CrossRef]
- Al-Eodawee, E.M.M. Direct immunodiagnosis of Echinococcus granulosus in feces of stray, companion, and policy dogs in Baghdad province—Iraq. AL-Qadisiyah J. Vet. Med. Sci. 2016, 15, 97–101. [Google Scholar]
- Mastin, A.; van Kesteren, F.; Torgerson, P.R.; Ziadinov, B.; Mytynova, M.T.; Rogan, T.; Tursunov, T.; Craig, P.S. Risk factors for Echinococcus coproantigen positivity in dogs from the Alay valley, Kyrgyzstan. J. Helminthol. 2015, 89, 655–663. [Google Scholar] [CrossRef]
- Satoskar, A.R.; Simon, G.L.; Hotez, P.J.; Tsuji, M. Medical Parasitology; Landes Bioscience: Austin, TX, USA, 2009; p. 320. [Google Scholar]
- Sadjjadi, S.M. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol. Int. 2006, 55, S197–S202. [Google Scholar] [CrossRef]
- Keyhani, A.; Sharifi, I.; Bamorovat, M.; Mohammadi, M.A.; Askari, A.; Ebrahimipou, M.; Harandi, M.F. Epidemiological and molecular studies on Echinococcus granulosus from free-roaming dogs in Southeast Iran. Vet. World 2020, 13, 739–745. [Google Scholar] [CrossRef]
- Bonelli, P.; Masu, G.; Dei Giudici, S.; Pintus, D.; Peruzzu, A.; Piseddu, T.; Santucciu, C.; Cossu, A.; Demurtas, N.; Masala, G. Cystic echinococcosis in a domestic cat (Felis catus) in Italy. Parasite 2018, 25, 25. [Google Scholar] [CrossRef]
- Wachira, T.M.; Macpherson, C.N.L.; Gathuma, J.M. Release and survival of Echinococcus eggs in different environments in Turkana, and their possible impact on the incidence of hydatidosis in man and livestock. J. Helminthol. 1991, 65, 55–61. [Google Scholar] [CrossRef]
- Rahif, R.H.; Al-Naimi, U.A.M. The Effect of environmental temperature in the growth and development of Echinococcus granulosus (Batsch1786). Iraqi J. Vet. Med. 2005, 29, 65–75. [Google Scholar] [CrossRef]
- Gemmell, M.A.; Roberts, M.G. Cystic Echinococcosis (Echinococcus granulosus). In Zoonoses; Palmer, S.R., Soulsby, E.J.L., Simpson, D.I.H., Eds.; Oxford University Press: Oxford, UK, 1998; pp. 665–688. [Google Scholar]
- Kozak, G.K.; MacDonald, D.; Landry, L.; Farber, J.M. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J. Food Prot. 2013, 76, 173–183. [Google Scholar] [CrossRef]
- Kang, G.; Mathew, M.S.; Ranjan, P.D.; Daniel, J.D.; Mathan, M.M.; Mathan, V.; Muliyil, J. Prevalence of intestinal parasites in rural Southern Indians. Trop. Med. Int. Health 1998, 3, 70–75. [Google Scholar] [CrossRef]
- Darchenkova, N.N.; Romanenko, N.A.; Chernyshenko, A.L. Current ascariasis situation in the Russian Federation. Meditsinskaia Parazitol. I Parazit. Bolezn. 2006, 4, 40–43. [Google Scholar]
- Taghipour, A.; Javanmard, E.; Haghighi, A.; Mirjalali, H.; Zali, M.R. The occurrence of Cryptosporidium sp., and eggs of soil-transmitted helminths in market vegetables in the north of Iran. Gastroenterol. Hepatol. Bed Bench 2019, 12, 364. [Google Scholar]
- Hama, A.A.; Mero, W.M.; Jubrael, J.M. Molecular identification of Echinococcus granulosus (G1) strain in human and animals. Sci. J. Univ. Zakho. 2013, 1, 1–7. [Google Scholar]
- Khan, H.; Ahmed, H.; Afzal, M.S.; Awan, U.A.; Khurram, M.; Simsek, S.; Cao, J. Detection of Anti-Echinococcus granulosus Antibodies in Humans: An Update from Pakistan. Pathogens 2022, 11, 29. [Google Scholar] [CrossRef]
- Dryden, M.W.; Payne, P.A.; Ridley, R.; Smith, V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther. 2005, 6, 15–28. [Google Scholar]
- Morseth, D.J. Ultrastructure of developing taeniid embryophores and associated structures. Exp. Parasitol. 1965, 16, 207–216. [Google Scholar] [CrossRef]
- Mathis, A.; Deplazes, P. Copro-DNA tests for diagnosis of animal taeniid cestodes. Parasitol Int. 2006, 55, 87–90. [Google Scholar] [CrossRef]
- Brummaier, T.; Archasuksan, L.; Watthanakulpanich, D.; Paris, D.H.; Utzinger, J.; McGready, R.; Proux, S.; Nosten, F. Improved detection of intestinal helminth infections with a formalin ethyl-acetate-based concentration technique compared to a crude formalin concentration technique. Trop. Med. Infect. Dis. 2021, 6, 51. [Google Scholar] [CrossRef]
- Bowles, J.; McManus, D.P. Molecular variation in Echinococcus. Acta Trop. 1993, 53, 291–305. [Google Scholar] [CrossRef]
