Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE
Abstract
:1. Introduction
2. Materials and Methods
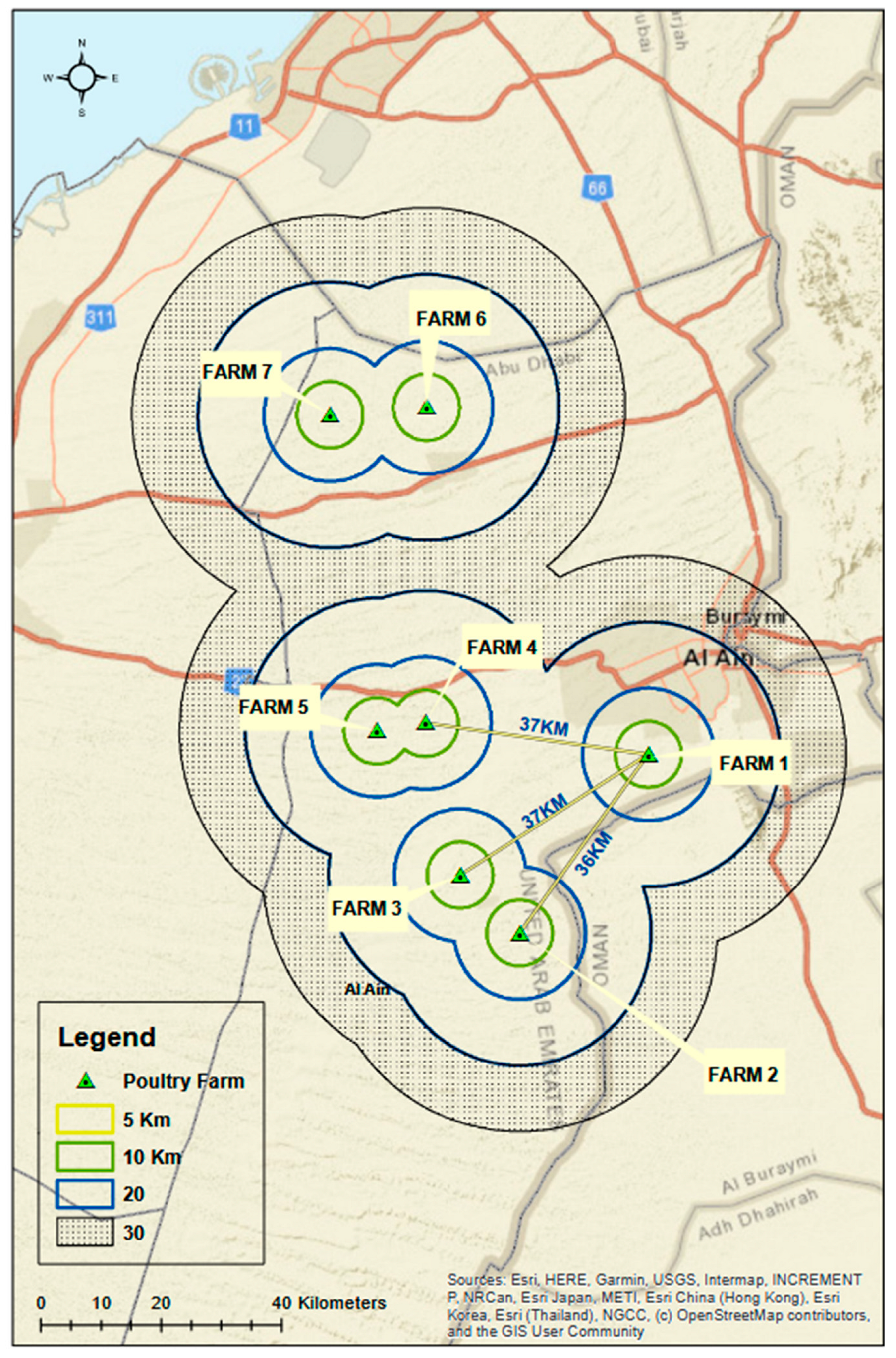
2.1. Epidemiological Data, Necropsy and Sampling
2.2. Histopathological Examination
2.3. SYBR Green-Based Quantitative Real-Time PCR (qPCR) of FAdV
2.4. Hexon Gene Sequencing and Phylogenetic Analysis
2.4.1. Hexon Gene Amplification
2.4.2. Sanger Sequencing
2.4.3. Sequence Alignment and Phylogenetic Analysis
3. Results
3.1. Epidemiological Data and Sampling
3.2. Clinical and Pathological: Findings
3.3. Histological Analysis
3.4. Molecular Analysis
3.4.1. The qPCR
3.4.2. Sequencing and Phylogenetic Analysis of FAdV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Yin, L.; Zhou, Q.; Peng, P.; Du, Y.; Liu, L.; Zhang, Y.; Xue, C.; Cao, Y. Epidemiological investigation of fowl adenovirus infections in poultry in China during 2015–2018. BMC Vet. Res. 2019, 15, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Ashraf, A.; Khan, M.; Rahman, M.; Habib, M.; Chughtai, M.; Qureshi, J. Fowl adenovirus: History, emergence, biology and development of a vaccine against hydropericardium syndrome. Arch. Virol. 2017, 162, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Harrach, B.; Benko, M. Phylogenetic analysis of adenovirus sequences. In Adenovirus Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2007; pp. 299–334. [Google Scholar]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef]
- Domanska-Blicharz, K.; Tomczyk, G.; Smietanka, K.; Kozaczynski, W.; Minta, Z. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult. Sci. 2011, 90, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-H.; Lee, H.-J.; Lee, D.-H.; Lee, Y.-N.; Park, J.-K.; Youn, H.-N.; Kim, M.-S.; Youn, H.-S.; Lee, J.-B.; Park, S.-Y. Identification and virulence characterization of fowl adenoviruses in Korea. Avian Dis. 2011, 55, 554–560. [Google Scholar] [CrossRef]
- Mase, M.; Nakamura, K.; Minami, F. Fowl adenoviruses isolated from chickens with inclusion body hepatitis in Japan, 2009–2010. J. Vet. Med. Sci. 2012, 74, 1087–1089. [Google Scholar] [CrossRef] [Green Version]
- Ojkic, D.; Martin, E.; Swinton, J.; Vaillancourt, J.-P.; Boulianne, M.; Gomis, S. Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathol. 2008, 37, 95–100. [Google Scholar] [CrossRef]
- Kim, J.N.; Byun, S.H.; Kim, M.J.; Kim, J.J.; Sung, H.W.; Mo, I.P. Outbreaks of hydropericardium syndrome and molecular characterization of Korean fowl adenoviral isolates. Avian Dis. 2008, 52, 526–530. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Qiu, L.; Han, Z.; Liu, S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect. Genet. Evol. 2016, 45, 230–241. [Google Scholar] [CrossRef]
