Histopathological and Immunohistochemical Evaluation of Canine Nerve Sheath Tumors and Proposal for an Updated Classification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Histopathology
2.3. Immunohistochemistry
- Strong (+++): dark staining that is clearly visible at low magnification and encompasses > 50% of cells.
- Moderate (++): focal darkly stained areas encompassing <50% of cells or moderate staining of >50% of cells.
- Weak (+): focal moderate staining in <50% of cells or pale staining in any proportion of cells that is not readily visible at low magnification.
- Negative (−): none of the above.
2.4. Statistical Analysis
3. Results
3.1. Clinical Findings
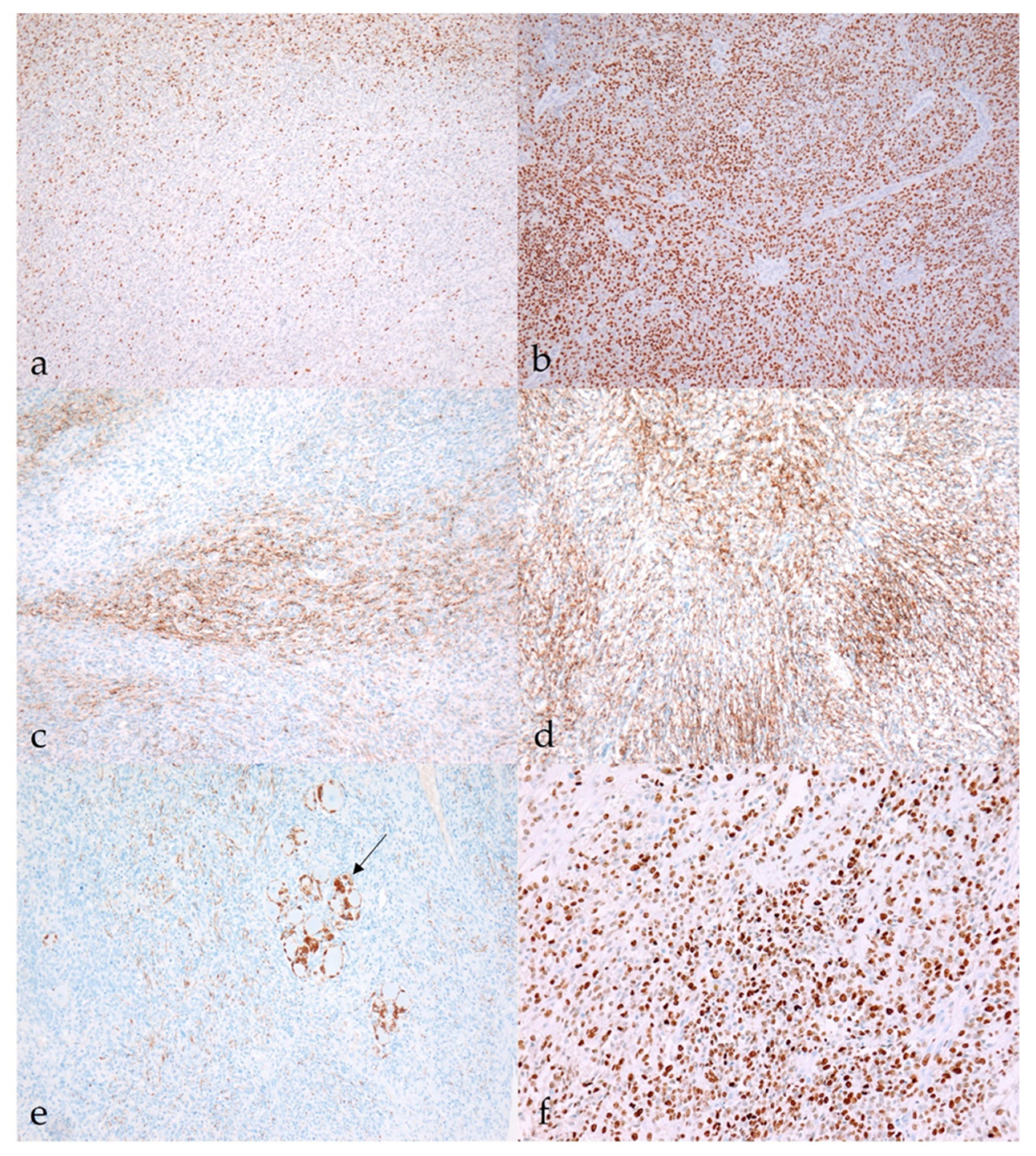
3.2. Histopathology and Immunohistochemistry
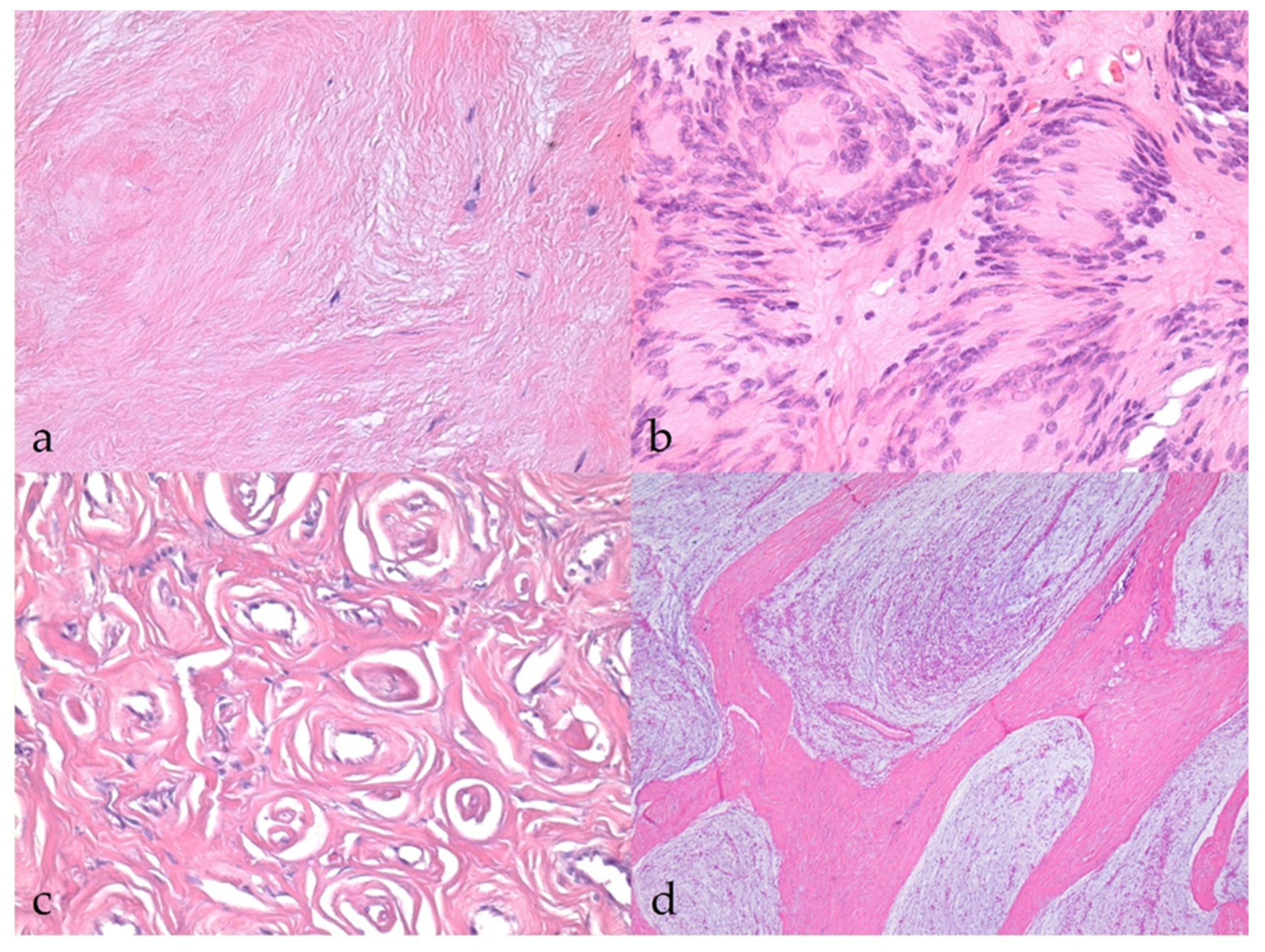
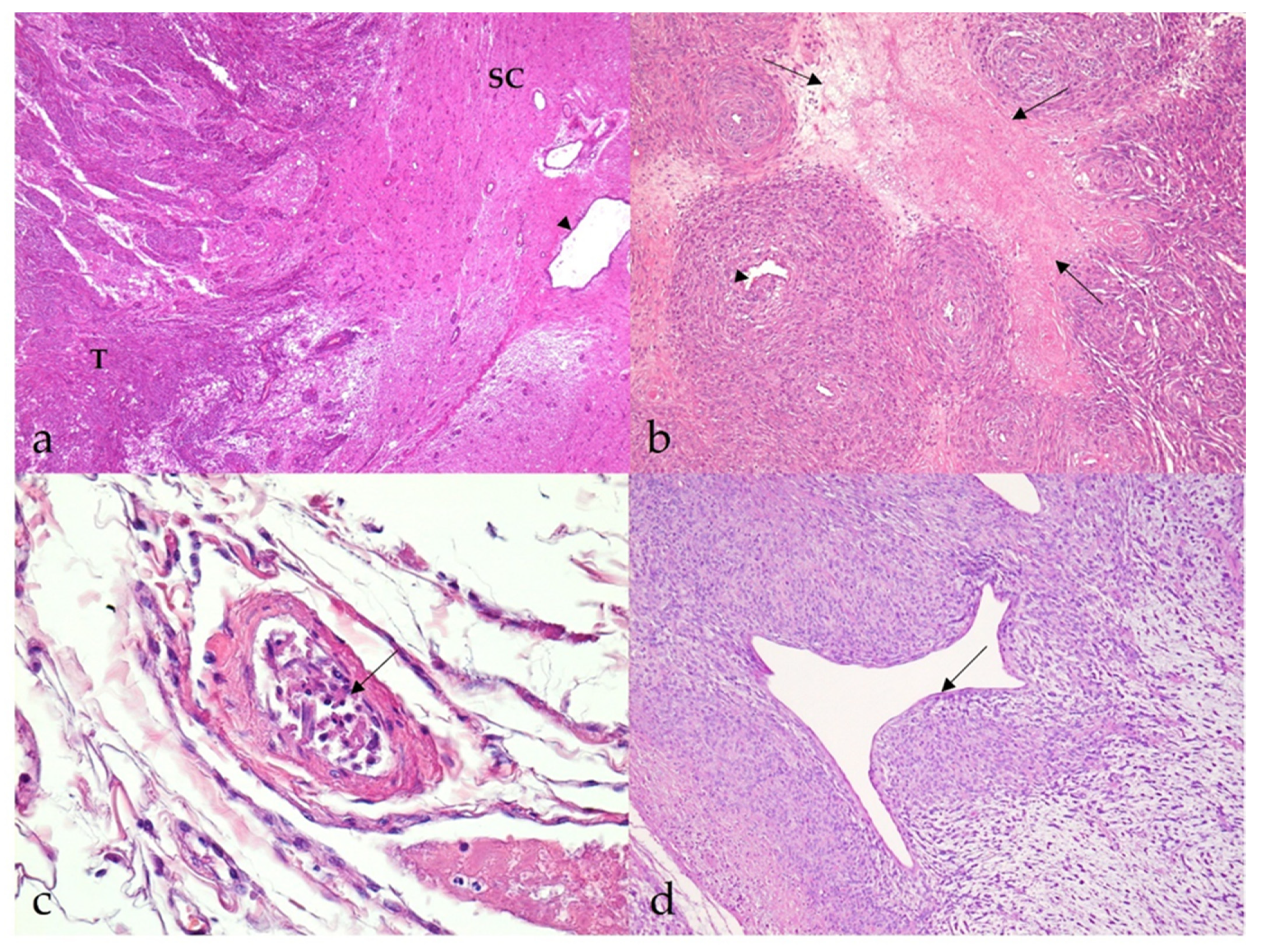
3.2.1. Benign Nerve Sheath Tumors
3.2.2. Malignant Nerve Sheath Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higgins, R.J.; Bollen, A.W.; Dickinson, P.J.; Sisó-Llonch, S. Tumors of the Nervous System. In Tumors in Domestic Animals; Wiley: Hoboken, NJ, USA, 2016; pp. 834–891. [Google Scholar]
- Lanigan, L.G.; Russell, D.S.; Woolard, K.D.; Pardo, I.D.; Godfrey, V.; Jortner, B.S.; Butt, M.T.; Bolon, B. Comparative Pathology of the Peripheral Nervous System. Vet. Pathol. 2021, 58, 10–33. [Google Scholar] [CrossRef] [PubMed]
- Schöniger, S.; Summers, B.A. Localized, plexiform, diffuse, and other variants of neurofibroma in 12 dogs, 2 horses, and a chicken. Vet. Pathol. 2009, 46, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Basa, R.M.; Crowley, A.M.; Johnson, K.A. Neurofibroma of the ulnar nerve in the carpal canal in a dog: Treatment by marginal neurectomy. J. Small Anim. Pract. 2020, 61, 512–515. [Google Scholar] [CrossRef] [Green Version]
- Vandevelde, M.; Higgins, R.J.; Oevermann, A. Veterinary Neuropathology: Essentials of Theory and Practice; Wiley-Blackwell: Oxford, UK, 2012; pp. 145–147. [Google Scholar]
- Gaitero, L.; Añor, S.; Fondevila, D.; Pumarola, M. Canine cutaneous spindle cell tumours with features of peripheral nerve sheath tumours: A histopathological and immunohistochemical study. J. Comp. Pathol. 2008, 139, 16–23. [Google Scholar] [CrossRef]
- Park, J.W.; Woo, G.H.; Jee, H.; Jung, D.W.; Youn, H.Y.; Choi, M.C.; Kim, D.Y. Malignant peripheral nerve sheath tumour in the liver of a dog. J. Comp. Pathol. 2011, 144, 223–226. [Google Scholar] [CrossRef]
- Sato, T.; Yamamoto, A.; Shibuya, H.; Sudo, H.; Shirai, W.; Amemori, T. Intraocular peripheral nerve sheath tumor in a dog. Vet. Ophthalmol. 2005, 8, 283–286. [Google Scholar] [CrossRef]
- Vom Hagen, F.; Romkes, G.; Kershaw, O.; Eule, J.C. Malignant peripheral nerve sheath tumor of the third eyelid in a 3-year-old Rhodesian Ridgeback. Clin. Case Rep. 2015, 3, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, W.; Burgener, I.A.; Roccabianca, P.; Rytz, U.; Welle, M. Primary splenic peripheral nerve sheath tumour in a dog. J. Comp. Pathol. 2009, 141, 195–198. [Google Scholar] [CrossRef]
- Ichikawa, M.; Suzuki, S.; Tei, M.; Nibe, K.; Uchida, K.; Ono, K.; Hirao, H. Malignant peripheral nerve sheath tumor originating from the adrenal gland in a dog. J. Vet. Med. Sci. 2018, 80, 1572–1575. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.M.; Dallaire, A.; Miller, L.M.; Miller, C.W. Peripheral nerve sheath tumor of the diaphragm with osseous differentiation in a one-year-old dog. J. Am. Anim. Hosp. Assoc. 1999, 35, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.O.; Goiozo, P.F.I.; Pereira, L.G.; Headley, S.A.; Bracarense, A. Concomitant Malignant Pulmonary Peripheral Nerve Sheath Tumour and Benign Cutaneous Peripheral Nerve Sheath Tumour in a Dog. J. Comp. Pathol. 2017, 157, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Baek, S.M.; Lee, A.R.; Kim, T.U.; Kim, D.; Kwon, Y.S.; Yun, S.; Park, S.J.; Hong, I.H.; Jeong, K.S.; et al. Malignant Peripheral Nerve Sheath Tumour in the Urinary Bladder of a Dog. J. Comp. Pathol. 2020, 175, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.J.; Folpe, A.L.; Giannini, C.; Perry, A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012, 123, 295–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, C.G.; Vasudevan, H.N.; Chen, W.C.; Magill, S.T.; Braunstein, S.E.; Jacques, L.; Dahiya, S.; Rodriguez, F.J.; Horvai, A.E.; Perry, A.; et al. Histopathologic findings in malignant peripheral nerve sheath tumor predict response to radiotherapy and overall survival. Neurooncol. Adv. 2020, 2, vdaa131. [Google Scholar] [CrossRef]
