Abstract
Thailand is a tropical country affected by global climate change and has high temperatures and humidity that cause heat stress in livestock. A temperature–humidity index (THI) is required to assess and evaluate heat stress levels in livestock. One of the livestock types in Thailand experiencing heat stress due to extreme climate change is crossbred dairy cattle. Genetic evaluations of heat tolerance in dairy cattle have been carried out for reproductive traits. Heritability values for reproductive traits are generally low (<0.10) because environmental factors heavily influence them. Consequently, genetic improvement for these traits would be slow compared to production traits. Positive and negative genetic correlations were found between reproductive traits and reproductive traits and yield traits. Several selection methods for reproductive traits have been introduced, i.e., the traditional method, marker-assisted selection (MAS), and genomic selection (GS). GS is the most promising technique and provides accurate results with a high genetic gain. Single-step genomic BLUP (ssGBLUP) has higher accuracy than the multi-step equivalent for fertility traits or low-heritability traits.
1. Introduction
As global temperatures have risen, heat stress in dairy cattle has become a severe concern for the worldwide dairy sector, especially in tropical countries. The average temperature on Earth increased by 1.09 °C during 2011–2020 [1]. Thailand is one of the countries that has experienced climate change. Thailand has a tropical climate with high relative humidity and high ambient temperatures. Thailand’s average temperature is 35.0–39.9 °C, with 72–74 percent relative humidity [2]. According to the Thai Meteorological Department, the annual mean temperature in Thailand increased by about 1.50 °C from 1970 to 2007 [3]. Hence, livestock in Thailand faces heat stress, especially dairy cattle.
Heat stress is caused by changes in the environment around the animal, including temperature, relative humidity, solar radiation, and wind speed [4]. The high temperature and humidity in Thailand may be the main factors causing heat stress in livestock. Livestock cannot dissipate body heat because of higher environmental heat intensity [5]. This causes animals to pass through the range of the thermoneutral zone, and heat stress will occur [6]. Heat stress also affects livestock reproduction. The direct effects are changes in endocrine regulation, interference of ovarian activity concerning steroid hormone synthesis, and altered progesterone concentrations [2]. Consequently, heat stress reduces estrous activity and impairs fertility performance, including long days open, a greater length of the calving interval, and a low conception rate [7]. Heat stress also has various adverse effects on milk yield and milk quality, thus harming farmers financially [8]. Optimizing feed and housing conditions are two techniques for minimizing the consequences of heat stress [9]. Although these methods can be successful in the short term, they may not be adequate for long-term strategies. Improving genetics, identifying and breeding animals with a natural resilience to extreme heat and humidity and establishing management approaches for long-term solutions should be performed in concert.
Understanding the genetic response to heat stress could help improve the management of feed or housing modifications for dairy cows. Ravagnolo and Misztal [10] proposed a strategy for examining the impact of heat stress on the genetic response of dairy cows using weather station data to predict the level of heat stress tolerance on the performance of dairy cattle in the United States. Tolerance to heat stress is the adaptive process that allows animals to endure the impacts of rising ambient temperature beyond the thermal neutral zone’s temperature–humidity index (THI) limit [11]. The THI limit value at which performance begins to decrease can determine the level of heat tolerance in animals. A THI value of 72 for production, and roughly 68 for reproduction was considered the threshold for heat stress [12].
Genetic differences in thermal tolerance between animals provide clues for selecting animals that are more tolerant to heat stress [13]. Previous studies on heat tolerance found genetic variation in the reproductive performance of dairy cattle under heat stress conditions [9,14,15]. Therefore, genetic improvement via the identification and selection for tolerance to heat in animals for reproductive traits is possible. In general, the genetic improvement of dairy cattle is cost-effective since it results in long-term and cumulative change [16]. However, due to low heritability values and large generation intervals, the traditional method’s rate of genetic improvement is low [17]. Compared to traditional breeding, genome selection is more appropriate for selecting heat tolerance, as it allows faster genetic gain [11]. To predict the breeding value of selection animals, genomic selection uses single-nucleotide polymorphisms (SNPs), genetic markers found throughout the genome [18]. This study aims to investigate the appropriate genetic approach for improving reproductive traits in Thai–Holstein crossbreeds under heat stress conditions.
2. Thai–Holstein Crossbred Cattle
In Thailand, dairy cattle are primarily crossbreeds of Bos taurus (Holstein, Jersey, Brown Swiss, and Red Dene) and Bos indicus (Sahiwal, Red Sindhi, Brahman, and Thai Native). However, most (>95%) are crosses between Holstein and Sahiwal cattle or Thai Native breeds [9]. According to the Thai Department of Livestock Development, Thailand’s dairy cattle population was reported to be 818,537 heads in 2021, with 358,500 cows on 24,764 farms, producing roughly 3000 tons of raw milk each day [19,20]. Most dairy farmers in Thailand (>80%) are smallholders, with only a few large commercial dairy farms [19].
The dairy cattle breeding improvement program in Thailand has been carried out for 60 years. In the beginning, both live purebred dairy cows and frozen semen were imported from subtropical countries, followed by an artificial insemination (AI) program in 1956. Thai Native cattle were inseminated with that semen, and now, they are being developed as crossbred Thai dairy cattle, with Holstein Friesian (HF) selected as the predominant breed [20]. The purpose of crossing those two breeds was to improve milk production, reproduction, and the tolerance of heat, tick-borne diseases, and other diseases of zebu cattle [21,22]. The main population in Thailand is now dairy crossbreds with ≥87.5% HF blood levels [20].
Currently, the development of crossbred Thai–Holstein cattle is still being carried out to select cattle with good performance and suitable for tropical conditions. Genetic evaluation of their economic traits is performed through a progeny testing program by the Thai Department of Livestock Development (DLD) [20].
3. Reproductive Traits in Dairy Cattle
3.1. Interval Traits
3.1.1. Age at First Calving (AFC)
AFC is the age at which heifers first calve and is also known as the start of the heifer’s productive life [23,24]. AFC influences the lifetime production, reproduction of the female, milk production, and lifetime calf crop [25]. Indirectly, AFC affects the cost invested for rearing cattle. The recommended age for the first calving in local breeds is three years, with many experts recommending between 23 and 25 months, which is regarded as optimal for increasing the dairy company’s profitability [24]. Previous studies on Thai–Holstein crossbred cattle reported an average AFC value of 32.39 ± 5.76 and 30.97 ± 6.65 months [26,27], higher than the recommended age. The same results were reported in Friesian × Zebu (F1), and Friesian × Arsi (F1) cattle were obtained as 35.3 and 33.9 months, respectively [25]. The lower values of AFC reported in US dairy cattle were 24.5 ± 2.73 months for Holstein, 22.9 ± 2.74 months for Jersey, and 26.3 ± 3.13 months for Brown Swiss [28]. The reports indicate that crossbreed heifers reach puberty later than pure breed cows. The variation in the reproductive performances of dairy cattle such as AFC is affected not only by their genetics but also by other aspects, including diet, management, health, and the environment. In Thailand, the AFC is not used as a selection criterion because it is entirely influenced by farmer management to determine the age and body condition score (BCS) at first service. However, AFC and body weight (BW) at calving have been found, in several studies, to have a considerable impact on future milk production and herd survival [29,30,31]. A greater BW or body condition score (BCS) at calving improves milk production later [29]. In contrast, excessively increased fat deposition throughout the raising period, resulting in heavier heifers at first calving, may compromise mammary development, reducing future milk production [32]. The long-term impact of AFC on reproductive success is still unclear. Calving heifers between the ages of 25 and 26 months had shorter recurrent calving intervals than heifers between the ages of 24 and older age groups [30].
3.1.2. Calving to First Service Interval (CFI)
The number of days between calving and the first artificial insemination or natural service in cows is defined as the CFI [33]. The recommended value of CFI in dairy cattle for good management is less than 70 days [34]. The CFI in Thai–Holstein crossbred cattle reached 101.06 ± 44.88 days [26], higher than Norway dairy cattle of 62.5 days and Japanese dairy cattle of 84.9 days [33,35]. In Arsi Negele, Ethiopia, for crossbred dairy cows, the value of CFI was reached at 184 days [36]. Those variations might be due to different genetic architectures, the environment, herd management and practices, and disease. Similarly, Softic et al. [37] also reported that extended CFI was influenced by several factors such as nutrition, endometritis, and a lack of estrus detection. The other factor, uterine infection, has been associated with extended CFI in dairy cattle and low reproductive performance [33,38]. Poor management techniques, such as failing to detect the presence of estrus in dairy cows, can also contribute to a long CFI [25].
3.1.3. Interval from First to Last Insemination (IFL)
The IFL is defined as interval days between the first and last service. It is one of the indicators used by farmers to cull cows after a series of inseminations because it can increase production costs [39]. The IFL days tend to be prolonged with the increasing number of services per conception (NSPC) in cattle. The IFL in Thai–Holstein crossbred cattle was reported to be 49.70 days [26]. The value is nearly the same as the IFL reported in Czech Holsteins of 43.95–50.13 days [40]. A lower IFL value was found in Japanese Black cattle, 28 days.
3.1.4. Days Open (DO)
DO is the time period between calving and successful conception. It is also the part of the calving interval (CI) that can be shortened by improved dairy management and genetics [25]. As a result, DO is one of the indicator variables most widely used to assess dairy cattle fertility. A long DO will cause a prolonged CI and can affect the overall economic income of farmers. Previous researchers reported that the average DO in Thai–Holstein crossbred cattle was 149.93 ± 78.60 days, 152 days, and 156.44 ± 20.77 days [9,26,41]. The other Frisian crossbreeds also have a higher DO, such as Friesian × Arsi (189 ± 9 days), but this did not occur in Friesian × Horro cattle (91.5 ± 1.20 days) and Jersey × Horro cattle (79.20 ± 3 days), which have lower values of DO [42,43]. Pure Holstein cattle are also reported to have a moderate DO of 139.5 days [4]. The high number of DO will affect the extra costs incurred per day [44]. On US dairy farms, an increase in DO from 112 to 166 causes economic losses of 3.2 USD to 5.1 USD per cow per day [45]. Dairy farmers of 44 farms in Central Thailand have to spend as much as 268,088 baht (8220.37 USD) for improving the reproductive performance program every production period [46].
3.1.5. Calving Interval (CI)
The CI is divided into three periods: postpartum anestrus, service period, and gestation [24]. The ideal CI is 12 to 13 months, assuming an average gestation period of 280 days and 85 days for DO [23]. The CI is one of the major aspects of reproductive performance that impacts the livestock production system [47]. The CI in Thai–Holstein crossbred cattle has been reported by a previous researcher to be 14.27 ± 2.62 months [26] higher than Friesian Holsteins of 13.12 months, Friesian × Arsi of 14.23 months, Ayrshire of 13.32 months, Guernsey of 13.10 months, and Jersey of 12.92 months [48,49]. An extended CI can result in a reduced milk yield, reduced lifetime productivity, and increased culling rates and replacement cost [23,35]. A prolonged CI may mainly be caused by a longer DO due to environmental variables such as management practices (poor nutrition, poor housing, estrus detection, semen handling), postpartum problems, and genetics [3,22]. Reproductive health problems result in uterine infection, delayed postpartum estrus, decreased fertility, and an extended CI [43].