- Lobanov, V.; Borisov, V.; Borisov, A.; Drygin, V.; Gusev, A.; Shmarov, M.; Akopian, T.; Naroditskiĭ, B. Sequence analysis of hexon gene from adenovirus KR95 inducing hydropericardium syndrome in chickens. Mol. Genet. Mikrobiol. Virusol. 2000, 30–36. [Google Scholar]
- Kaján, G.L.; Affranio, I.; Bistyák, A.T.; Kecskeméti, S.; Benkő, M. An emerging new fowl adenovirus genotype. Heliyon 2019, 5, e01732. [Google Scholar] [CrossRef] [Green Version]
- Okuda, Y.; Ono, M.; Shibata, I.; Sato, S. Pathogenicity of serotype 8 fowl adenovirus isolated from gizzard erosions of slaughtered broiler chickens. J. Vet. Med. Sci. 2004, 66, 1561–1566. [Google Scholar] [CrossRef] [Green Version]
- Okuda, Y.; Ono, M.; Shibata, I.; Sato, S.; Akashi, H. Comparison of the polymerase chain reaction-restriction fragment length polymorphism pattern of the fiber gene and pathogenicity of serotype-1 fowl adenovirus isolates from gizzard erosions and from feces of clinically healthy chickens in Japan. J. Vet. Diagn. Investig. 2006, 18, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asrani, R.; Gupta, V.; Sharma, S.; Singh, S.; Katoch, R. Hydropericardium-hepatopathy syndrome in Asian poultry. Vet. Rec. 1997, 141, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Cizmecigil, U.Y.; Umar, S.; Yilmaz, A.; Bayraktar, E.; Turan, N.; Tali, B.; Aydin, O.; Tali, H.E.; Yaramanoglu, M.; Yilmaz, S.G.; et al. Characterisation of fowl adenovirus (FAdV-8b) strain concerning the geographic analysis and pathological lesions associated with inclusion body hepatitis in broiler flocks in Turkey. J. Vet. Res. 2020, 64, 231. [Google Scholar] [CrossRef] [PubMed]
- De Herdt, P.; Timmerman, T.; Defoort, P.; Lycke, K.; Jaspers, R. Fowl adenovirus infections in Belgian broilers: A ten-year survey. Vlaams Diergeneeskd. Tijdschr. 2013, 82, 125–133. [Google Scholar] [CrossRef]
- Maartens, L.H.; Joubert, H.W.; Aitchison, H.; Venter, E.H. Inclusion body hepatitis associated with an outbreak of fowl adenovirus type 2 and type 8b in broiler flocks in South Africa. J. S. Afr. Vet. Assoc. 2014, 85, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Sun, Q.; Zhu, M.; Zhao, J.; Zhang, G.; Liu, X.; Xiao, Y.; Liu, S. Molecular epidemiology and phylogenetic analysis of fowl adenoviruses caused hydropericardium outbreak in China during 2015. Poult. Sci. 2018, 97, 803–811. [Google Scholar] [CrossRef]
- Günes, A.; Marek, A.; Grafl, B.; Berger, E.; Hess, M. Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A to FAdV-E). J. Virol. Methods 2012, 183, 147–153. [Google Scholar] [CrossRef]
- Mase, M.; Mitake, H.; Inoue, T.; Imada, T. Identification of group I-III avian adenovirus by PCR coupled with direct sequencing of the hexon gene. J. Vet. Med. Sci. 2009, 71, 1239–1242. [Google Scholar] [CrossRef] [Green Version]