- Dennis, M.M.; McSporran, K.D.; Bacon, N.J.; Schulman, F.Y.; Foster, R.A.; Powers, B.E. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol. 2011, 48, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coindre, J.M. Grading of soft tissue sarcomas: Review and update. Arch. Pathol. Lab. Med. 2006, 130, 1448–1453. [Google Scholar] [CrossRef]
- Adams, E.J.; Green, J.A.; Clark, A.H.; Youngson, J.H. Comparison of different scoring systems for immunohistochemical staining. J. Clin. Pathol. 1999, 52, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Pekmezci, M.; Folpe, A.L.; Ersen, A.; Horvai, A.E. Diagnostic utility of SOX10 to distinguish malignant peripheral nerve sheath tumor from synovial sarcoma, including intraneural synovial sarcoma. Mod. Pathol. 2014, 27, 55–61. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 30 October 2021).
- Chijiwa, K.; Uchida, K.; Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol. 2004, 41, 307–318. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. (Eds.) World Health Organization Histological Classification of Tumours of the Central Nervous System, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Cornelis, I.; Chiers, K.; Maes, S.; Kramer, M.; Ducatelle, R.; De Decker, S.; Van Ham, L. Claudin-1 and glucose transporter 1 immunolabelling in a canine intraneural perineurioma. J. Comp. Pathol. 2012, 147, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.J.; Dickinson, P.J.; Jimenez, D.F.; Bollen, A.W.; Lecouteur, R.A. Canine intraneural perineurioma. Vet. Pathol. 2006, 43, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Ramos, A.T.; Viott, A.d.M.; Adeodato, A.G.; Graça, D.L. Canine intraneural perineurioma. Braz. J. Vet. Pathol. 2010, 3, 66–69. [Google Scholar]
- Ahlawat, S.; Fayad, L.M. Revisiting the WHO classification system of soft tissue tumours: Emphasis on advanced magnetic resonance imaging sequences. Part 1. Pol. J. Radiol. 2020, 85, e396–e408. [Google Scholar] [CrossRef]
- Khashaba, H.; Hafez, E.; Burezq, H. Nerve Sheath Myxoma: A rare tumor, a case report and literature review. Int. J. Surg. Case Rep. 2020, 73, 183–186. [Google Scholar] [CrossRef]
- Tafti, D.A.; Dearborn, M.C.; Ornoff, A.; Moeck, A.R.; Cecava, N.D. Nerve Sheath Myxoma in the Lower Extremity: A Rare Case with Description of Magnetic Resonance Imaging and Sonographic Findings. Am. J. Case Rep. 2021, 22, e927922-1–e927922-7. [Google Scholar] [CrossRef]
- Martin, E.; Acem, I.; Grünhagen, D.J.; Bovée, J.; Verhoef, C. Prognostic Significance of Immunohistochemical Markers and Genetic Alterations in Malignant Peripheral Nerve Sheath Tumors: A Systematic Review. Front. Oncol. 2020, 10, 594069. [Google Scholar] [CrossRef]
- Watanabe, T.; Oda, Y.; Tamiya, S.; Kinukawa, N.; Masuda, K.; Tsuneyoshi, M. Malignant peripheral nerve sheath tumours: High Ki67 labelling index is the significant prognostic indicator. Histopathology 2001, 39, 187–197. [Google Scholar] [CrossRef]
- Pekmezci, M.; Reuss, D.E.; Hirbe, A.C.; Dahiya, S.; Gutmann, D.H.; von Deimling, A.; Horvai, A.E.; Perry, A. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod. Pathol. 2015, 28, 187–200. [Google Scholar] [CrossRef]