3.2. Binary Traits
3.2.1. First Service Conception Rate (FSCR)
The FSCR is the percentage of cows that become pregnant after calving at the first insemination (35). The values for the FSCR in Thai–Holstein crossbred cattle were reported to be 54% and 46% [26,50], similar to dairy crossbred cattle in Ethiopia of 41 to 45% [51]. Haile and Yoseph [35] reported that the FSCR was significantly affected by the parity number and season of calving. Similar results were reported by Hammoud et al. [52] and Lemma et al. [53], who associated enhanced fertility with an appropriate ambient temperature and the availability of forage.
3.2.2. Conception Rate (CR)
Most Interbull-participating countries divide the CR into three categories: the heifer conception rate (HCR), cow conception rate (CCR), and daughter pregnancy rate (DPR). The HCR is defined as the percentage of inseminated heifers that become pregnant at each service [54]. The CCR is a measure of the female’s fertility, whereas the SCR is a measure of the bull’s fertility [55]. The optimum recommended range of CR in dairy cattle is 80–85% [35]. CCR in dairy cattle in Thailand is reported to be low, at <40% [56]. In contrast, the CCR value of crossbred dairy cows in Ethiopia’s central highlands was previously found to be 79.3% in a previous study [57]. Haile and Yoseph [35] reported that the CR was significantly (p < 0.05) affected by the season of calving and parity. The CR of cows in the rainy season was higher than in dry rainy seasons; this might be because of moderate climatic conditions and the availability of forage.
3.2.3. Pregnancy within 90 Days after First Service (P90)
The successful insemination date within 90 days of the first insemination is referred to as P90. Previous studies showed that the values of the P90 in Thai Crossbred cattle were 75% and 82.17% [26,50], lower than Spanish dairy cattle of 83% [58]. The previous report by Buaban et al. [50] and González-Recio and Alenda [58] showed that the P90 had positive genetic correlations with FSCR and test-day milk yield ranging from 0.29 to 0.69 and 0.92 to 0.97. Otherwise, a negative genetic correlation was found in the P90 inseminations per lactation (INS) of −0.54 [58].
3.3. Count Traits
The Number of Services per Conception (NSPC)
The number of services required for a successful conception is referred to as the NSPC [57]. The NSPC is a measure of reproductive efficiency that expresses the fertility of dairy cows [59]. In a dairy farm, the NSP largely depends on the breeding program used. A lack of knowledge and experience in the timing of insemination, unqualified technicians, and disease and hygiene problems are the most common reasons for a high rate of the NSCP [60]. In natural mating, the NSPC is less controlled than using artificial insemination (AI), and NSPC values higher than 2 can be considered poor [43]. A previous study reported that natural service conception has lower values (1.18) than artificial insemination users (1.5 up to 2.3). This might indicate that many environmental factors affect the AI method, including the availability of feed, the insemination time, and estrus detection in cattle [24]. The average values of the NSCP of Thai–Holstein crossbred cattle were reported to be 2.29 ± 1.72 and 1.90 ± 1.37 [26,50]. Those were higher than dairy cows in midland and low-land areas in Ethiopia reported as 1.54 ± 0.55 and 1.82 ± 0.65, respectively [60]. Different locations may offer different management and affect the NSPC. Feed and high environmental temperature were also reported to have an effect on the NSPC [59]. NSCP also has an impact on farmers’ economies. In Korea, dairy calves that were unable to conceive cost an additional USD 55.40 for reproductive treatment and palpation [61].
4. Heat Stress and Its Effect on Reproductive Traits
Heat stress is one of the significant factors affecting fertility [62]. Heat stress is a physiological condition in which an animal’s body temperature rises over its typical range of activity due to total heat gain from both internal and external sources [63]. Hence, evaporative cooling through sweating is a significant part of the heat-dissipation process in a high-temperature environment [64]. Since sweating plays an important role in the mechanism of heat dissipation, skin morphology must also be involved. Previous studies have found effects between different breeds of Sahiwal (B. indicus), HF (B. taurus), crossbred with 75% and 87.5% HF, respectively, and the interaction effect between skin color and the genetic fraction of HF on skin morphology [65]. The study revealed that Sahiwal had the highest density and volume of sweat glands, as well as the highest density of hair follicles (1058 glands/cm2; 1.60 μ3 × 10−6) compared with pure HF (920 glands/cm2; 0.51 μ3 × 10−6 and crossbred with 75% and 87.5% HF (709 glands/cm2; 0.68 μ3 × 10−6; and 691 glands/cm2; 0.61 μ3 ×10−6), respectively (p < 0.01). However, the components of skin blood flow, such as the capillary diameter, capillary circumference, and capillary surface, were higher in the HF purebreds (8.33 μm, 26.48 μm, and 2.07 μm per cm2, respectively), 87.5% HF (7.13 μm, 22.40 μm, and 1.95 μm per cm2, respectively), and 75% HF (7.85 μm, 24.92 μm, 1.83 μm per cm2, respectively) than in the Sahiwal (7.24 μm, 22.49 μm, 1.79 μm per cm2) (p < 0.01). This suggests that skin morphology may influence cutaneous evaporative heat-loss capabilities and heat tolerance in crossbred cattle.
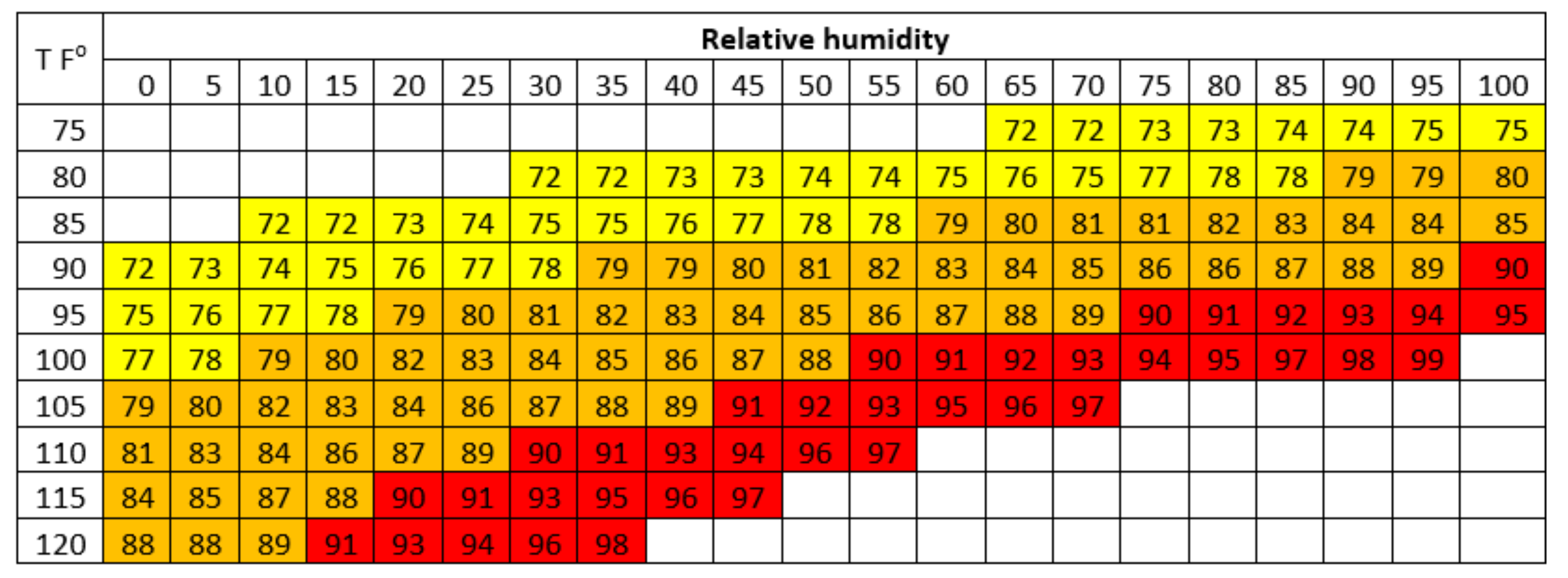
The level of heat tolerance in animals can be determined by the THI limit, where livestock performance begins to decline. The THI value is used to assess heat stress levels in dairy cattle by combining the effects of temperature and humidity [2]. The THI chart for estimating heat stress levels in dairy cattle is presented in Figure 1. The THI in Thailand could reach the highest range (82.62–82.80) from March to May [2]. Therefore, this situation can cause heat stress and has an impact on livestock performance, especially in reproductive performance.
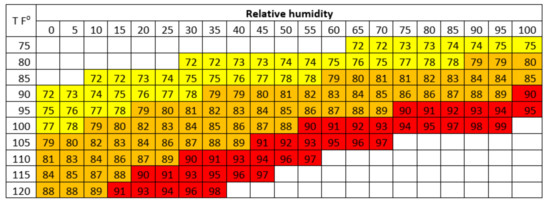
Figure 1.
THI for dairy cows. THI 72 to 78 represents mild heat stress; THI 79 to 89 represents moderate heat stress; THI > 89 represents severe heat stress. (Source: Habeeb et al. [66]).
The effects of heat stress on livestock reproduction altered endocrine regulation in the hypothalamic–pituitary–ovarian axis (HPO axis), impaired ovarian activity, and impaired progesterone concentrations, which reduced estrous behavior and caused a prolonged number of days open and low conception rate [7,67]. Kornmatitsuk et al. [68] reported that the days open for Thai dairy cattle in the hot season were higher (111.2 ± 8.9) than in the cool season (97.4 ± 5.8). The reduction in CR during heat stress can be up to 30%, with a clear seasonal pattern of estrus detection [62]. Increased ambient temperatures have a detrimental impact on the cow’s ability to perform natural mating behavior by reducing the duration and intensity of estrus expression [69].
A previous study stated that heat stress also affected the service period, FSCR, CFI, and CI in dairy cattle. The cows that calved under heat stress conditions had a longer service period, 299 ± 11 days, than in the normal condition, reported as 133 ± 7 days [70]. The FSCR rate during the cool season in a commercial farm of Thailand was also reported as higher, 23.1%, than the hot season, 9.8% [68]. In the same way, Kananub et al. [71] stated that the FSCR in the winter season had a three-times greater chance of success compared to the summer season. In Argentina, the CFI in the cows that received their service during the hot season was longer (97 days) than those that had their service from October to December (83 days) [72]. In North Africa, cows calving in the absence of heat stress (THI ≤ 70) have a shorter CI of 420 ± 15.1 days than cows calving in the presence of heat stress (THI ≥ 80) of 487 ± 12.8 days [73].