- Maged Gomaa Hemida, M.A.-H. Prevalence and molecular characteristics of fowl adenovirus serotype 4 in eastern Saudi Arabia. Turk. J. Vet. Anim. Sci. 2017, 41, 506–513. [Google Scholar] [CrossRef]
- Abdul-Aziz, T.; Al-Attar, M. New syndrome in Iraqi chicks. Vet. Rec. 1991, 129, 272. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M. Avian Adenoviruses Infections with Special Attention to Inclusion Body Hepatitis/Hydropericardium. Pak. J. Zool. 2009, 41, 269–276. [Google Scholar]
- Sharif, N.; Mehmood, M.D.; Naqvi, S.Z.H.; Ul-Haq, H.A.; Ahmed, S.S.; Ghani, M.U.; Shoaib, M.; Hussain, M. PCR Based Detection and Phylogenetic Analysis of Fowl Adenovirus Strains Isolated from 2019 Epidemic from Punjab and Sindh, Pakistan. Am. J. Mol. Biol. 2020, 10, 246–258. [Google Scholar] [CrossRef]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl adenovirus-induced diseases and strategies for their control–a review on the current global situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef]
- Ledwoń, A.; Bailey, T.; O’Donovan, D.; Mckeown, S.; Lloyd, C.; Więckowski, T.; Kinne, J.; Silvanose, C.; Szeleszczuk, P.; Wernery, U. Prevalence of circovirus and adenovirus in pigeons in Dubai. Med. Weter 2011, 67, 752–756. [Google Scholar]
- Bello, A.; Umaru, M.; Baraya, Y.; Adamu, Y.; Jibir, M.; Garba, S.; Hena, S.; Raji, A.; Saidu, B.; Mahmuda, A. Postmortem procedure and diagnostic avian pathology. J. Zool. 2012, 229, 1–5. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Abghour, S.; Zro, K.; Mouahid, M.; Tahiri, F.; Tarta, M.; Berrada, J.; Kichou, F. Isolation and characterization of fowl aviadenovirus serotype 11 from chickens with inclusion body hepatitis in Morocco. PLoS ONE 2019, 14, e0227004. [Google Scholar] [CrossRef]
- Madden, T. The BLAST Sequence Analysis Tool, 2nd ed.; US National Center for Biotechnology Information: Bethesda, MD, USA, 2003. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Winterfield, R.; Fadly, A.; Gallina, A. Adenovirus infection and disease. I. Some characteristics of an isolate from chickens in Indiana. Avian Dis. 1973, 17, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Naeem, K.; Niazi, T.; Malik, S.; Cheema, A. Immunosuppressive potential and pathogenicity of an avian adenovirus isolate involved in hydropericardium syndrome in broilers. Avian Dis. 1995, 39, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.K.; Hussain, I.; Arshad, M.; Muhammad, G.; Hussain, M.H.; Mehmood, M.S. Molecular characterization of fowl adenovirus serotype 4 (FAV-4) isolate associated with fowl hydropericardium-hepatitis syndrome in Pakistan. Pak. J. Zool. 2009, 41, 269–276. [Google Scholar]
