Effects of Red Ginseng Byproducts on Rumen Fermentation, Growth Performance, Blood Metabolites, and mRNA Expression of Heat Shock Proteins in Heat-Stressed Fattening Hanwoo Steers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Diets and Chemical Analysis
2.2. In Vitro Fermentation
2.3. In Vivo Experimental Design
2.4. Temperature–Humidity Index
2.5. Respiration Rate and Rectal Temperature
2.6. Rumen Fermentation Characteristics
2.7. Analysis of Blood Metabolites
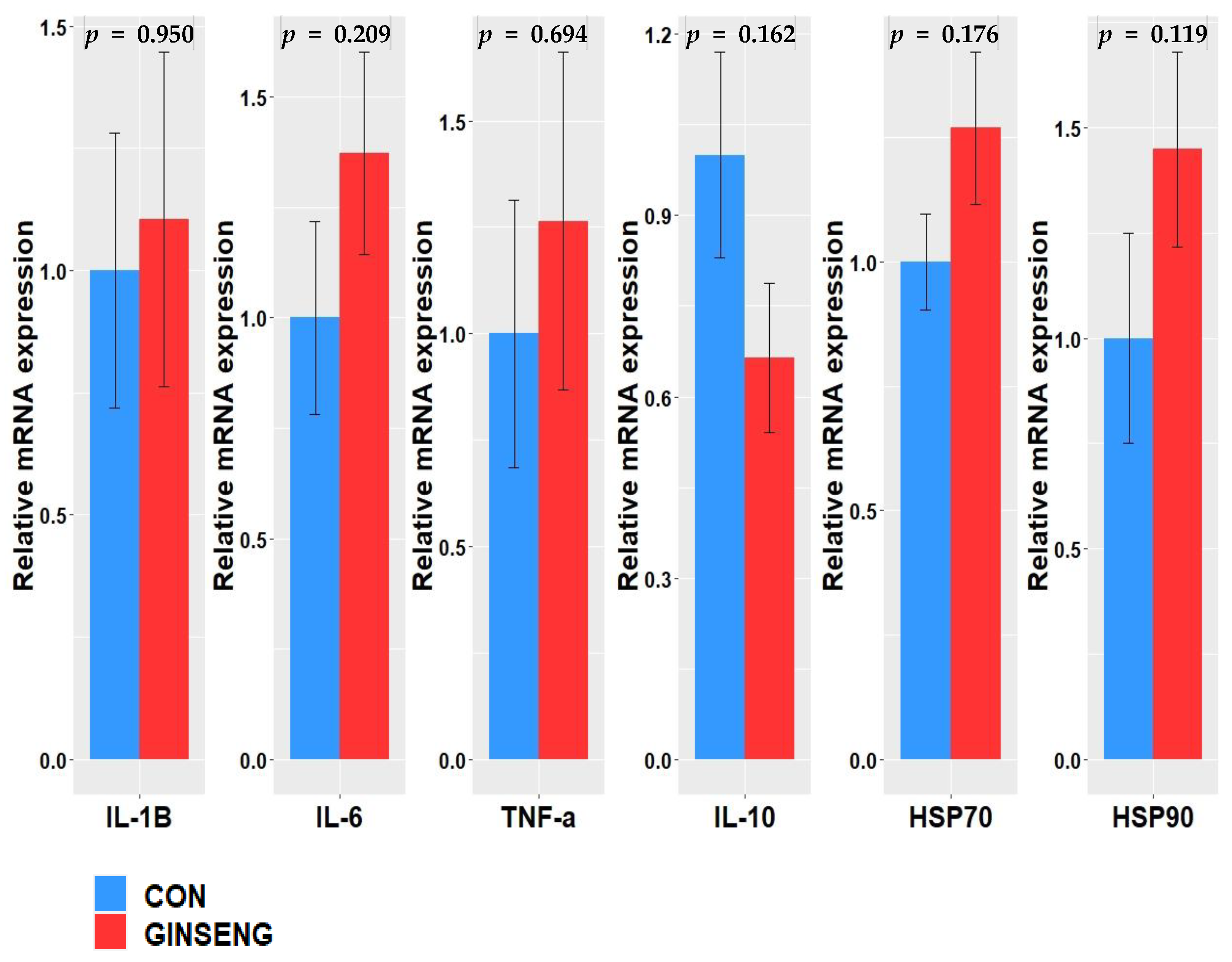
2.8. Total RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
3.1. In Vitro Experiment
3.2. In Vivo Experiment
4. Discussion
4.1. In Vitro Experiment
4.2. In Vivo Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-S.; Kang, S.; Kim, M.-J.; Han, S.-G.; Lee, H.-G. Dietary supplementation with combined extracts from garlic (Allium sativum), brown seaweed (Undaria pinnatifida), and pinecone (Pinus koraiensis) improves milk production in Holstein cows under heat stress conditions. Asian-Australas. J. Anim. Sci. 2020, 33, 111. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Compa. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bu, D.; Wang, J.; Huo, X.; Guo, T.; Wei, H.; Zhou, L.; Rastani, R.; Baumgard, L.; Li, F. Effect of saturated fatty acid supplementation on production and metabolism indices in heat-stressed mid-lactation dairy cows. J. Dairy Sci. 2010, 93, 4121–4127. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.A.; Cooke, R.F.; Millican, A.A.; Schubach, K.M.; Scatolin, G.N.; Rett, B.; Brandão, A.P. Supplementing an immunomodulatory feed ingredient to improve thermoregulation and performance of finishing beef cattle under heat stress conditions. J. Anim. Sci. 2019, 97, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.-H.; Guo, J.; Sun, X.; Li, N.; Yang, X.; Gao, Y.; Qiu, D.; Li, X.; Wang, Y.; Feng, M. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J. Anim. Sci. 2018, 96, 4444–4457. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Kim, S.-K.; Cha, K.-M.; Jeong, M.-S.; Ghosh, P.; Rhee, D.-k. Korean Red Ginseng alleviates neuroinflammation and promotes cell survival in the intermittent heat stress-induced rat brain by suppressing oxidative stress via estrogen receptor beta and brain-derived neurotrophic factor upregulation. J. Ginseng. Res. 2020, 44, 593–602. [Google Scholar] [CrossRef]