5. Genetic Parameters and a Model for Reproductive Traits in Dairy Cattle Experiencing Heat Stress
5.1. Heritability (h2)
Heritability is the difference in the phenotype between animals caused by genetic factors. Broad-sense heritability is defined as the proportion of phenotypic variation (VP) due to genetic values (VG), which may include dominance (VD) and epistasis (VI) effects. Narrow-sense heritability, however, only represents the proportion of genetic variation owing to additive genetic values (VA) [74]. Narrow-sense heritability is commonly utilized in animal breeding because the response to artificial (and natural) selection is dependent on additive genetic variation. Furthermore, additive genetic variation is the primary driver of relative similarity [74].
Heritability ranges from 0 to 1. A higher value of heritability means that most of the differences between the phenotypes of animals are genetic [75]. In contrast, while a low heritability indicates a low VA, it says a lot about VG, but it does not account for total genetic variance. As a result, a character h2 might still have a lot of genetic diversity at the loci that contribute to the observed trait variance. When a variable has a heritability value of near zero (0), it means that all phenotypic variation in the population is attributable to non-additive genetic influences and environmental factors. As a result, (VA ≈ 0) does not mean that the trait has no genetic basis; rather, it implies that the observed trait variation within the population under consideration cannot be explained by additive genetic variance [74]. Several previous studies of heritability for reproductive traits in some dairy cattle breeds, including Thai–Holstein crossbred cattle, are presented in Table 1.

Table 1.
Heritability (h2) of reproductive traits in dairy cattle.
The heritability values for reproductive traits in both Thai–Holstein crossbreeds and the other dairy cattle were low (Table 1). The difference in heritability values is due to different genetic and phenotypic variations in each population. The heritability of AFC, NSPC, CFI, IFL, FSCR, CR, DO, P90, and CI ranged from 0.01 to 0.08. The low heritability values mean the genetic component of these traits was low, and most of the variation of reproduction traits in these populations is due to management. In addition, a low value of heritability indicates that genetic improvement for these traits would be slow.
Faster genetic gain might be achieved by enhancing the accuracy of the heritability estimate, especially for reproductive traits. Berry et al. [81] reported that the on-farm usage of low-cost genomic technologies could help in a more precise parentage assignment, enhancing not only heritability but also genetic assessments. Poor record keeping with incorrect phenotypic data and pedigree could be a serious factor that can reduce the accuracy of the analysis. As a result, the recording system must be improved. More detailed records would allow researchers to partition the genetic effects between traits and result in more accurate fertility evaluations. In addition, standardized environmental circumstances can increase heritability by decreasing non-genetic variations among animals. Modern milking facilities, improved nutrition, and well-trained management personnel increased the possibility of genetic improvement in reproductive traits [74].
According to Table 1, the most popular model used in analyzing the variance components for these reproductive traits was MTM. Guo et al. [82] and Karaman et al. [83] reported that MTM outperformed STM for traits with poor heritability and a small number of records because phenotypic information for all traits of interest is not always accessible for all animals in a reference population; this is critical in practical breeding efforts. For example, there is typically a limited quantity of data for difficult or expensive traits to assess, such as carcass quality, feed efficiency, and disease traits. For traits with limited phenotypic data, the accuracy of EBV or GEBV derived via an STM will be low. An MTM will increase the accuracy derived by including information from associated and more readily measurable traits. Prediction using the MTM model gives more reliable results. Budhlakoti et al. [84] also reported that MTM has great potential to increase genetic gain and contributes to estimating the breeding value more precisely.
5.2. Genetic Correlation (rg)
Genetic correlations measure how closely two traits are related genetically [85]. Pleiotropy of genes is the major cause of the association, although linkage disequilibrium can also play a role. Pleiotropy refers to a gene’s ability to alter more than one character [74]. Genetic correlations are determined from the additive genetic variance and covariance between traits, , where are traits, and , are the variances of the traits.
Genetic correlations ranged from −1.0 to 1.0, and they can be positive or negative. The presence of positive or negative coefficients merely denotes whether the relationship is direct or inverse. Genetic correlations reveal how traits “covary” or change in tandem. When genetic correlations are near zero, each attribute is controlled by a distinct set of genes, and selection for one trait has minimal impact on the other. If the genetic correlation is positive, selection for one characteristic will increase the other, while selection for the other trait will reduce it if the genetic correlation is negative [74]. Genetic correlations are used by farmers to increase accuracy, minimize assessment time, and broaden the area of genetic evaluation [75]. The genetic correlation between reproduction traits in Thai–Holstein crossbred cattle is presented in Table 2.

Table 2.
Genetic correlations within reproductive traits in Thai–Holstein crossbred cattle.
Genetic correlations values in this study ranged from 0.36 to 1.00 and are included in the high category (Table 2). Strong positive genetic correlations (≥90) were observed between NSPC-IFL, CFI-DO, CFI-CI, IFL-DO, IFL-P90, and DO-CI. High positive genetic correlation values (0.36–0.89) were found between NSPC-CFI, NSPC-DO, NSPC-CI, CFI-IFL, IFL-CI, and FSCR-P90. Similar findings for NSPC-IFL, IFL-DO, and DO-CI were reported by González-Recio and Alenda [58] in Spanish dairy cattle: 0.91, 0.99, and 0.99, respectively. The values of genetic correlation between DO and CI in this study were also similar to those reported by Rahbar et al. [14] and Ghiasi et al. [86], of 0.98 and 0.99, respectively. The values between NSPC-CFI and CFI-IFL in Thai–Holstein crossbred cattle were higher than Spanish dairy cattle reported by González-Recio and Alenda [58] of 0.11 and 0.50, respectively. Differences in the additive variance and covariance of each trait in the population might explain the disparity in these results. High positive values imply that these reproductive traits are nearly genetically identical and are affected by the same genes. This means that the selection between traits with positive genetic correlations would be followed by an increase in other traits.
The high negative values of genetic correlations (>−0.53) in Thai–Holstein crossbred cattle were found between NSPC-FSCR, NSPC-P90, CFI-FSCR, CFI-P90, IFL-FSCR, FSCR-DO, FSCR-CI, DO-P90, and P90-CI (Table 2). A negative value of genetic correlations indicates that these traits have an inverse relationship with one another. For example, selection for a lower NSPC, for example, might result in a higher number of FSCR and P90, and a selection within traits that have a negative genetic correlation value will also be the same.
A study of Thai–Holstein crossbred dairy cattle also found a positive genetic correlation between reproductive traits (P90) and milk yield (MY) of 0.69 [50]. This result revealed that the selection of the P90 trait would increase milk production. The other study on tropical dairy cattle in Ethiopia showed negative genetic correlations between AFC-MY, CI-MY, and DO-MY of −0.24 ± 0.11, −0.10 ± 0.13, and −0.02 ± 0.14, respectively [87]. Conversely, the negative genetic correlation between CI-MY and DO-MY suggests that when milk output increases, fertility can be improved in this herd.
5.3. THI and Genetic Model for Heat Stress
5.3.1. THI Model
THI is calculated from temperature (°C) and relative humidity. Several formulas in the previous studies reported by Nascimento et al. [88] were compared to calculate the THI; however, this was the best model that produced the most accurate results according to Berman et al. [5]:
where T is the average daily temperature (°C) and RH denotes the average daily relative humidity (%). Then, as a dummy variable, f(THI), a function of THI, was developed to quantify the reduction in reproductive performance under heat stress situations.
THIs = 3.43 + 1.058 × T − 0.293 × RH + 0.0164 × T × RH + 35.7
were investigated at several different critical levels or threshold points, and the optimal model was determined by the lowest 2logL Akaike information criterion (AIC) and Bayesian information criterion (BIC) [15,89,90].
5.3.2. Genetic Model
Several models were applied to evaluate variance components for heat tolerance in dairy cattle (Table 1). Sigdel et al. [15] and Aguilar et al. [89] purposed a multi-trait linear-repeatability test-day model (REP) to analyze the variance components for conception per insemination (binary trait) and multiple lactations at heat stress levels. Furthermore, Aguilar et al. [89] utilized the multiple-trait model (MTM) on DIM and f(THI) with the same effects as the REP model. Boonkum et al. [9] also used the MTM to estimate the variance components of DO. In agreement with Boonkum et al. [9], Oseni et al. [91] purposed the MTM and random regression model (RRM) to analyze the DO. In another study, Rahbar et al. [14] used an animal linear mixed model and univariate threshold models to analyze variance components for FSCR, gestation length (GL), NSPC, insemination outcome (IO), CI, calving birth weight (CBW), and DO. The (co)variance structure of each study was created according to the model, objectives, and problems of each study.
Some software is used by researchers to support the data analysis of each model. Aguilar et al. [89] used GIBBS2F90 to conduct their MTM on multiple lactations; the software implements Gibb’s sampling with a joint sampling of random correlated effects and traits [92]. Boonkum et al. [9] and Oseni et al. [91] used the SAS’s GLM method and AIREMLF90 program for their analysis of DO. Rahbar et al. [14] also used AI-REML in the DMU software package [93] to estimate variance components of reproductive traits using their genetic model.
6. The Approach of Genetic Selection for Reproductive Traits in Dairy Cattle
6.1. Traditional Breeding Method
The goal of livestock breeding is to select animals that will produce superior animals with higher yields [94]. The traditional breeding method (TAB) used the information of phenotypic records or pedigree for animal selection. This method is complicated for traits with low heritability values, such as reproductive traits, because they are more difficult to improve, and there are long generation intervals [95].
Furthermore, the new method was designed with the combination of the phenotypic and those of relatives into the estimated breeding value (EBV), called the best linear unbiased prediction (BLUP) [94]. This method successfully increases the genetic response to selection by improving the reliability of the EBV [96]. In addition, this method includes all systematic effects and exploits all pedigree information of the animals using a numerator relationship matrix to account for increases in additive genetic variance caused by inbreeding or assortative mating [97].
However, according to Ibtisham et al. [94], there are still a few limitations in using the BLUP method: (1) making accurate early selection decisions by regularly and timely recording of phenotypes is costly; (2) for some economics traits with low heritability, longevity traits, sex-limited traits, or expensive or difficult traits such as meat quality and disease resistance, it is less accurate and inefficient; (3) the breeding process is lengthy but necessary to collect sufficient phenotypic data to increase precision in performing the genetic evaluation.
Many models have been utilized for the genetic evaluation of reproductive traits, especially for interval traits, including the sire model (SM), sire–dam model (SDM), and the animal model (AM). However, a study comparison between these models revealed that AM could improve stability and accuracy in evaluating fertility traits in dairy cattle [98]. The SDM is comparable to the AM in terms of performance, although it is not significantly different. The AM has the benefit of delivering EBV of cows with intermediate reliability, and therefore it is better for interval traits.
The several traits of animal reproduction are observed as discrete outcomes. A threshold model has been proposed and applied to the problems of animal breeding which assumes that no observable normal variable is defined as a threshold. Evaluations based on threshold models for categorical traits showed that they could provide a greater response to selection than those derived from linear models [99].