- Grillo, F.; Bruzzone, M.; Pigozzi, S.; Prosapio, S.; Migliora, P.; Fiocca, R.; Mastracci, L. Immunohistochemistry on old archival paraffin blocks: Is there an expiry date? J. Clin. Pathol. 2017, 70, 988–993. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Zachos, T.A.; Sams, A.E.; Aitken, M.L. Malignant nerve-sheath tumor with divergent and glandular differentiation in a dog: A case report. Vet. Pathol. 2002, 39, 406–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.S.; Bronner-Fraser, M. Review: The role of neural crest cells in the endocrine system. Endocr. Pathol. 2009, 20, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Cho, D.Y.; Kim, D.Y.; Lee, J.; Taylor, H.W. Malignant peripheral nerve sheath tumor with divergent mesenchymal differentiations in a dog. J. Vet. Diagn. Investig. 2003, 15, 174–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakab, C.; Gálfi, P.; Jerzsele, Á.; Szabó, Z.; Németh, T.; Sterczer, Á.; Rusvai, M.; Ózsvári, L. Expression of claudin-1 in canine peripheral nerve sheath tumours and perivascular wall tumours. Immunohistochemical study. Histol. Histopathol. 2012, 27, 905–917. [Google Scholar] [CrossRef]
- García, P.; Sánchez, B.; Sánchez, M.A.; González, M.; Rollán, E.; Flores, J.M. Epithelioid malignant peripheral nerve sheath tumour in a dog. J. Comp. Pathol. 2004, 131, 87–91. [Google Scholar] [CrossRef]
- Pumarola, M.; Añor, S.; Borràs, D.; Ferrer, I. Malignant epithelioid schwannoma affecting the trigeminal nerve of a dog. Vet. Pathol. 1996, 33, 434–436. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Erlandson, R.A.; Lieberman, P.H. Canine malignant melanotic schwannomas: A light and electron microscopic study of two cases. Vet. Pathol. 1984, 21, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Warren, A.L.; Miller, A.D.; de Lahunta, A.; Kortz, G.; Summers, B.A. Four Cases of the Melanotic Variant of Malignant Nerve Sheath Tumour: A Rare, Aggressive Neoplasm in Young Dogs with a Predilection for the Spinal Cord. J. Comp. Pathol. 2020, 178, 1–8. [Google Scholar] [CrossRef]
- Park, Y.T.; Minamoto, T. Laparoscopic resection of retroperitoneal paraganglioma close to caudal vena cava in a dog. Vet. Med. Sci. 2021, 7, 2191–2197. [Google Scholar] [CrossRef]
- Treggiari, E.; Pedro, B.; Dukes-McEwan, J.; Gelzer, A.R.; Blackwood, L. A descriptive review of cardiac tumours in dogs and cats. Vet. Comp. Oncol. 2017, 15, 273–288. [Google Scholar] [CrossRef] [Green Version]
- Kelsh, R.N. Sorting out Sox10 functions in neural crest development. Bioessays 2006, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, D.; Chiriboga, L.; Rubin, B.P. Sox10: A pan-schwannian and melanocytic marker. Am. J. Surg. Pathol. 2008, 32, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, J.R.; Nielsen, T.O.; van de Rijn, M.; West, R.B. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucenas, S. Perineurial glia. Cold Spring Harb. Perspect. Biol. 2015, 7, a020511. [Google Scholar] [CrossRef] [Green Version]
- Ersen, A.; Pekmezci, M.; Folpe, A.L.; Tihan, T. Comparision of New Diagnostic Tools for Malignant Peripheral Nerve Sheath Tumors. Pathol. Oncol. Res. 2017, 23, 393–398. [Google Scholar] [CrossRef]
- Piña-Oviedo, S.; Ortiz-Hidalgo, C. The normal and neoplastic perineurium: A review. Adv. Anat. Pathol. 2008, 15, 147–164. [Google Scholar] [CrossRef]
- Folpe, A.L.; Billings, S.D.; McKenney, J.K.; Walsh, S.V.; Nusrat, A.; Weiss, S.W. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am. J. Surg. Pathol. 2002, 26, 1620–1626. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Tascos, N.A.; Parr, J.; Gonatas, N.K. Immunocytochemical study of the glial fibrillary acidic protein in human neoplasms of the central nervous system. Hum. Pathol. 1982, 13, 454–458. [Google Scholar] [CrossRef]
- Gray, M.H.; Rosenberg, A.E.; Dickersin, G.R.; Bhan, A.K. Glial fibrillary acidic protein and keratin expression by benign and malignant nerve sheath tumors. Hum. Pathol. 1989, 20, 1089–1096. [Google Scholar] [CrossRef]