- Jung, J.S.; Shin, J.A.; Park, E.M.; Lee, J.E.; Kang, Y.S.; Min, S.W.; Kim, D.H.; Hyun, J.W.; Shin, C.Y.; Kim, H.S. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: Critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J. Neurochem. 2010, 115, 1668–1680. [Google Scholar] [CrossRef]
- Kim, K.-J.; Yoon, K.-Y.; Hong, H.-D.; Lee, B.-Y. Role of the red ginseng in defense against the environmental heat stress in Sprague Dawley rats. Molecules 2015, 20, 20240–20253. [Google Scholar] [CrossRef]
- Nam, K.-Y. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng CA Meyer). J. Ginseng. Res. 2005, 29, 1–18. [Google Scholar]
- Hong, J.-K.; Bong, M.-H.; Park, J.-C.; Moon, H.-K.; Lee, S.-C.; Lee, J.-H.; Hwang, S.-G. Effect of feeding red ginseng marc on vital reaction in laying hens under stress task. Korean J. Poultry Sci. 2012, 39, 63–70. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-K.; Choe, Y.-H.; Kim, G.-S.; Kim, H.-Y.; Kim, B.-S. Effect of Korean red ginseng marc fermented by Bacillus subtilis on swine immunity. Korean J. Vet. Serv. 2018, 41, 141–147. [Google Scholar]
- Kim, J.E.; Jang, S.G.; Lee, C.H.; Lee, J.Y.; Park, H.; Kim, J.H.; Lee, S.; Kim, S.H.; Park, E.Y.; Lee, K.W. Beneficial effects on skin health using polysaccharides from red ginseng by-product. J. Food Biochem. 2019, 43, e12961. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Shreve, B.; Thiex, N.; Wolf, M. National Forage Testing Association Reference Method: Dry Matter by Oven Drying for 3 Hours at 105 C. In NFTA Reference Methods; National Forage Testing Association: Omaha, NB, USA, 2006; pp. 1–4. [Google Scholar]
- Aoac, C. Official Methods of Analysis of the Association of Analytical Chemists International; Official Methods: Gaithersburg, MD, USA, 2005.