6.2. Marker-Assisted Selection (MAS)
MAS is the combination between traditional breeding methods and molecular genetic methods. This method was introduced in 1900, with the concept of using specific markers on genes that affected economic traits in animals [94]. MAS requires accurate information about the relationship between genetic markers and related quantitative traits loci (QTL) for more precise results [100]. It provides an opportunity to predict the genotype of superior animals in the next generation, regardless of environmental conditions.
MAS has several advantages compared with the TAB method: (1) the process is faster since the phenotype of an individual may be forecasted at an early stage; (2) it is more profitable for sex-limited characteristics and difficult late-life traits; and (3) it is more useful for crossbreeding programmed by interrogating animals with superior genetics and better breeding value into local breeds [94]. However, the MAS technique uses a small number of loci that only account for a small amount of genetic variation, and can thus result in a small genetic gain [101]. Another limitation of MAS is that this method is quite expensive and economically inefficient when applied in commercial breeding programs [102]. The application of MAS in breeding programs is also limited because only a few markers have been proven to have a significant impact on animals’ economic traits, and they only account for a small percentage of genetic diversity [103].
Identifying and characterizing candidate genes and genetic variations associated with economically significant traits is essential in animal breeding. Each candidate gene has its own regulatory region or coding region [104]. Several potential genes associated with reproductive traits in dairy cattle were discovered in previous studies (Table 3).

Table 3.
Candidate genes that were previously associated with fertility traits in cattle.
6.3. Genomic Selection (GS) Method
Many SNPs covering whole genomes in animals have been discovered with genome-sequencing technology. Based on the genotypic information from whole genomes, a genomic selection (GS) method was proposed by using markers to estimate breeding values with high accuracy. Genomic selection is a useful tool and has been proven to raise genetic gain for low-heritability traits or difficult-to-measure traits [108]. Hence, this tool is suitable for reproductive traits in dairy cattle. Studies of the GS application strategies, the factors that influence GS accuracy, and the implementation of GS have been conducted in animal breeding [103].
In principle, GS predicts the breeding value of each genotyped individual using high-density (HD) markers that span the entire genome, which is subsequently referred to as genomic estimated breeding value (GEBV). Several approaches for determining the GEBV for cattle have been proposed, including a single-step Genomic Best Linear Unbiased Prediction (ssGBLUP) and a weighted ssGBLUP (WssGBLUP). A hybrid matrix (H) in the ssGBLUP is created by combining the pedigree-based relationship matrix (A) with the genomic relationship matrix (G), and when compared to multi-step genomic predictions, this advantage improves the accuracy and decreases the prediction bias of GEBVs [109]. However, the ssGBLUP does not assume the exact differences between the SNPs and their biological point of view; hence, the WssGBLUP method has been proposed [110]. The WssGBLUP accounts for locus-specific variance and uses various SNP weights when computing the G matrix [111]. These two approaches have been compared and contrasted in various studies and are commonly used in genomic prediction studies [112,113].
GS has the potential to overcome the limitations of MAS and more correctly forecast breeding values [114]. In MAS, only a few markers have been proven to significantly impact economically relevant traits. GS can fully utilize genotypic information from whole genomes in the genetic evaluation of animals, and breeding values can be predicted with high accuracy using genetic markers alone. GS is the latest technology and promises greater genetic advantages than TAB methods in dairy cattle. According to Gutierrez-Reinoso et al. [115], GS has higher reliability of 73.3% to 93.5% compared to TAB of 46% to 72%, depending on the number of progenies studied. GS also requires a relatively shorter time than a TAB to obtain progeny testing results. Although it requires more expensive equipment, the technology advances constantly, decreasing its cost. GS is more accurate and unbiased for modeling with unknown parent groups to predict the genetic merit [115]. For genetic variance estimation using pedigree matrices, GS produces high-reliability values for young bulls without pedigree or known sires [115]. GS also produces a high genetic gain of 50–100% for yield traits [116].
The application of GS in dairy cattle significantly increased the economic efficiency of animal breeding programs [117]. The benefits of GS in the dairy industry are generally due to a shorter generation interval, a reduction in costs for progeny testing, and an increase in the accuracy of EBV and genetic gains [118,119]. According to García-Ruiz et al. [116], the introduction of GS decreases the generation interval, especially for the sire(s) of bulls and dam(s) of bulls path from 7 to 2.5 years and from 4 to 2.5 years, respectively. In addition, Lund et al. [120] and Wiggans et al. [121] stated that the application of GS might improve genomic prediction reliability by 0.8 for production traits and 0.7 for fertility traits. Positive genetic trends also occur in low-heritability traits such as the productive life, daughter pregnancy rate, and somatic cell score [116]. Therefore, the benefit of GS for reproductive traits or traits with low heritability is high compared to TAB and MAS.
6.3.1. Bayesian Approaches
The data are modeled at two levels using Bayesian estimation. The first is a model at the data level, while the second is a model at the variances of the chromosomal segments [122]. Several Bayesian statistical approaches have been applied in genomic assessment, with the hypothesis of marker effect distributions differing. Meuwissen et al. [108] presented distinct a priori distributions between the Bayes A and Bayes B approaches for simulating the variances of the effects of the markers.
The Bayes A technique assumes that the variance-of-marker effect varies across loci. The scaled inverted chi-square distribution is used to simulate the variances, χ−2 (v, S), where S denotes the scale parameter, and v denotes the degree of freedom. This is a valuable option since the resultant posterior distribution is similarly a scaled inverted chi-square when the information from the previous distribution is joined with the information from the data [108].
The Bayes B approach assumes variances of SNP effects in a genomic assessment context, with many SNP contributing to zero effects and a few contributing to substantial impacts on the trait [122]. Meuwissen et al. [108] presented a model in which a fraction of the markers have no impact (arbitrarily set at 0.95). Because some markers have a high likelihood of having zero variance, Gibbs sampling cannot be utilized to estimate the effects and variances of the Bayes B model. According to Meuwissen et al. [108], the Bayes B approach is commonly regarded as the “benchmark” in terms of genome prediction, although it is exceedingly time consuming in terms of computing.
Habier et al. [123] expanded the Bayesian alphabet by introducing the Bayes C approach, in which the effect of SNP markers on is considered to be zero, and a normal prior distribution is assumed for the variances relevant to the remaining (1 − ) markers, and the mixing parameter, , may be inferred from the data rather than presumed to be known. Other approaches, such as the Bayesian LASSO reported by de los Campos et al. [124], assume that a double exponential prior distribution has also been utilized in animal breeding. Furthermore, Erbe [125] presented the Bayes R approach, which assumes a combination of normal prior distributions and accommodates SNPs with zero, small, medium, and high effects more effectively. In instances where one or more QTL with moderate or high effects exist, Bayesian regression approaches outperform GBLUP by a small margin, but when the trait is impacted by many genes, each with a small effect, GBLUP performs as well as or better than the Bayesian regression model [126].
Bayesian methods have some drawbacks. According to Legara et al. [127], the SNP effects might be modeled using a Bayesian approaches if the individual SNP variances were known and kept in the diagonal matrix B. This is different from the single-step genomic model, which allows for flexible modeling of SNP effects in terms of the number and (co)variance structure of fitted SNP markers. Moreover, for Bayesian SNP models assuming heterogeneous SNP variances for the various qualities, it is no longer easy to deduce correlations of traits at the SNP level from those at the genome level. This makes the Bayesian models more challenging to extend to multiple traits than the BLUP SNP models [127].
6.3.2. Multi-Step Genomic Evaluation
Several strategies have been developed since genomic selection was initially proposed in animal breeding. A multi-step procedure was introduced by VanRaden et al. [101] for GS in dairy cattle using field datasets. The steps in the multi-step procedure are: (1) estimating the traditional breeding values, (2) generating fictitious observations for genotyped animals (bulls) such as daughter yield deviations (DYD) and the degressed evaluation (DD), (3) determining the direct genomic values (DGV) for genotyped animals using approaches based on SNP effects or genomic connections with DYD or DD as phenotypic observations, and (4) constructing an index based on pedigree and genomic predictions to estimate GEBV by the formula:
where PA denotes the parent average, and PI denotes the parental index derived from genotyped animals’ pedigree relationships. Because only a small percentage of animals in a lineage are genotyped, the quantity of phenotypic data required to compute DGV is often less than that required to calculate PA [101]. In this scenario, using DGV to account for the normal sire–dam PA, as well as the PA computed from a selection of genotyped ancestors in an index, can assist to enhance genomic predictions [128]. Furthermore, a previous study found that expanding a genotyped group’s reference population had a greater influence on prediction accuracy than increasing the number of SNP markers. Pseudo-observation might be computed as degressed proofs (DRP) instead of DYD, which is time-consuming and involves considerable work [121].
GEBV = w1PA + w2DGV − w3PI
The multi-step approach was well suited to situations where the datasets belong to separate organizations and are condensed into a limited number of animals to genotype [128]. This includes a population with large numbers of average bulls with many daughters. The multi-step method has some limitations. It is difficult to create a DRP that is free of double counting when the population comprises both males and females, especially when genotyping includes parents and their progeny [127]. Weighting factors, such as variance components or selection index coefficients, are downsides of this strategy, as is information loss [101,129,130]. Moreover, these disadvantages may negatively affect the use of marker-assisted selection, especially for cows [131]. According to Misztal et al. [128] the multi-step approach is rather complex, and it relies on the existence of animals (bulls) with high correct EBVs based on pedigree information in its first form. Conversely, this method also needs to construct pseudo-observations that become biased by genomic preselection when a population contains non-genotyped animals with phenotypes because of BLUP, and the move to a single step is necessary [132].
6.3.3. Single-Step Genomic Evaluation
Single-step genomic BLUP (ssGBLUP) was designed to solve possible issues by combining phenotypes, by merging phenotypic, pedigree, and genomic links into a combined relationship matrix. Furthermore, by substituting the connection matrix with the combined matrix, pedigree-only studies may be converted to genomic analyses, and pseudo-phenotypes are no longer required. This method also allows for the simultaneous evaluation of non-genotyped animals and genotyped animals. It is a simple and more accurate method that can reduce the number of steps in the evaluation [109].
Compared to multi-step genomic predictions, the ssGBLUP combines the pedigree-based relationship matrix (A) and the genomic relationship matrix (G) into a hybrid matrix (H). This benefit boosts the accuracy and minimizes the prediction bias of GEBVs [109]. In addition, Patry and Ducrocq [132] reported that ssGBLUP accounts for preselection due to pedigree BLUP, which can minimize bias, and is different from multi-step that does not take this into account. However, the ssGBLUP does not assume the exact differences between the SNPs and their biological point of view; hence, the WssGBLUP method has been proposed [110]. The WssGBLUP accounts for locus-specific variance and uses various SNP weights when computing the G matrix [111]. These two methods have been evaluated in various studies and are commonly used in genomic prediction studies [112,113].