- Kawahara, E.; Oda, Y.; Ooi, A.; Katsuda, S.; Nakanishi, I.; Umeda, S. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors. A comparative study of immunoreactivity of GFAP, vimentin, S-100 protein, and neurofilament in 38 schwannomas and 18 neurofibromas. Am. J. Surg. Pathol. 1988, 12, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Memoli, V.A.; Brown, E.F.; Gould, V.E. Glial fibrillary acidic protein (GFAP) immunoreactivity in peripheral nerve sheath tumors. Ultrastruct. Pathol. 1984, 7, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Perentes, E.; Collins, V.P.; Rubinstein, L.J. GFA protein reactivity in nerve sheath tumors: A polyvalent and monoclonal antibody study. J. Neuropathol. Exp. Neurol. 1987, 46, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Carey, E.M.; Herschkowitz, N. Immunohistochemical localization of myelin basic protein and 2′,3′-cyclic nucleotide 3′-phosphohydrolase in flattened membrane expansions produced by cultured oligodendrocytes. Neuroscience 1989, 28, 181–188. [Google Scholar] [CrossRef]
- Sprinkle, T.J. 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit. Rev. Neurobiol. 1989, 4, 235–301. [Google Scholar]
- Nielsen, A.B.; Jensen, H.E.; Leifsson, P.S. Immunohistochemistry for 2′,3′-cyclic nucleotide-3′-phosphohydrolase in 63 bovine peripheral nerve sheath tumors. Vet. Pathol. 2011, 48, 796–802. [Google Scholar] [CrossRef]


| Differentiation Score | |
| 1 | Well-differentiated MNSTs arising in transition from neurofibroma |
| 2 | Conventional, monomorphous spindle cell MNSTs |
| 3 | Highly pleomorphic MNSTs, as well as MNSTs with divergent differentiation |
| Mitotic count | |
| 1 | 0–9 mitoses/10 HPF |
| 2 | 10–19 mitoses/10 HPF |
| 3 | >19 mitoses/10 HPF |
| Tumor necrosis | |
| 0 | No necrosis |
| 1 | ≤50% necrosis |
| 2 | >50% necrosis |
| Histological grade * | |
| I | ≤3 |
| II | 4–5 |
| III | ≥6 |
| Primary Antibody, Clone, and Catalogue Number | Manufacturer | Antigen Retrieval | Antibody Dilution | Time and Temperature of Incubation of the Primary Antibody | Detection System | IHC Automated Stainer |
|---|---|---|---|---|---|---|
| Ki-67, MIB-1, (M7240) | Dako, Denmark | CC1, pH 8.5, 60 min, 25 °C | 1/50 | 32 min, 37 °C | UltraView Universal DAB Detection Kit (Ventana Medical Systems Inc., Tucson, AZ, USA) | Ventana Benchmark XT (USA) |
| CNPase, 11-5B, (ab6319) | Abcam, UK | Citrate buffer, pH 6.0, MW (1100 W), 20 min | 1/750 | 60 min, 23 °C | DAKO REALTM EnVision Detection System Peroxidase/DAB+, Rabbit/Mouse (Dako, Denmark) | / |
| Claudin-1, (ab15098) | Abcam, UK | ULTRA CC1, pH 8.45–8.65, 56 min, 25 °C | 1/50 | 20 min, 37 °C | OptiView DAB Detection Kit (Ventana Medical Systems Inc., Tucson, AZ, USA) | Ventana Benchmark ULTRA (USA) |
| GFAP, EP672Y, (05269784001) | Ventana, USA | ULTRA CC1, pH 8.45–8.65, 56 min, 25 °C | RTU * | 16 min, 37 °C | OptiView DAB Detection Kit (Ventana Medical Systems Inc., Tucson, AZ, USA) | Ventana Benchmark ULTRA (USA) |
| Sox10, EP268 (383R-15) | Cell Marque, USA | Citrate buffer, pH 6.0, MW (1100 W), 20 min | 1/100 | 60 min, 23 °C | DAKO REALTM EnVision Detection System Peroxidase/DAB+, Rabbit/Mouse (Dako, Denmark) | / |
| No. | Breed | Age (Years) | Sex | Clinical Presentation | Location | Group |
|---|---|---|---|---|---|---|
| 1 | Cocker Spaniel | 13 | M | Central vestibular syndrome. Compulsive gait. | Left V. and VII. cranial nerve. | A |
| 2 | Labrador Retriever | 6 | M | Head tilt to the left. | Left V. nerve. | A |
| 3 | French Bulldog | 6 | M | NR | Lateral mass on the left medulla oblongata and pons. | A |
| 4 | Golden Retriever | 10 | M | NR | Right pontomesencephalic extra-axial neoplasia. | A |
| 5 | Mixed breed | 7 | M | Paralysis of the right VII., IX., and X. cranial nerves. | Right VII., IX., and X. cranial nerves. | A |
| 6 | Mixed breed | 12 | F | NR | Left trigeminal nerve. | A |
| 7 | Mixed breed | 13 | M | NR | Extradural mass of the right cervical spinal cord segment (C1–C6). | B |
| 8 | Yorkshire Terrier | 12 | F | NR | Intramedullary lesions at the level of C2 and C6. | B |
| 9 | Labrador Retriever | 4 | M | NR | Lardaceous extradural neoplasia C1–C2. | B |
| 10 | Rottweiler | 9 | F | NR | Tumor of the right C1–C2. | B |