- Mero, W.M.S.; Jubrael, J.M.S.; Hama, A.A. Prevalence of Hydatid Disease among Slaughtered Animals in Slemani Province/Kurdistan-Iraq. Sci. J. Uni. Zakho 2018, 2, 33–38. [Google Scholar] [CrossRef]
- Kohansal, M.H.; Nourian, A.; Haniloo, A.; Fazaeli, A. Molecular detection of Taenia spp. in dogs’ feces in Zanjan Province, Northwest of Iran. Vet. World 2017, 10, 445–449. [Google Scholar] [CrossRef][Green Version]
- Khamesipour, F.; Shojaat, S.; Basirpour, B.; Kheiri, P.; Afzal, S.; Chelgerdi, B.; Nezaratizadeh, S.; Hejazi, S.H. Infection status of hydatid cysts in Iran: A review. Infect. Diseases Herb. Med. 2021, 2. [Google Scholar] [CrossRef]
- Kawasmeh, Z.A.; Cheema, A.H.; Shigidi, M.T. Prevalence of Echinococcosis/hydatidosis in stray dogs and slaughtered animals in Al-Hassa region. In Symposium on Biological Aspects of Saudi Arabia; Abu-Zinada, A.H., Ed.; Saudi Arabia Biological Society: Riyadh, Saudi Arabia, 1984; pp. 79–80. [Google Scholar]
- Hassan, Z.I.; Meerkhan, A.A.; Boufana, B.; Hama, A.A.; Ahmed, B.D.; Mero, W.M.S.; Orsten, S.; Interisano, M.; Pozio, E.; Casulli, A. Two haplotype clusters of Echinococcus granulosus sensu stricto in northern Iraq (Kurdistan region) support the hypothesis of a parasite cradle in the Middle East. Acta Trop. 2017, 172, 201–207. [Google Scholar] [CrossRef]
- Hama, A.A.; Hassan, Z.I.; Salih Mero, W.M.; Interisano, M.; Boufana, B.; Casulli, A. A Morphologically Unusual Echinococcus granulosus (G1 Genotype) Cyst in a Cow from Kurdistan—Iraq. Epidemiol. Open Access 2015, 5, 2161-1165. [Google Scholar]
- Ahmadi, N.A.; Dalimi, A. Molecular characterization of Echinococcus granulosus isolated from sheep and camel in Iran. Arch. Razi Inst. 2002, 53, 47–56. [Google Scholar]
- Bart, J.M.; Abdukader, M.; Zhang, Y.L.; Lin, R.Y.; Wang, Y.H.; Nakao, M.; Ito, A.; Craig, P.S.; Piarroux, R.; Vuitton, D.A.; et al. Genotyping of human cystic Echinococcosis in Xinjiang, PR China. Parasitology 2006, 133, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Varcasia, A.; Canu, S.; Kogkos, A.; Pipia, A.P.; Scala, A.; Garippa, G.; Seimenis, A. Molecular characterization of Echinococcus granulosus in sheep and goats of Peloponnesus, Greece. Parasitol. Res. 2007, 101, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Bera, A.K.; Bera, B.C.; Maity, A.; Das, S.K. Genotypic characterization of Indian cattle, buffalo, and sheep isolates of Echinococcus granulosus. Vet. Parasitol. 2007, 143, 371–374. [Google Scholar] [CrossRef] [PubMed]


| Area | Total Samples | Negative Samples | Positive Samples | Percentage of Positive from Total Samples | Statistical Analysis Chi-Square Test |
|---|---|---|---|---|---|
| Sulaimani City | 130 | 100 | 30 | 7.50 | χ2 = 5.58 p = 0.1335 |
| Halabja | 115 | 87 | 28 | 7.00 | |
| Kalar | 120 | 95 | 25 | 6.25 | |
| Rzgari | 35 | 21 | 14 | 3.50 | |
| Total | 400 | 303 | 97 | 24.25 |
| Study Area | DNA Extraction from Dog Faecal Samples | Positive by PCR |
|---|---|---|
| Sulaimani | 15 | 14 (93.3%) |
| Halabja | 12 | 10 (83.3%) |
| Kalar | 13 | 11 (84.6%) |
| Rzgari | 10 | 5 (50%) |
| Total | 50 | 40 (80%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, H.M.; Hama, A.A.; Salih, M.A.H.; Ditta, A. Prevalence and Molecular Characterization of Echinococcus granulosus Sensu Lato Eggs among Stray Dogs in Sulaimani Province—Kurdistan, Iraq. Vet. Sci. 2022, 9, 151. https://doi.org/10.3390/vetsci9040151
Aziz HM, Hama AA, Salih MAH, Ditta A. Prevalence and Molecular Characterization of Echinococcus granulosus Sensu Lato Eggs among Stray Dogs in Sulaimani Province—Kurdistan, Iraq. Veterinary Sciences. 2022; 9(4):151. https://doi.org/10.3390/vetsci9040151
Chicago/Turabian StyleAziz, Hazhar M., Abdullah A. Hama, Mariwan A. Hama Salih, and Allah Ditta. 2022. "Prevalence and Molecular Characterization of Echinococcus granulosus Sensu Lato Eggs among Stray Dogs in Sulaimani Province—Kurdistan, Iraq" Veterinary Sciences 9, no. 4: 151. https://doi.org/10.3390/vetsci9040151
APA StyleAziz, H. M., Hama, A. A., Salih, M. A. H., & Ditta, A. (2022). Prevalence and Molecular Characterization of Echinococcus granulosus Sensu Lato Eggs among Stray Dogs in Sulaimani Province—Kurdistan, Iraq. Veterinary Sciences, 9(4), 151. https://doi.org/10.3390/vetsci9040151