The previous study suggested that the ssGBLUP method is a better option than the WssGBLUP method for crossbreeding with polygenic traits [111]. When comparing WssGBLUP to ssGBLUP for less-polygenic variables, Lourenco et al. [133] found that WssGBLUP might be more accurate. WssGBLUP can be beneficial for phenotypes with a small number of causal genes. It assumes that the traits are affected by a finite number of markers [111]. Below are some interesting factors of ssGBLUP according to Leggara et al. [127]:
- All relatives of genotyped individuals are automatically accounted for, as are their performances.
- Fitting genetic data and estimating additional effects are performed at the same time (e.g., contemporary groups). As a result, there will be no data loss.
- Feedback: all of a genotyped individual’s relatives benefit from the greater accuracy.
- Extensions are simple. Because this is a linear BLUP-like estimator, it can be immediately applied to more complex models (multiple traits, threshold traits, test day records). Any model that can be fitted with relationship matrices may also be fitted with combined relationship matrices.
Previously, the single step method has been used to evaluate the genetics for production traits of Thai–Holstein crossbred cattle. In a recent study, Sungkhapreecha et al. [134,135] proposed the ssGBLUP and ssGREML method to investigate the effect of heat stress on genetics for milk yield traits. The results revealed that the ssGBLUP technique was more accurate than the BLUP method, while ssGREML results are not much different from the REML method in genomic predictions. However, Buaban et al. [136] generated GEBV using a single-step random regression test-day model (ssRR-TDM) in contrast to an estimated breeding value (EBV) based on the pedigree-based model by utilizing the RR-TDM approach. The results showed that ssRR-TDM raised individual accuracies by 0.22 and validation accuracies by 0.07 in comparison to RR-TDM, when only bull genotypes were employed. Individual accuracies also increased by 0.02 with cow genotypes, while validation accuracies increased by 0.06. Based on these findings, a single-step method is commonly used in Thai–Holstein crossbred cattle because it generates a greater accuracy for genomic prediction. It is also possible to use the technique in genetic evaluation for reproductive traits in the future.
6.3.4. Accuracy of GS
GS could increase the accuracy of EBV testing at an early age [137]. The square root of the proportion of genetic variation captured by the SNP panel (q) and the precision with which the genetic effects captured by SNPs can be assessed are used to calculate the accuracy of GEBV (r) [138]. The size of the reference population, the heritability of the phenotypes in the reference dataset, and the number of different QTLs that impact the trait are additional factors [139].
GS is now being used on a broad scale in cattle; however, it is mostly focused on production traits [18,139]. The accuracy of GS for dairy traits in developed countries varies from 0.50 to 0.85 for production traits with medium to high heritability to roughly 0.20–0.50 for fertility and survival variables with lower heritability [140,141]. According to Hayes et al. [142], ssGBLUP has a greater accuracy of 0.69 and 0.60 for production traits (protein yield and protein percentage), respectively, than the multi-step approach of 0.67 and 0.54. In other studies related to milk yield and fat percentage, the accuracy of these steps is higher than multi-step methods [143,144]. Recently, in Thai dairy cattle, a GS study on milk production traits and somatic cell score using a single-step method revealed increased average individual accuracies by 0.22 [136].
Several studies on GS were reported in dairy cattle regarding fertility traits, including AFC, the non-return rate, and calving ease [143]. The accuracy of prediction for AFC using the ssGBLUP model was 0.299 in the Thai crossbred cattle [144] and 0.56 in the Gyr dairy cattle breed [145]. For the non-return rate, the accuracy of genomic prediction was 0.39 in Canadian Holstein cows [145]. A GS study on calving ease in Holsteins (Canadian) showed that the accuracy performance was 0.69 [109]. Most researchers use the ssGBLUP method for reproductive traits because it has higher accuracy. In a previous study, ssGBLUP had higher accuracy than multi-step analysis, 0.43 vs. 0.42, respectively [142].
6.3.5. Challenges and Opportunities in Applying GS
Thailand has had a dairy cattle breeding development program for about 60 years. They have attempted to produce its tropical dairy breed based on Holstein Friesian (HF) and indigenous Thai cattle through selection and inter se mating. Improving reproductive performance and resistance to heat is one of the main objectives of this crossbreeding program. The central database system of the Bureau of Biotechnology for Livestock Production, Department of Livestock Development, hosts milk-production data, reproduction data, and pedigree information. Several studies on genetic evaluation of reproductive traits in Thai–Holstein crossbred cattle have been carried out using these data [9,50,146]. THI may be estimated using climate data acquired from the nearest meteorological center based on postal code to evaluate heat resistance [12,135]. The small number of prospective bulls selected from Thailand’s dairy population, however, might be a problem. This matter will limit the intensity of selection and inaccurate prediction of young animals if they are still using the traditional evaluation method. To increase the size of the reference population, Thai government’s bureau began collecting genotypes of daughter-proven bulls and phenotyped cows in 2015 [136]. These genotypic data could be used to estimate GEBV, which gives a higher accuracy value than traditional methods. Sungkhapreecha et al. [134,135] previously estimated GEBV using the ssGBLUP method for milk yield and heat tolerance in Thai–Holstein crossbreed cattle. The results indicated that the ssGBLUP technique’s predictions were more accurate than the BLUP method’s, with a ratio of accuracy of 32–54% [134]. There is still no research on GS for fertility traits in Thai dairy cows under heat stress; hence, this is an excellent opportunity, considering the availability of data from the Thai government’s bureau. The application of GS in Thai–Holstein crossbred cattle is expected to reduce generation intervals to simplify the selection process and reduce maintenance costs. Using the ssGBLUP method will also provide an overview of the GS research reference model for improving reproductive traits under heat stress, especially in tropical countries.
Most dairy farmers in Thailand (>80%) are smallholders with an average of 30 animals per farm [146]. Smallholder farmers have two key challenges: (i) lack of infrastructure and (ii) human capacity, notably in the areas of technological competency, data processing, and interpretation [147]. The small genetic trends in Thai dairy populations imply that farmer capacity to learn new technology and absorb new information for improving dairy performance and profitability may be limited [148,149]. To overcome these limitations, Thai dairy farmers will require a program that incorporates systematic training and ongoing assistance to increase milk production and earnings in a long-term way. Furthermore, strengthening collaboration is needed among academics, industry, farmers’ groups, the public sector, charitable organizations, and development agencies to advance the development and implementation of genetic improvement programs.
7. Conclusions
Genetic evaluations for reproductive traits have been carried out in dairy cattle, especially in Thailand. The genetic variance for reproductive traits in livestock is generally low, indicated by low heritability values (<0.1). However, there were moderate to strong genetic correlations between reproductive traits and between reproductive traits and production traits. THI should be included in the model for genetics evaluation under heat stress. Several approaches have been used for the genetic selection of reproductive traits, and GS may be the appropriate genetic technique for Thai dairy cattle. For reproductive traits in dairy cattle, the single-step genomic BLUP (ssGBLUP) technique is more accurate than the multi-step method. The implementation of GS for reproductive traits in Thai–Holstein cattle is interesting to study in the future because it has not been widely adopted.
Author Contributions
Conceptualization, A.F., M.D., V.C., and W.B.; methodology, A.F., M.D., and W.B.; writing—original draft preparation, writing—review and editing, A.F., M.D., and W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the KKU Scholarship for ASEAN and GMS Countries No. 588/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021: The Physical Science Basis, the Working Group I contribution to the Sixth Assessment Report; Cambridge University Press: Cambridge, UK, 2022; pp. 1–35. [Google Scholar]
- Thammahakin, P.; Yawongsa, A.; Rukkwamsuk, T. Effect of Heat Stress on Reproductive Performance of Dairy Cows under Tropical Climate: A Review. J. Kasetsart Vet. 2020, 30, 122–133. [Google Scholar]
- Kachenchart, B.; Kamlangkla, C.; Puttanapong, N.; Limsakul, A. Urbanization effects on surface air temperature trends in Thailand during 1970–2019. Environ. Eng. Res. 2020, 26, 200378. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.; Horovitz, T.; Kaim, M.; Gacitua, H. A comparison of THI indices leads to a sensible heat-based heat stress index for shaded cattle that aligns temperature and humidity stress. Int. J. Biometeorol. 2016, 60, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bjerg, B.S.; Choi, C.Y.; Zong, C.; Zhang, G. A review and quantitative assessment of cattle-related thermal indices. J. Therm. Biol. 2018, 77, 24–37. [Google Scholar] [CrossRef]
- Schüller, L.; Michaelis, I.; Heuwieser, W. Impact of heat stress on estrus expression and follicle size in estrus under field conditions in dairy cows. Theriogenology 2017, 102, 48–53. [Google Scholar] [CrossRef]
- Nasr, M.A.; El-Tarabany, M.S. Impact of three THI levels on somatic cell count, milk yield and composition of multiparous Holstein cows in a subtropical region. J. Therm. Biol. 2017, 64, 73–77. [Google Scholar] [CrossRef]
- Boonkum, W.; Misztal, I.; Duangjinda, M.; Pattarajinda, V.; Tumwasorn, S.; Buaban, S. Short communication: Genetic effects of heat stress on days open for Thai Holstein crossbreds. J. Dairy Sci. 2011, 94, 1592–1596. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I. Effect of Heat Stress on Nonreturn Rate in Holsteins: Fixed-Model Analyses. J. Dairy Sci. 2002, 85, 3101–3106. [Google Scholar] [CrossRef]
- Thulani, S.; Maliviwe, M.; Ayodeji, I.P. Heat tolerance level in dairy herds: A review on coping strategies to heat stress and ways of measuring heat tolerance. J. Anim. Behav. Biometeorol. 2019, 7, 39–51. [Google Scholar] [CrossRef]
- Boonkum, W.; Misztal, I.; Duangjinda, M.; Pattarajinda, V.; Tumwasorn, S.; Sanpote, J. Genetic effects of heat stress on milk yield of Thai Holstein crossbreds. J. Dairy Sci. 2011, 94, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Bohlouli, M.; Shodja, J.; Alijani, S.; Eghbal, A. The relationship between temperature-humidity index and test-day milk yield of Iranian Holstein dairy cattle using random regression model. Livest. Sci. 2013, 157, 414–420. [Google Scholar] [CrossRef]
- Rahbar, R.; Aminafshar, M.; Abdullahpour, R.; Chamani, M. Genetic analysis of fertility traits of Holstein dairy cattle in warm and temperate climate. Acta Sci. Anim. Sci. 2016, 38, 333–340. [Google Scholar] [CrossRef]
- Sigdel, A.; Liu, L.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Genetic dissection of reproductive performance of dairy cows under heat stress. Anim. Genet. 2020, 51, 511–520. [Google Scholar] [CrossRef]
- Wall, E.; Simm, G.; Moran, D. Developing breeding schemes to assist mitigation of greenhouse gas emissions. Animal 2010, 4, 366–376. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bowman, P.J.; Haile-Mariam, M.; Pryce, J.; Hayes, B. Genomic selection for tolerance to heat stress in Australian dairy cattle. J. Dairy Sci. 2016, 99, 2849–2862. [Google Scholar] [CrossRef]
- Silva, M.V.; dos Santos, D.J.; Boison, S.A.; Utsunomiya, A.; Carmo, A.S.; Sonstegard, T.S.; Cole, J.B.; Van Tassell, C.P. The development of genomics applied to dairy breeding. Livest. Sci. 2014, 166, 66–75. [Google Scholar] [CrossRef]
- Department of Livestock Development. Summary of Livestock Information by Animal Species, Fiscal Year 2021. Available online: https://ict.dld.go.th/webnew/images/stories/stat_web/monthly/2564/jun64/2---Dairy.pdf (accessed on 30 July 2021).