| 11 | German Shepherd | 4 | M | NR | Medullary lesion of the cervical spine. | B |
| 12 | Beagle | 8 | M | NR | Intradural extramedullary neoplasia C4–C5. | B |
| 13 | Cane Corso | 8 | M | Bilateral flexor hyporeflexia and proprioceptive deficit of the right forelimb. Neck pain. | Neoplasia of the right C4 with medullary infiltration. | B |
| 14 | Mixed breed | 8 | M | Left hemiparesis. | Intradural extramedullary mass on the left C2–C3. | B |
| 15 | French Bulldog | 8 | F | NR | Neoplasia of C2 with compression of the spinal cord. | B |
| 16 | Golden Retriever | 4 | M | Progressive tetraparesis. | Epidural lesion C2–C4. | B |
| 17 | Staffordshire Terrier | 9 | M | NR | Neoplasia of the right C2 root with endocanalar extension and spinal cord compression. | B |
| 18 | Mixed breed | 6 | F | Progressive ataxia with severe proprioceptive deficits and cervical pain. | Intradural, extramedullary mass at the level of right C2. | B |
| 19 | Mixed breed | 8 | M | Lameness/paresis of the thoracic limbs (LMN and UMN type). | Below the spine at the level of C6–T1, extending upward through the foramina and infiltrating the epidural space. | C |
| 20 | Mixed breed | 7.5 | F | Atrophy of the right shoulder. Right pleurothotonus. | Extra- and intradural lesions at the level of C6–C7 | C |
| 21 | English Setter | 7 | M | Postural deficit, hyporeflexia of the right forelimb. | Nerve roots involvement at the level of the cervicothoracic spine. | C |
| 22 | Mixed breed | 8 | M | NR | Nerve roots at the level of C7–T1 | C |
| 23 | Dalmatian | 11 | M | Lameness of the left forelimb with muscular atrophy. Reduced forelimbs proprioception. Neck pain. | Nerve roots at the level of the C6–T1. | C |
| 24 | Yorkshire Terrier | 7 | M | NR | Intradural extramedullary lesion at the level of C7–T1. | C |
| 25 | Mixed breed | 11 | M | Chronic lameness and paresis of the left forelimb. EMG: denervation atrophy. | Nerve roots—T1. | C |
| 26 | Mixed breed | 8 | M | Neck pain and lameness of the right forelimb. | Intradural extramedullary neoplasia of the roots C6–C7. | C |
| 27 | German Shepherd | 12 | M | NR | Nerve root C8. | C |
| 28 | German Shepherd | 12 | M | Progressive left hemiparesis, progressing to recumbency. | Extra- and intravertebral neoplasm at the level of the left foramina C5–C6. | C |
| 29 | German Shepherd | 6 | M | NR | Left axillary region—the T2 root. | C |
| 30 | Maltese | 6 | F | NR | Involvement of the nerve roots C7–T1. | C |
| 31 | Mixed breed | 12 | M | Lameness of the right forelimb associated with hypomyotrophy. | Tumor of the nerve roots at the cervicothoracic spinal cord. | C |
| 32 | Mixed breed | 6 | F | Atrophy of the muscles of the shoulder and left forelimb. | Tumor of the nerve roots at the cervicothoracic spinal cord. | C |
| 33 | Czechoslovakian Wolfdog | 11 | M | NR | Nerve root C8. | C |
| 34 | Mixed breed | 11 | M | NR | Nerve root of the left C7. | C |
| 35 | Mixed breed | 7 | M | NR | Tumor of the nerve roots at the cervicothoracic spinal cord. | C |
| 36 | Labrador Retriever | 7 | M | NR | Endocanalar, extramedullary C6 lesion. | C |
| 37 | West Highland White Terrier | 12 | M | Progressive hemiparesis for 15 days. | Intradural extramedullary mass involving the nerve roots at the level of the cervicothoracic spinal cord. | C |
| 38 | Dogo Argentino | 5 | F | NR | Tumor of the right C6. | C |
| 39 | Mixed breed | 11 | F | Right forelimb lameness, decreased proprioception, and pain. Right Horner syndrome. Absence of panniculus reflex cranial to right T11. | Right axillary mass extending to the spinal cord by multiple nerve roots. | C |
| 40 | German Shepherd | 10 | F | Right forelimb lameness, flexor areflexia, and muscular atrophy. | Right C8 nerve. | C |
| 41 | German Shepherd | 7 | F | Left forelimb paresis and hyporeflexia. | Left C8 nerve root. | C |
| 42 | Bernese Mountain dog | 7 | M | Ataxia of the four limbs and neck pain. | NR | C |
| 43 | French Bulldog | 10.5 | F | NR | Neoplasia of the right C7 root. | C |