- Parthiban, M.; Manoharan, S.; Roy, P.; Chandran, N.; Aruni, A.W.; Koteeswaran, A. Nucleotide sequence analysis of the L1 loop variable region of hexon gene of fowl adenovirus 4 isolates from India. Acta Virol. 2005, 49, 65–68. [Google Scholar]
- Hess, M.; Raue, R.; Prusas, C. Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol. 1999, 28, 433–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Tian, K.; Wang, Z.; Yang, X.; Gao, D.; Zhang, Y.; Fu, J.; Wang, H.; Zhao, J. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, A.; Wang, Y.; Cui, H.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Li, K.; Gao, L.; et al. A Single Amino Acid at Residue 188 of the Hexon Protein Is Responsible for the Pathogenicity of the Emerging Novel Virus Fowl Adenovirus 4. J. Virol. 2021, 95, JVI0060321. [Google Scholar] [CrossRef]






| Farm | Outbreak | Age | Sampling Date | Population | Infected | Death | Morbidity Rate (%) | Mortality Rate (%) | Case Fatality Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | NA | 2 March 2020 | NA | |||||
| 2 | 7–14 days | 11 April 2020 | NA | ||||||
| 2 | 3 | 25 days | 28 July 2020 | 9000 | 9000 | 6000 | 100% | 67% | 67% |
| 3 | 4 | 21 days | 22 September 2020 | 20,000 | 20,000 | 16,000 | 100% | 80% | 80% |
| 4 | 5 | 17 days | 27 September 2020 | 16,000 | 8000 | 7000 | 50% | 44% | 88% |
| 5 | 6 | 17 days | 28 September 2020 | 9000 | 9000 | 9000 | 100% | 100% | 100% |
| 6 | 7 | NA | 28 September 2020 | 107,839 | 4000 | 916 | 3.70% | 85% | 23% |
| 8 | |||||||||
| 7 | 9 | 14 days | 24 November 2020 | 11,000 | 11,000 | 11,000 | 100% | 100% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishag, H.Z.A.; Terab, A.M.A.; El Tigani-Asil, E.T.A.; Bensalah, O.K.; Khalil, N.A.H.; Khalafalla, A.I.; Al Hammadi, Z.M.A.H.; Shah, A.A.M.; Al Muhairi, S.S.M. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Vet. Sci. 2022, 9, 154. https://doi.org/10.3390/vetsci9040154
Ishag HZA, Terab AMA, El Tigani-Asil ETA, Bensalah OK, Khalil NAH, Khalafalla AI, Al Hammadi ZMAH, Shah AAM, Al Muhairi SSM. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Veterinary Sciences. 2022; 9(4):154. https://doi.org/10.3390/vetsci9040154
Chicago/Turabian StyleIshag, Hassan Zackaria Ali, Abdelnasir Mohammed Adam Terab, El Tigani Ahmed El Tigani-Asil, Oum Keltoum Bensalah, Nasereldien Altaib Hussein Khalil, Abdelmalik Ibrahim Khalafalla, Zulaikha Mohamed Abdel Hameed Al Hammadi, Asma Abdi Mohamed Shah, and Salama Suhail Mohammed Al Muhairi. 2022. "Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE" Veterinary Sciences 9, no. 4: 154. https://doi.org/10.3390/vetsci9040154