- Buaban, S.; Puangdee, S.; Duangjinda, M.; Boonkum, W. Estimation of genetic parameters and trends for production traits of dairy cattle in Thailand using a multiple-trait multiple-lactation test day model. Asian-Australas. J. Anim. Sci. 2020, 33, 1387–1399. [Google Scholar] [CrossRef]
- VanRaden, P.; Sanders, A. Economic Merit of Crossbred and Purebred US Dairy Cattle. J. Dairy Sci. 2003, 86, 1036–1044. [Google Scholar] [CrossRef]
- Chanvijit, K.; Duangjinda, M.; Pattarajinda, V.; Reodecha, C. Model comparison for genetic evaluation of milk yield in crossbred Holsteins in the tropics. J. Appl. Genet. 2005, 46, 387–393. [Google Scholar]
- Adisu, A.; Zewdu, W. Reproductive Performance of Indigenous Cow Breeds of Ethiopia: A Review. J. Anim. Health Behav. Sci. 2021, 5, 105. [Google Scholar]
- Tadesse, G. Reproductive Performance and Wastage in Large Ruminant (Cattle) in Ethiopia-Review. J. Dairy Vet. Sci. 2018, 8, 555729. [Google Scholar] [CrossRef]
- Alem, W.T. Review on Reproductive and Productive Performance of Dairy Cow in Ethiopia. Int. J. Ecotoxicol. Ecobiol. 2021, 6, 14. [Google Scholar]
- Buaban, S.; Sanpote, J.; Duangjinda, M. Estimates of genetic parameters for dairy crossbred female fertility traits. Khon Kaen Agric. J. 2018, 46, 767–778. [Google Scholar]
- Yodklaew, P.; Koonawootrittriron, S.; Elzo, M.A.; Suwanasopee, T.; Laodim, T. Genome-wide association study for lactation characteristics, milk yield and age at first calving in a Thai multibreed dairy cattle population. Agric. Nat. Resour. 2017, 51, 223–230. [Google Scholar] [CrossRef]
- Hutchison, J.; VanRaden, P.; Null, D.; Cole, J.; Bickhart, D. Genomic evaluation of age at first calving. J. Dairy Sci. 2017, 100, 6853–6861. [Google Scholar] [CrossRef]
- Ettema, J.; Santos, J. Impact of Age at Calving on Lactation, Reproduction, Health, and Income in First-Parity Holsteins on Commercial Farms. J. Dairy Sci. 2004, 87, 2730–2742. [Google Scholar] [CrossRef]
- Evans, R.; Wallace, M.; Garrick, D.; Dillon, P.; Berry, D.; Olori, V. Effects of calving age, breed fraction and month of calving on calving interval and survival across parities in Irish spring-calving dairy cows. Livest. Sci. 2006, 100, 216–230. [Google Scholar] [CrossRef]
- Pirlo, G.; Miglior, F.; Speroni, M. Effect of Age at First Calving on Production Traits and on Difference Between Milk Yield Returns and Rearing Costs in Italian Holsteins. J. Dairy Sci. 2000, 83, 603–608. [Google Scholar] [CrossRef]
- Cooke, J.S.; Cheng, Z.; Bourne, N.E.; Wathes, D.C. Association between growth rates, age at first calving and subsequent fertility, milk production and survival in Holstein-Friesian heifers. Open J. Anim. Sci. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Softic, A.; Asmare, K.; Granquist, E.G.; Godfroid, J.; Fejzić, N.; Skjerve, E. The serostatus of Brucella spp., Chlamydia abortus, Coxiella burnetii and Neospora caninum in cattle in three cantons in Bosnia and Herzegovina. BMC Vet. Res. 2018, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Nakao, T.; Yoshida, C.; Long, S.T.; Gautam, G.; Ranasinghe, R.B.K.; Koike, K.; Hayashi, A. Days in Milk at First AI in Dairy Cows; Its Effect on Subsequent Reproductive Performance and Some Factors Influencing It. J. Reprod. Dev. 2011, 57, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Haile, B. Reproductive Performance of Holstein Friesian Dairy Cows at Alage Dairy Farm, Ethiopia. J. Dairy Vet. Sci. 2018, 7, 555713. [Google Scholar] [CrossRef]
- Semara, L.; Mouffok, C.; Madani, T.; Radi, F.; Rezig, N. Environmental Factors Affecting Reproductive Traits in Cows on Algerian Smallholder Farms. Int. J. Agric. Sci. Vet. Med. 2014, 2, 85–95. [Google Scholar]
- Softic, A.; Martin, A.; Skjerve, E.; Fejzic, N.; Goletic, T.; Kustura, A.; Granquist, E.G. Reproductive Performance in a Selected Sample of Dairy Farms in Una-Sana Canton, Bosnia and Herzegovina. Vet. Med. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Elkjær, K.; Ancker, M.-L.; Gustafsson, H.; Friggens, N.; Waldmann, A.; Mølbak, L.; Callesen, H. Uterine bacterial flora in postpartum Danish Holstein dairy cows determined using DNA-based fingerprinting: Correlation to uterine condition and calving management. Anim. Reprod. Sci. 2013, 138, 39–48. [Google Scholar] [CrossRef][Green Version]
- Setiaji, A.; Oikawa, T. Genetics of heifer reproductive traits in Japanese Black cattle. Asian-Australas. J. Anim. Sci. 2020, 33, 197–202. [Google Scholar] [CrossRef]
- Zavadilová, L.; Zink, V. Genetic relationship of functional longevity with female fertility and milk production traits in Czech Holsteins. Czech J. Anim. Sci. 2013, 58, 554–565. [Google Scholar] [CrossRef]
- Kaewlamun, W.; Chayaratanasin, R.; Virakul, P.; Ponter, A.A.; Humblot, P.; Suadsong, S.; Tummaruk, P.; Techakumphu, M. Differences of Periods of Calving on Days Open of Dairy Cows in Different Regions and Months of Thailand. Thai J. Vet. Med. 2011, 41, 315–320. [Google Scholar]
- Negussie, E.; Brännäng, E.; Banjaw, K.; Rottmann, O.J. Reproductive performance of dairy cattle at Asella livestock farm, Arsi, Ethiopia. I: Indigenous cows versus their F1 crosses. J. Anim. Breed. Genet. 1998, 115, 267–280. [Google Scholar] [CrossRef]
- Ibrahim, N.; Seid, A. Review on Reproductive Performance of Crossbred Dairy Cattle in Ethiopia. J. Reprod. Infertil. 2017, 8, 88–94. [Google Scholar]
- Kalantari, A.; Armentano, L.; Shaver, R.; Cabrera, V. Economic impact of nutritional grouping in dairy herds. J. Dairy Sci. 2016, 99, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A. Economic Value of Pregnancy in Dairy Cattle. J. Dairy Sci. 2006, 89, 3876–3885. [Google Scholar] [CrossRef]
- Hall, D.; Ehui, S.; Shapiro, B. Economic analysis of the impact of adopting herd health control programs on smallholder dairy farms in Central Thailand. Agric. Econ. 2004, 31, 335–342. [Google Scholar] [CrossRef]
- Fodor, I.; Abonyi-Tóth, Z.; Ózsvári, L. Management practices associated with reproductive performance in Holstein cows on large commercial dairy farms. Animal 2018, 12, 2401–2406. [Google Scholar] [CrossRef]
- Mostert, B.; Van Der Westhuizen, R.; Theron, H. Calving interval genetic parameters and trends for dairy breeds in South Africa. S. Afr. J. Anim. Sci. 2010, 40, 57288. [Google Scholar] [CrossRef]
- Duguma, B.; Kechero, Y.; Janssens, G.P.J. Productive and Reproductive Performance of Zebu X Holstein-Friesian Crossbred Dairy Cows in Jimma Town, Oromia, Ethiopia. Glob. Vet. 2012, 8, 67–72. [Google Scholar]
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Genetic relationships of fertility traits with test-day milk yield and fat-to-protein ratio in tropical smallholder dairy farms. Anim. Sci. J. 2015, 87, 627–637. [Google Scholar] [CrossRef]
- Mureda, E.; Mekuriaw, Z.Z. Reproductive Performance of Crossbred Dairy Cows in Eastern Lowlands of Ethiopia. Livest. Res. Rural Dev. 2007, 19, 11. [Google Scholar]
- Hammoud, M.H.; El-Zarkouny, S.Z.; Oudah, E.Z.M. Effect of Sire, Age at First Calving, Season and Year of Calving and Parity on Reproductive Performance of Friesian Cows under Semiarid Conditions in Egypt. Arch. Zootech. 2010, 13, 60–82. [Google Scholar]
- Lemma, H.; Belihu, K.; Sheferaw, D. Study on the Reproductive Performance of Jersey Cows at Wolaita Sodo Dairy Farm, Southern Ethiopia. Ethiop. Vet. J. 2010, 14, 53–70. [Google Scholar]
- Galliou, J.M.; Kiser, J.N.; Oliver, K.F.; Seabury, C.M.; Moraes, J.G.N.; Burns, G.W.; Spencer, T.E.; Dalton, J.; Neibergs, H.L. Identification of Loci and Pathways Associated with Heifer Conception Rate in U.S. Holsteins. Genes 2020, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, T.; Hutchison, J.; Norman, H.; Cole, J.; Fok, G.; Lourenco, D.; VanRaden, P. Investigating conception rate for beef service sires bred to dairy cows and heifers. J. Dairy Sci. 2020, 103, 10374–10382. [Google Scholar] [CrossRef] [PubMed]
- Suadsong, S. Alleviating Heat Stress Leads to Improved Cow Reproductive Performance; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Shiferaw, Y.; Tenhagen, B.-A.; Bekana, M.; Kassa, T. Reproductive performance of crossbred dairy cows in different production systems in the Central Highlands of Ethiopia. Trop. Anim. Health Prod. 2003, 35, 551–561. [Google Scholar] [CrossRef]
- González-Recio, O.; Alenda, R. Genetic Parameters for Female Fertility Traits and a Fertility Index in Spanish Dairy Cattle. J. Dairy Sci. 2005, 88, 3282–3289. [Google Scholar] [CrossRef]
- Kumar, N.; Eshetie, A.; Tesfaye, A.; Yizengaw, H.A. Productive performance of indigenous and HF crossbred dairy cows in Gondar, Ethiopia. Vet. World 2014, 7, 177–181. [Google Scholar] [CrossRef]
- Ayeneshet, B.; Abera, M.; Wondifraw, Z. Reproductive and Productive Performance of Indigenous Dairy Cows under Smallholder Farmers Management System in North. J. Fish. Livest. Prod. 2018, 6, 261. [Google Scholar] [CrossRef]
- Ill-Hwa, K.; Jeong, J.K. Risk factors limiting first service conception rate in dairy cows, and their economic impact. Asian-Australas. J. Anim. Sci. 2019, 32, 519–526. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Kadzere, C.; Murphy, M.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Jian, W.; Duangjinda, M.; Vajrabukka, C.; Katawatin, S. Differences of skin morphology in Bos indicus, Bos taurus, and their crossbreds. Int. J. Biometeorol. 2013, 58, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.A.; Gad, A.E.; Atta, M.A. Temperature-Humidity Indices as Indicators to Heat Stress of Climatic Conditions with Relation to Production and Reproduction of Farm Animals. Int. J. Biotechnol. Recent Adv. 2018, 1, 35–50. [Google Scholar] [CrossRef]