| 44 | German Shepherd | 11 | F | Acute paraparesis-paraplegia. | Tumor at the level of T8-T9 with involvement of the left nerve root. | D |
| 45 | Labrador Retriever | 10 | M | NR | Extradural mass at the level of L1–L2—lateralized on the left. | D |
| 46 | Labrador Retriever | 3 | M | NR | Lesion of the T9–T10. | D |
| 47 | Mixed breed | 11 | M | Right paraparesis, ataxia, and proprioceptive deficit. | Nerve root T13. | D |
| 48 | Mixed breed | 10 | M | NR | Neoplasia of the left root L3. Invasion of the spinal canal—intramedullary growth. | D |
| 49 | Fox Terrier | 10 | F | Vestibular syndrome, facial paralysis, bilateral progressive paraparesis (LMN type), paralysis of the urinary bladder. | Nerve roots at the level of L3–L5. | D, E |
| 50 | Mixed breed | 13 | F | NR | T13 and L5 nerve roots. | D, E |
| 51 | Mixed breed | 8 | M | Paraplegia (LMN type) and absence of deep pain perception. | Lumbosacral extra- and intradural lesion | E |
| 52 | Newfoundland dog | 1.5 | M | Lameness of the left hindlimb with impaired proprioception. | Tumor located ventral to the left transverse process of L7, adjacent to the nerve root L6. The tumor is encapsulated proximally and continues distally within the nerve. | E |
| 53 | Mixed breed | 9 | M | NR | Nerve roots involvement at the level of the lumbosacral spinal cord. | E |
| 54 | Mixed breed | 4 | M | Paraparesis with proprioceptive deficit, urinary and fecal incontinence. | Intradural, intramedullary mass L4–L7. | E |
| 55 | Cavalier King Charles Spaniel | 12 | M | NR | Lumbar paravertebral lesion on the left side. | E |
| 56 | Staffordshire Terrier | 8 | F | Right hindlimb paresis. | Extramedullary mass at the level of the L4–L5 nerve roots. | E |
| 57 | Mixed breed | 6 | M | NR | Right brachial plexus (C6–C7) | F |
| 58 | Beagle | 8 | M | NR | Brachial plexus. | F |
| 59 | German Wirehaired Pointer | 8 | M | Left forelimb paresis.Absence of spinal reflexes. Pain on palpation of the axilla. | Extramedullary centripetal lesion at the root of the left radial nerve. | F |
| 60 | Shih-Tzu | 4 | F | Pulmonary and brain metastases. | Brachial plexus. | F |
| 61 | Labrador Retriever | 6 | M | NR | Right brachial plexus. | F |
| 62 | Mixed breed | 6 | M | NR | Left brachial plexus. | F |
| 63 | English Setter | 11 | M | NR | Neoplasia of the left brachial plexus (C7–T1). | F |
| 64 | Boston Terrier | 8 | M | NR | Brachial plexus. | F |
| 65 | Mixed breed | 9 | M | NR | Brachial plexus. | F |
| 66 | German Shepherd | 8 | M | NR | Brachial plexus. | F |
| 67 | Mixed breed | 11 | M | NR | Right brachial plexus. | F |
| 68 | Mixed breed | 11 | M | Progressive lameness of left forelimb. | A mass in the left shoulder region—involving the brachial plexus and cervicothoracic spinal cord C4–T7. | F |
| 69 | Mixed breed | 11 | M | NR | Left brachial plexus. | F |
| 70 | Papillon | 7 | M | Progressive pain of the right forelimb (radicular syndrome) and neck pain. | Right brachial plexus tumor (C1–T2). | F |
| 71 | Mixed breed | 8 | F | Chronic paresis of the left forelimb. | Left brachial plexus. | F |
| 72 | Jack Russel Terrier | 6.5 | M | Chronic lameness of the right forelimb. | Neoplasia of the right brachial plexus extending to the C6–T1 nerve roots. | F |
| 73 | West Highland White Terrier | 10 | M | NR | Lumbosacral plexus. | G |
| 74 | Mixed breed | 9 | M | NR | Lumbar plexus (L6–L7). | G |
| 75 | Labrador Retriever | 7 | F | Lameness and muscle atrophy of the right pelvic limb | Right femoral nerve. | H |
| 76 | Labrador Retriever | 5 | M | NR | Left sciatic nerve. | H |
| 77 | German Shepherd | 12 | M | NR | Left radial nerve. | H |
| 78 | Bernese Mountain dog | 12 | M | NR | Brachial nerve. | H |
| 79 | Boxer | 6 | M | Chronic lameness and pain of the left forelimb. | Left ulnar nerve. | H |
| No. | Tumor Type | Histological Grade (If Malignant) | Sox10 Expression | Claudin-1 Expression | GFAP Expression | CNPase Expression | Proliferation Index Ki-67 (%) |