- Ozawa, M.; Tabayashi, D.; Latief, T.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef]
- Kornmatitsuk, B.; Chantaraprateep, P.; Kindahl, H. Different Types of Postpartum Luteal Activity Affected by the Exposure of Heat Stress and Subsequent Reproductive Performance in Holstein Lactating Cows. Reprod. Domest. Anim. 2008, 43, 515–519. [Google Scholar] [CrossRef]
- Orihuela, A. Some factors affecting the behavioural manifestation of oestrus in cattle: A review. Appl. Anim. Behav. Sci. 2000, 70, 1–16. [Google Scholar] [CrossRef]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet. World 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Kananub, S.; VanLeeuwen, J.A.; Arunvipas, P. Association between milk urea nitrogen and first service conception in smallholder dairy farms under heat and humidity stress. Vet. World 2018, 11, 1604–1608. [Google Scholar] [CrossRef]
- Recce, S.; Huber, E.; Notaro, U.S.; Rodríguez, F.M.; Ortega, H.H.; Rey, F.; Signorini, M.L.; Salvetti, N.R. Association between heat stress during intrauterine development and the calving-to-conception and calving-to-first-service intervals in Holstein cows. Theriogenology 2021, 162, 95–104. [Google Scholar] [CrossRef]
- Djelailia, H.; Bouraoui, R.; Jemmali, B.; Najar, T. Effects of heat stress on reproductive efficiency in Holstein dairy cattle in the North African arid region. Reprod. Domest. Anim. 2020, 55, 1250–1257. [Google Scholar] [CrossRef]
- Toghiani, S. Quantitative Genetic Application in the Selection Process for Livestock Production; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Bennett, G.; Pollak, E.; Kuehn, L.; Snelling, W. Breeding: Animals. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 173–186. [Google Scholar] [CrossRef]
- Almeida, T.P.; Kern, E.L.; Daltro, D.D.S.; Neto, J.B.; McManus, C.; Neto, A.T.; Cobuci, J.A. Genetic associations between reproductive and linear-type traits of Holstein cows in Brazil. Rev. Bras. Zootec. 2017, 46, 91–98. [Google Scholar] [CrossRef]
- Eriksson, S.; Johansson, K.; Axelsson, H.H.; Fikse, W. Genetic trends for fertility, udder health and protein yield in Swedish red cattle estimated with different models. J. Anim. Breed. Genet. 2017, 134, 308–321. [Google Scholar] [CrossRef]
- Þórarinsdóttir, Þ.; Eriksson, S.; Albertsdóttir, E. Genetic parameters and genetic trends of female fertility in Icelandic dairy cattle. Livest. Sci. 2021, 251, 104628. [Google Scholar] [CrossRef]
- Häggman, J.; Christensen, J.M.; Mäntysaari, E.A.; Juga, J. Genetic parameters for endocrine and traditional fertility traits, hyperketonemia and milk yield in dairy cattle. Animal 2019, 13, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Muuttoranta, K.; Tyrisevä, A.-M.; Mäntysaari, E.A.; Pösö, J.; Aamand, G.P.; Lidauer, M.H. Genetic parameters for female fertility in Nordic Holstein and Red Cattle dairy breeds. J. Dairy Sci. 2019, 102, 8184–8196. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Wall, E.; Pryce, J. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 2014, 8, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhao, F.; Wang, Y.; Zhang, Y.; Du, L.; Su, G. Comparison of single-trait and multiple-trait genomic prediction models. BMC Genet. 2014, 15, 30. [Google Scholar] [CrossRef]
- Karaman, E.; Lund, M.S.; Su, G. Multi-trait single-step genomic prediction accounting for heterogeneous (co)variances over the genome. Heredity 2019, 124, 274–287. [Google Scholar] [CrossRef]
- Budhlakoti, N.; Mishra, D.C.; Rai, A.; Lal, S.; Chaturvedi, K.K.; Kumar, R.R. A Comparative Study of Single-Trait and Multi-Trait Genomic Selection. J. Comput. Biol. 2019, 26, 1100–1112. [Google Scholar] [CrossRef]
- Sodini, S.M.; Kemper, K.; Wray, N.R.; Trzaskowski, M. Comparison of Genotypic and Phenotypic Correlations: Cheverud’s Conjecture in Humans. Genetics 2018, 209, 941–948. [Google Scholar] [CrossRef]
- Ghiasi, H.; Pakdel, A.; Nejati-Javaremi, A.; Mehrabani-Yeganeh, H.; Honarvar, M.; Gonzalez-Recio, O.; Carabaño, M.J.; Alenda, R. Genetic variance components for female fertility in Iranian Holstein cows. Livest. Sci. 2011, 139, 277–280. [Google Scholar] [CrossRef]
- Ayalew, W.; Aliy, M.; Negussie, E. Estimation of genetic parameters of the productive and reproductive traits in Ethiopian Holstein using multi-trait models. Asian-Australas. J. Anim. Sci. 2017, 30, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.G.D.O.; Aguiar, H.C.P.; Rodrigues, G.M.; Guimarães, E.C.; Nascimento, M.R.B.D.M. What is the best temperature-humidity index equation to indicate heat stress in crossbred dairy calves in a tropical environment? Cienc. Rural 2019, 49, 132. [Google Scholar] [CrossRef]
- Aguilar, I.; Misztal, I.; Tsuruta, S. Genetic components of heat stress for dairy cattle with multiple lactations. J. Dairy Sci. 2009, 92, 5702–5711. [Google Scholar] [CrossRef] [PubMed]
- Boonkum, W.; Duangjinda, M. Estimation of genetic parameters for heat stress, including dominance gene effects, on milk yield in Thai Holstein dairy cattle. Anim. Sci. J. 2014, 86, 245–250. [Google Scholar] [CrossRef]
- Oseni, S.; Misztal, I.; Tsuruta, S.; Rekaya, R. Genetic Components of Days Open Under Heat Stress. J. Dairy Sci. 2004, 87, 3022–3028. [Google Scholar] [CrossRef]
- Misztal, I.; Tsuruta, S.; Strabel, T.; Auvray, B.; Druet, T.; Lee, D.H. Blupf90 and Related Programs (Bgf90). In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002. [Google Scholar]
- Madsen, P.; Jensen, J. DMU-A Package for Analyzing Multivariate Mixed Models in quantitative Genetics and Genomics. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production (WCGALP), Vancouver, BC, Canada, 17–22 August 2013. [Google Scholar]
- Ibtisham, F.; Zhang, L.; Xiao, M.; An, L.; Ramzan, M.B.; Nawab, A.; Zhao, Y.; Li, G.; Xu, Y. Genomic Selection and Its Application in Animal Breeding. Thai J. Vet. Med. 2017, 47, 301–310. [Google Scholar]
- Calus, M.; de Haas, Y.; Pszczola, M.; Veerkamp, R. Predicted accuracy of and response to genomic selection for new traits in dairy cattle. Animal 2013, 7, 183–191. [Google Scholar] [CrossRef]
- Dekkers, J.C. Application of Genomics Tools to Animal Breeding. Curr. Genom. 2012, 13, 207–212. [Google Scholar] [CrossRef]
- Cowling, W.A.; Stefanova, K.T.; Beeck, C.P.; Nelson, M.N.; Hargreaves, B.L.W.; Sass, O.; Gilmour, A.R.; Siddique, K. Using the Animal Model to Accelerate Response to Selection in a Self-Pollinating Crop. G3 Genes Genomes Genet. 2015, 5, 1419–1428. [Google Scholar] [CrossRef]
- Sun, C.; Madsen, P.; Nielsen, U.; Zhang, Y.; Lund, M.; Su, G. Comparison between a sire model and an animal model for genetic evaluation of fertility traits in Danish Holstein population. J. Dairy Sci. 2009, 92, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Misztal, I.; Gianola, D.; Foulley, J. Computing Aspects of a Nonlinear Method of Sire Evaluation for Categorical Data. J. Dairy Sci. 1989, 72, 1557–1568. [Google Scholar] [CrossRef]
- Weller, J.I.; Ron, M. Invited review: Quantitative trait nucleotide determination in the era of genomic selection. J. Dairy Sci. 2011, 94, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Vanraden, P.M.; Van Tassell, C.P.; Wiggans, G.R.; Sonstegard, T.S.; Schnabel, R.D.; Taylor, J.F.; Schenkel, F.S. Invited Review: Reliability of genomic predictions for North American Holstein bulls. J. Dairy Sci. 2009, 92, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.C.M. Commercial application of marker- and gene-assisted selection in livestock: Strategies and lessons. J. Anim. Sci. 2004, 82, E313–E328. [Google Scholar]
- Goddard, M.E.; Hayes, B.J. Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nat. Rev. Genet. 2009, 10, 381–391. [Google Scholar] [CrossRef]
- Ortega, M.S. Identification of genes associated with reproductive function in dairy cattle. Anim. Reprod. 2018, 15, 923–932. [Google Scholar] [CrossRef]
- Ortega, M.S.; Denicol, A.C.; Cole, J.B.; Null, D.J.; Taylor, J.F.; Schnabel, R.D.; Hansen, P.J. Association of single nucleotide polymorphisms in candidate genes previously related to genetic variation in fertility with phenotypic measurements of reproductive function in Holstein cows. J. Dairy Sci. 2017, 100, 3725–3734. [Google Scholar] [CrossRef]
- Jecminkova, K.; Müller, U.; Kyselova, J.; Sztankoova, Z.; Zavadilova, L.; Stipkova, M.; Majzlik, I. Association of leptin, toll-like receptor 4, and chemokine receptor of interleukin 8 C-X-C motif single nucleotide polymorphisms with fertility traits in Czech Fleckvieh cattle. Asian-Australas. J. Anim. Sci. 2018, 31, 1721–1728. [Google Scholar] [CrossRef]
- Cañizares-Martínez, M.A.; Parra-Bracamonte, G.M.; Segura-Correa, J.C.; Magaña-Monforte, J.G. Effect of Leptin, Pituitary Transcription Factor and Luteinizing Hormone Receptor Genes Polymorphisms on Reproductive Traits and Milk Yield in Holstein Cattle. Braz. Arch. Biol. Technol. 2021, 64, 643. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Guarini, A.; Lourenco, D.; Brito, L.; Sargolzaei, M.; Baes, C.; Miglior, F.; Misztal, I.; Schenkel, F. Comparison of genomic predictions for lowly heritable traits using multi-step and single-step genomic best linear unbiased predictor in Holstein cattle. J. Dairy Sci. 2018, 101, 8076–8086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lourenco, D.; Aguilar, I.; Legarra, A.; Misztal, I. Weighting Strategies for Single-Step Genomic BLUP: An Iterative Approach for Accurate Calculation of GEBV and GWAS. Front. Genet. 2016, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, A.; Veroneze, R.; De Oliveira, H.R.; Marques, D.B.D.; Lopes, P.S.; Silva, F.F.; Brito, L.F. Comparing Alternative Single-Step GBLUP Approaches and Training Population Designs for Genomic Evaluation of Crossbred Animals. Front. Genet. 2020, 11, 263. [Google Scholar] [CrossRef]
- Pocrnic, I.; Lourenco, D.A.L.; Chen, C.-Y.; Herring, W.; Misztal, I. Crossbred evaluations using single-step genomic BLUP and algorithm for proven and young with different sources of data1. J. Anim. Sci. 2019, 97, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Guarini, A.; Lourenco, D.; Brito, L.; Sargolzaei, M.; Baes, C.; Miglior, F.; Misztal, I.; Schenkel, F. Genetics and genomics of reproductive disorders in Canadian Holstein cattle. J. Dairy Sci. 2019, 102, 1341–1353. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Ding, X. Advances in genomic selection in domestic animals. Chin. Sci. Bull. 2011, 56, 2655–2663. [Google Scholar] [CrossRef][Green Version]
- Gutierrez-Reinoso, M.; Aponte, P.; Garcia-Herreros, M. Genomic Analysis, Progress and Future Perspectives in Dairy Cattle Selection: A Review. Animals 2021, 11, 599. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Cole, J.B.; VanRaden, P.M.; Wiggans, G.R.; Ruiz-López, F.J.; Van Tassell, C.P. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. USA 2016, 113, E3995–E4004. [Google Scholar] [CrossRef]
- König, S.; Simianer, H.; Willam, A. Economic evaluation of genomic breeding programs. J. Dairy Sci. 2009, 92, 382–391. [Google Scholar] [CrossRef]
- Shumbusho, F.; Raoul, J.; Astruc, J.M.; Palhiere, I.; Elsen, J.M. Potential benefits of genomic selection on genetic gain of small ruminant breeding programs1. J. Anim. Sci. 2013, 91, 3644–3657. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.; De Koning, D.-J. Genomic selection needs to be carefully assessed to meet specific requirements in livestock breeding programs. Front. Genet. 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.S.; de Roos, A.P.; de Vries, A.G.; Druet, T.; Ducrocq, V.; Fritz, S.; Guillaume, F.; Guldbrandtsen, B.; Liu, Z.; Reents, R.; et al. A common reference population from four European Holstein populations increases reliability of genomic predictions. Genet. Sel. Evol. 2011, 43, 43. [Google Scholar] [CrossRef] [PubMed]
- Wiggans, G.; VanRaden, P.; Cooper, T. The genomic evaluation system in the United States: Past, present, future. J. Dairy Sci. 2011, 94, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Ben Zaabza, H.; Ben Gara, A.; Rekik, B. Bayesian Modeling in Genetics and Genomicsvvv; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Habier, D.; Fernando, R.L.; Kizilkaya, K.; Garrick, D.J. Extension of the bayesian alphabet for genomic selection. BMC Bioinform. 2011, 12, 186. [Google Scholar] [CrossRef]
- Campos, G.D.L.; Naya, H.; Gianola, D.; Crossa, J.; Legarra, A.; Manfredi, E.; Weigel, K.; Cotes, J.M. Predicting Quantitative Traits with Regression Models for Dense Molecular Markers and Pedigree. Genetics 2009, 182, 375–385. [Google Scholar] [CrossRef]
- Erbe, M.; Hayes, B.J.; Matukumalli, L.K.; Goswami, S.; Bowman, P.J.; Reich, C.M.; Mason, B.A.; Goddard, M.E. Improving accuracy of genomic predictions within and between dairy cattle breeds with imputed high-density single nucleotide polymorphism panels. J. Dairy Sci. 2012, 95, 4114–4129. [Google Scholar] [CrossRef]
- Genomic selection of dairy cattle:A review of methods, strategies, and impact. J. Anim. Breed. Genom. 2017, 2017, 1. [CrossRef]
- Legarra, A.; Christensen, O.F.; Aguilar, I.; Misztal, I. Single Step, a general approach for genomic selection. Livest. Sci. 2014, 166, 54–65. [Google Scholar] [CrossRef]
- Misztal, I.; Lourenco, D.; Legarra, A. Current status of genomic evaluation. J. Anim. Sci. 2020, 98, 101. [Google Scholar] [CrossRef]
- Legarra, A.; Aguilar, I.; Misztal, I. A relationship matrix including full pedigree and genomic information. J. Dairy Sci. 2009, 92, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, F.; Fritz, S.; Boichard, D.; Druet, T. Short Communication: Correlations of Marker-Assisted Breeding Values with Progeny-Test Breeding Values for Eight Hundred Ninety-Nine French Holstein Bulls. J. Dairy Sci. 2008, 91, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Neuner, S.; Edel, C.; Emmerling, R.; Thaller, G.; Götz, K.-U. Precision of genetic parameters and breeding values estimated in marker assisted BLUP genetic evaluation. Genet. Sel. Evol. 2009, 41, 26. [Google Scholar] [CrossRef] [PubMed]
- Patry, C.; Ducrocq, V. Evidence of biases in genetic evaluations due to genomic preselection in dairy cattle. J. Dairy Sci. 2011, 94, 1011–1020. [Google Scholar] [CrossRef]
- Lourenco, D.A.L.; Misztal, I.; Tsuruta, S.; Aguilar, I.; Ezra, E.; Ron, M.; Shirak, A.; Weller, J.I. Methods for Genomic Evaluation of a Relatively Small Genotyped Dairy Population and Effect of Genotyped Cow Information in Multiparity Analyses. J. Dairy Sci. 2014, 97, 1742–1752. [Google Scholar] [CrossRef]
- Sungkhapreecha, P.; Chankitisakul, V.; Duangjinda, M.; Buaban, S.; Boonkum, W. Determining Heat Stress Effects of Multiple Genetic Traits in Tropical Dairy Cattle Using Single-Step Genomic BLUP. Vet. Sci. 2022, 9, 66. [Google Scholar] [CrossRef]
- Sungkhapreecha, P.; Misztal, I.; Hidalgo, J.; Steyn, Y.; Buaban, S.; Duangjinda, M.; Boonkum, W. Changes in genetic parameters for milk yield and heat tolerance in the Thai Holstein crossbred dairy population under different heat stress levels and over time. J. Dairy Sci. 2021, 104, 12703–12712. [Google Scholar] [CrossRef]
- Buaban, S.; Prempree, S.; Sumreddee, P.; Duangjinda, M.; Masuda, Y. Genomic prediction of milk-production traits and somatic cell score using single-step genomic best linear unbiased predictor with random regression test-day model in Thai dairy cattle. J. Dairy Sci. 2021, 104, 12713–12723. [Google Scholar] [CrossRef]
- Dekkers, J.C.M.; Su, H.; Cheng, J. Predicting the accuracy of genomic predictions. Genet. Sel. Evol. 2021, 53, 55. [Google Scholar] [CrossRef]
- Dekkers, J.C.M. Prediction of response to marker-assisted and genomic selection using selection index theory. J. Anim. Breed. Genet. 2007, 124, 331–341. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Villanueva, B.; Woolliams, J.A. Accuracy of Predicting the Genetic Risk of Disease Using a Genome-Wide Approach. PLoS ONE 2008, 3, e3395. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Khatkar, M.S.; Hayes, B.J.; Raadsma, H.W. Accuracy of direct genomic values in Holstein bulls and cows using subsets of SNP markers. Genet. Sel. Evol. 2010, 42, 37. [Google Scholar] [CrossRef] [PubMed]
- Wiggans, G.R.; Cole, J.B.; Hubbard, S.M.; Sonstegard, T.S. Genomic Selection in Dairy Cattle: The USDA Experience. Annu. Rev. Anim. Biosci. 2017, 5, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.J.; Bowman, P.; Chamberlain, A.; Goddard, M. Invited review: Genomic selection in dairy cattle: Progress and challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef]
- Shao, B.; Sun, H.; Ahmad, M.J.; Ghanem, N.; Abdel-Shafy, H.; Du, C.; Deng, T.; Mansoor, S.; Zhou, Y.; Yang, Y.; et al. Genetic Features of Reproductive Traits in Bovine and Buffalo: Lessons from Bovine to Buffalo. Front. Genet. 2021, 12, 617128. [Google Scholar] [CrossRef]
- Laodim, T.; Elzo, M.A.; Koonawootrittriron, S.; Suwanasopee, T.; Jattawa, D. Genomic-polygenic and polygenic predictions for milk yield, fat yield, and age at first calving in Thai multibreed dairy population using genic and functional sets of genotypes. Livest. Sci. 2018, 219, 17–24. [Google Scholar] [CrossRef]
- Boison, S.A.; Utsunomiya, A.T.H.; Santos, D.J.A.; Neves, H.H.R.; Carvalheiro, R.; Mészáros, G.; Utsunomiya, Y.T.; do Carmo, A.S.; Verneque, R.S.; Machado, M.A.; et al. Accuracy of genomic predictions in Gyr (Bos indicus) dairy cattle. J. Dairy Sci. 2017, 100, 5479–5490. [Google Scholar] [CrossRef]
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Short communication: Genetic analysis for fertility traits of heifers and cows from smallholder dairy farms in a tropical environment. J. Dairy Sci. 2015, 98, 4990–4998. [Google Scholar] [CrossRef]
- Burrow, H.M.; Mrode, R.; Mwai, A.O.; Coffey, M.P.; Hayes, B.J. Challenges and Opportunities in Applying Genomic Selection to Ruminants Owned by Smallholder Farmers. Agriculture 2021, 11, 1172. [Google Scholar] [CrossRef]
- Koonawootrittriron, S.; Elzo, M.; Thongprapi, T. Genetic trends in a Holstein × other breeds multibreed dairy population in Central Thailand. Livest. Sci. 2009, 122, 186–192. [Google Scholar] [CrossRef]
- Sarakul, M.; Koonawootrittriron, S.; Suwanasopee, T.; Hirunwong, A.; Tongprapi, T. Situation and attitude for production and sire selection of dairy farmers in Thailand. In Proceeding of the 47th Kasetsart University Conference, Bangkok, Thailand, 17–21 March 2009. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).