|---|---|---|---|---|---|---|---|
| 1 | MNST—divergent | III | − | − | − | − | ND |
| 2 | MNST—conventional | III | + | + | − | − | ND |
| 3 | MNST—conventional | III | + | + | + | − | 11.8 |
| 4 | MNST—conventional | II | − | − | − | − | 19.0 |
| 5 | MNST—conventional | II | − | ++ | − | − | 9.7 |
| 6 | MNST—conventional | II | + | ++ | − | − | 11.0 |
| 7 | MNST—conventional | II | − | − | − | − | ND |
| 8 | MNST—conventional | II | + | − | − | − | 15.8 |
| 9 | MNST—conventional | III | − | − | − | − | 27.7 |
| 10 | Schwannoma—classic | NA | +++ | − | ++ | + | 1.5 |
| 11 | MNST—conventional | II | + | + | − | − | 9.1 |
| 12 | Nerve sheath myxoma | NA | ++ | ++ | ++ | − | 3.2 |
| 13 | MNST—conventional | III | − | + | − | − | 39.2 |
| 14 | MNST—conventional | II | + | − | − | − | 5.6 |
| 15 | MNST—conventional | I | ++ | ++ | − | − | 20.1 |
| 16 | MNST—conventional | III | − | − | − | − | 43.6 |
| 17 | Neurofibroma | NA | ++ | +++ | ++ | − | 10.0 |
| 18 | Neurofibroma | NA | ++ | ++ | + | − | 6.4 |
| 19 | MNST—conventional | III | − | − | − | − | ND |
| 20 | MNST—conventional | II | ++ | ++ | + | − | ND |
| 21 | MNST—conventional | III | (++) | (++) | (++) | − | ND |
| 22 | MNST—conventional | I | ++ | ++ | ++ | − | ND |
| 23 | Hybrid BNST— perineurioma/neurofibroma | NA | ND | ++ | ND | − | ND |
| 24 | MNST—conventional | I | ++ | ++ | (+) | − | ND |
| 25 | MNST—conventional | I | − | − | − | − | ND |
| 26 | MNST—conventional | II | +++ | + | − | − | ND |
| 27 | MNST—conventional | I | + | +++ | + | − | ND |
| 28 | MNST—conventional | III | + | + | − | − | ND |
| 29 | Hybrid BNST— perineurioma/schwannoma | NA | ++ | ++ | + | − | ND |
| 30 | MNST—conventional | II | ++ | ++ | + | − | 23.2 |
| 31 | Neurofibroma | NA | ++ | +++ | ++ | − | 11.0 |
| 32 | MNST—conventional | I | ++ | ++ | ++ | − | 10.5 |
| 33 | MNST—perineural | II | − | +++ | − | − | 35.3 |
| 34 | MNST—conventional | I | ++ | ++ | + | − | 14.2 |
| 35 | MNST—conventional | II | ++ | ++ | + | − | 45.3 |
| 36 | MNST—conventional | II | + | + | + | − | 23.5 |
| 37 | MNST—conventional | I | ++ | ++ | − | − | 16.4 |
| 38 | Nerve sheath myxoma | NA | ++ | ++ | + | − | 8.2 |
| 39 | MNST—divergent | III | ++ | ++ | + | − | 71.4 |
| 40 | Neurofibroma—plexiform | NA | ++ | ++ | ++ | − | 0.9 |
| 41 | MNST—conventional | III | ++ | +++ | − | − | 48.7 |
| 42 | Neurofibroma | NA | ++ | + | ++ | − | 2.0 |
| 43 | MNST—conventional | II | + | + | − | − | 32.1 |
| 44 | MNST—divergent | III | − | − | − | − | ND |
| 45 | MNST—conventional | II | − | + | − | − | ND |
| 46 | Neurofibroma | NA | − | ++ | + | − | 0.8 |
| 47 | MNST—conventional | II | + | − | − | − | 11.3 |
| 48 | MNST—conventional | II | + | ++ | + | − | 20.7 |
| 49 | MNST—conventional | I | + | ++ | − | − | ND |
| 50 | MNST—conventional | II | − | ++ | − | − | 7.8 |
| 51 | MNST—conventional | II | + | + | − | − | ND |
| 52 | Nerve sheath myxoma | NA | + | + | + | − | ND |
| 53 | MNST—conventional | III | − | + | − | − | 16.2 |
| 54 | MNST—conventional | I | + | (+) | (+) | − | 2.4 |
| 55 | MNST—conventional | III | + | + | + | − | 13.0 |
| 56 | MNST—conventional | III | − | − | − | − | 19.6 |
| 57 | MNST—conventional | II | − | − | − | − | ND |
| 58 | MNST—perineural | II | − | +++ | − | − | ND |
| 59 | MNST—conventional | II | + | + | + | − | ND |
| 60 | MNST—conventional | III | − | − | − | − | ND |
| 61 | MNST—conventional | I | ++ | ++ | + | − | 9.1 |
| 62 | MNST—conventional | III | + | + | − | − | 28.5 |
| 63 | MNST—perineural | III | − | +++ | − | − | 20.1 |
| 64 | MNST—conventional | III | ++ | + | − | − | 21.0 |
| 65 | MNST—perineural | III | − | +++ | − | − | 64.3 |
| 66 | MNST—divergent | III | − | ++ | − | − | 22.1 |
| 67 | MNST—conventional | I | + | ++ | − | − | 29.7 |
| 68 | MNST—conventional | II | ++ | ++ | − | − | 30.2 |
| 69 | MNST—conventional | III | + | ++ | − | − | 46.4 |
| 70 | MNST—divergent | III | ++ | ++ | ++ | − | 31.7 |
| 71 | MNST—conventional | II | + | + | + | − | 47.0 |
| 72 | MNST - conventional | II | + | +++ | ++ | − | 29.3 |
| 73 | MNST—conventional | II | − | − | − | − | 27.4 |
| 74 | MNST—conventional | II | ++ | + | + | − | 30.1 |
| 75 | MNST—conventional | I | ++ | − | − | − | ND |
| 76 | MNST—conventional | I | + | + | + | − | 14.4 |
| 77 | MNST—conventional | I | + | − | − | − | 15.7 |
| 78 | MNST—divergent | III | ++ | − | − | − | 11.2 |
| 79 | MNST—epithelioid | II | +++ | − | − | − | 20.4 |
| Tumor Type | Sox10 Expression | Claudin-1 Expression | GFAP Expression | CNPase Expression |
|---|---|---|---|---|
| BNST | ||||
| Neurofibroma * | −/++ | +/++/+++ | +/++ | − |
| Schwannoma * | +++ | − | +/++ | −/+ |
| Perineurioma * | − | ++/+++ | − | − |
| Nerve sheath myxoma | +/++ | +/++ | +/++ | − |
| MNST | ||||
| Conventional | −/+/++/++ | −/+/++/+++ | −/+/++ | − |
| Divergent | −/++ | −/++ | −/+/++ | − |
| Perineural | − | +++ | − | − |
| Epithelioid | +++ | − | − | − |
| Tumor Type | N | Sox10 Expression, n (%) | Claudin-1 Expression, n (%) | GFAP Expression, n (%) | CNPase Expression, n (%) | Proliferation Index Ki-67 (%) Range **** (Mean, SD) |
|---|---|---|---|---|---|---|
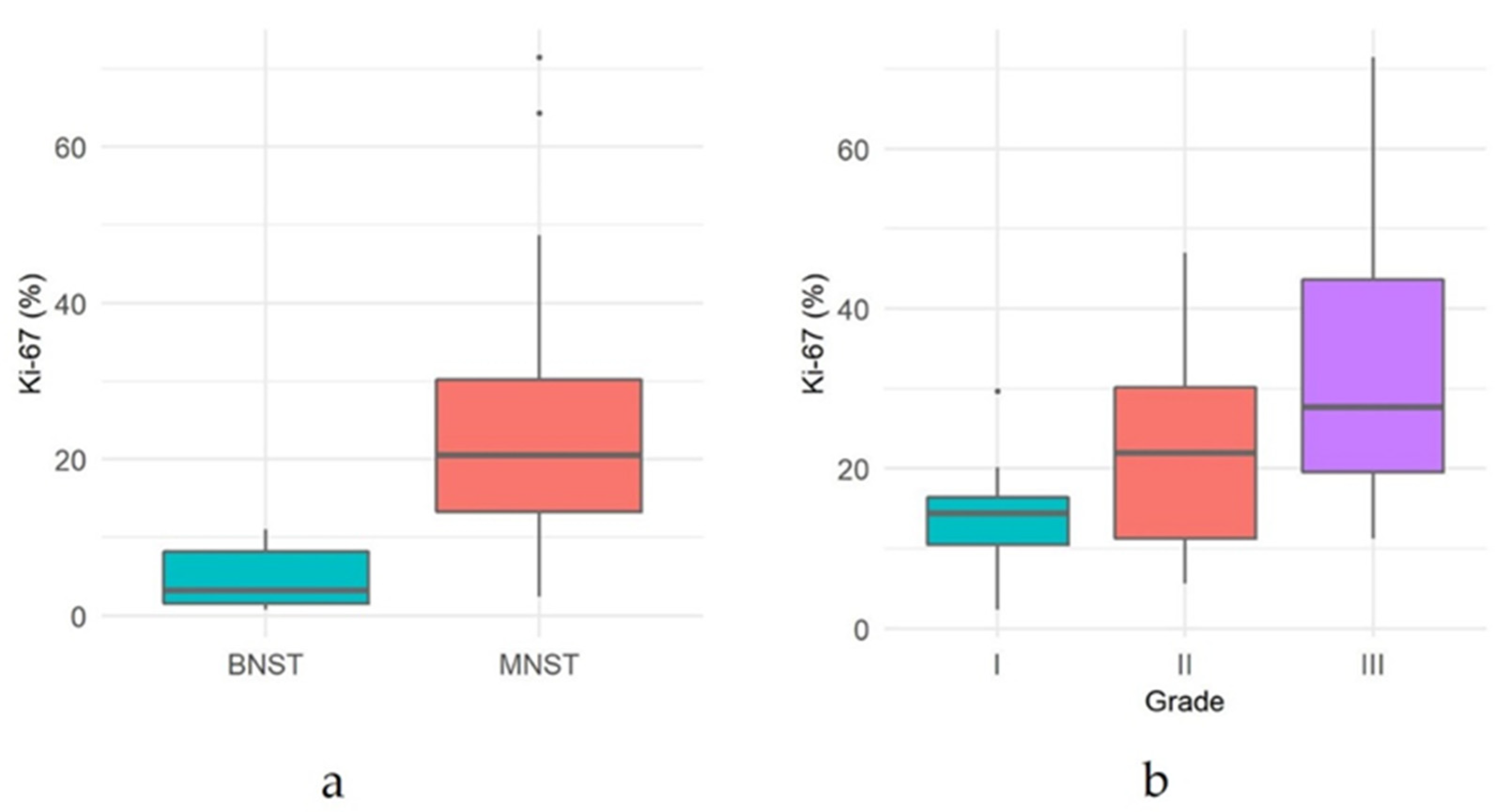
| NST | 79 | 54/77 (68.4) ** | 59/79 (74.7) | 33/78 (43.0) * | 1/79 (0.1) | 0.8–71.4 (21.2 ± 15.6) |
| BNST | 12 | 10/11 (90.9) * | 11/12 (91.7) | 11/11 (100) * | 1/12 (0.8) | 0.8–11.0 (4.9 ± 4.1) |
| Neurofibroma *** | 7 | 5/6 (83.3) * | 7/7 (100) | 6/6 (100) * | 0/7 (0) | 0.8–11.0 (5.1 ± 4.6) |
| Schwannoma *** | 2 | 2/2 (100) | 0/2 (0) | 2/2 (100) | 1/2 (50.0) | 1.5 |
| Perineurioma *** | 2 | 0/1 (0) * | 2/2 (100) | 0/1 (0) * | 0/2 (0) | ND |
| Nerve sheath myxoma | 3 | 3/3 (100) | 3/3 (100) | 3/3 (100) | 0/3 (0) | 3.2–8.2 (5.7 ± 3.5) |
| MNST | 67 | 44/66 (66.7) * | 48/67 (71.6) | 22/67 (32.8) | 0/67 (0) | 2.4–71.4 (24.4 ± 15.1) |
| Conventional | 56 | 40/55 (72.7) * | 41/56 (73.2) | 20/56 (35.7) | 0/56 (0) | 2.4–48.7 (22.3 ± 12.6) |
| Grade I | 15 | 13/14 (91.7) * | 12/15 (76.9) | 8/15 (46.2) | 0/15 (0) | 2.4–29.7 (14.7 ± 7.6) |
| Grade II | 25 | 18/25 (72.0) | 18/25 (72.0) | 9/25 (36.0) | 0/25 (0) | 5.6–47.0 (22.1 ± 12.3) |
| Grade III | 16 | 9/16 (56.3) | 11/16 (68.8) | 3/16 (18.8) | 0/16 (0) | 11.8–48.7 (28.7 ± 13.7) |
| Divergent | 6 | 3/6 (50.0) | 3/6 (50.0) | 2/6 (33.3) | 0/6 (0) | 11.2–71.4 (34.1 ± 26.2) |
| Grade III | 6 | 3/6 (50.0) | 3/6 (50.0) | 2/6 (33.3) | 0/6 (0) | 11.2–71.4 (34.1 ± 26.2) |
| Perineural | 4 | 0/4 (0) | 4/4 (100) | 0/4 (0) | 0/4 (0) | 20.1–64.3 (39.9 ± 22.5) |
| Grade II | 2 | 0/2 (0) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 35.3 |
| Grade III | 2 | 0/2 (0) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 20.1–64.3 (45.2 ± 31.3) |
| Epithelioid | 1 | 1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 20.4 |
| Grade II | 1 | 1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 20.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekavec, K.; Švara, T.; Knific, T.; Gombač, M.; Cantile, C. Histopathological and Immunohistochemical Evaluation of Canine Nerve Sheath Tumors and Proposal for an Updated Classification. Vet. Sci. 2022, 9, 204. https://doi.org/10.3390/vetsci9050204
Tekavec K, Švara T, Knific T, Gombač M, Cantile C. Histopathological and Immunohistochemical Evaluation of Canine Nerve Sheath Tumors and Proposal for an Updated Classification. Veterinary Sciences. 2022; 9(5):204. https://doi.org/10.3390/vetsci9050204
Chicago/Turabian StyleTekavec, Kristina, Tanja Švara, Tanja Knific, Mitja Gombač, and Carlo Cantile. 2022. "Histopathological and Immunohistochemical Evaluation of Canine Nerve Sheath Tumors and Proposal for an Updated Classification" Veterinary Sciences 9, no. 5: 204. https://doi.org/10.3390/vetsci9050204
APA StyleTekavec, K., Švara, T., Knific, T., Gombač, M., & Cantile, C. (2022). Histopathological and Immunohistochemical Evaluation of Canine Nerve Sheath Tumors and Proposal for an Updated Classification. Veterinary Sciences, 9(5), 204. https://doi.org/10.3390/vetsci9050204