- Van Soest, P.v.; Robertson, J.B.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dairy Cattle; National Research Press: Washington, DC, USA, 2001. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.; Pell, A. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Pell, A.; Schofield, P. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 1993, 76, 1063–1073. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef]
- Griesbeck-Zilch, B.; Osman, M.; Kühn, C.; Schwerin, M.; Bruckmaier, R.; Pfaffl, M.; Hammerle-Fickinger, A.; Meyer, H.; Wellnitz, O. Analysis of key molecules of the innate immune system in mammary epithelial cells isolated from marker-assisted and conventionally selected cattle. J. Dairy Sci. 2009, 92, 4621–4633. [Google Scholar] [CrossRef]
- Strandberg, Y.; Gray, C.; Vuocolo, T.; Donaldson, L.; Broadway, M.; Tellam, R. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 2005, 31, 72–86. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, G.M.; Park, S.D.; Hill, E.W.; Meade, K.G.; Mitchell, L.C.; Agaba, M.; Gibson, J.P.; Hanotte, O.; Naessens, J.; Kemp, S.J. Cytokine mRNA profiling of peripheral blood mononuclear cells from trypanotolerant and trypanosusceptible cattle infected with Trypanosoma congolense. Physiol. Genom. 2006, 28, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Nejad, J.G.; Peng, D.; Jung, U.; Kim, M.; Jo, Y.; Jo, J.; Lee, J.; Lee, H. Identification of heat shock protein gene expression in hair follicles as a novel indicator of heat stress in beef calves. Anim. 2020, 14, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Singh, D.D.; Mukesh, M.; Kataria, R.; Yadav, A.; Mohanty, A.; Mishra, B. Identification of suitable housekeeping genes for expression analysis in mammary epithelial cells of buffalo (Bubalus bubalis) during lactation cycle. Livest. Sci. 2012, 147, 72–76. [Google Scholar] [CrossRef]
- Hamid, M.M.A.; Moon, J.; Yoo, D.; Kim, H.; Lee, Y.K.; Song, J.; Seo, J. Rumen fermentation, methane production, and microbial composition following in vitro evaluation of red ginseng byproduct as a protein source. J. Anim. Sci. Technol. 2020, 62, 801. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Joo, J.; Lee, S.; Kim, D.; Maeng, W. Effects of substitution level of ginseng meal for alfalfa hay on the ruminal fermentation characteristics In vitro. Korean J. Anim. Nutr. Feedstuffs 1994, 18, 481–490. [Google Scholar]
- Millen, D.D.; Arrigoni, M.D.B.; Pacheco, R.D.L. Rumenology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhu, W.; Zhang, B.; Yao, K.; Yoon, I.; Chung, Y.; Wang, J.; Liu, J. Effects of supplemental levels of Saccharomyces cerevisiae fermentation product on lactation performance in dairy cows under heat stress. Asian-Australas. J. Anim. Sci. 2016, 29, 801. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Chen, J.; Peng, D.; Gu, X. Predicting rectal temperature and respiration rate responses in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2020, 103, 5466–5484. [Google Scholar] [CrossRef]
- Davison, C.; Michie, C.; Hamilton, A.; Tachtatzis, C.; Andonovic, I.; Gilroy, M. Detecting heat stress in dairy cattle using neck-mounted activity collars. Agriculture 2020, 10, 210. [Google Scholar] [CrossRef]
- Abeni, F.; Calamari, L.; Stefanini, L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int. J. Biometeorol. 2007, 52, 87–96. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: Evidence from studies with heat-stressed Caco-2 cells, C. elegans and growing broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-K.; Kim, J.-H.; Kim, K.-H.; Choi, C.-W.; Kang, S.-W.; Nam, I.-S.; Kim, D.-H.; Song, M.-K.; Kim, C.-W.; Park, K.-K. Effects of level and degradability of dietary protein on ruminal fermentation and concentrations of soluble non-ammonia nitrogen in ruminal and omasal digesta of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2008, 21, 392–403. [Google Scholar] [CrossRef]
- Zenobi, M.; Lardner, H.; Jefferson, P.; McKinnon, J. Effect of feeding strategically blended feed pellets on rumen fermentation and nutrient digestion. Can. J. Anim. Sci. 2015, 95, 243–254. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Lee, S.; Oh, Y.; Chung, C.; Kim, K. Effects of total mixed rations on ruminal characteristics, digestibility and beef production of Hanwoo steers. J. Anim. Sci. Technol. 2003, 45, 387–396. [Google Scholar] [CrossRef][Green Version]
- Baek, Y.C.; Kim, M.S.; Reddy, K.E.; Oh, Y.K.; Jung, Y.H.; Yeo, J.M.; Choi, H. Rumen fermentation and digestibility of spent mushroom (Pleurotus ostreatus) substrate inoculated with Lactobacillus brevis for Hanwoo steers. Rev. Colomb. Cienc. Pecu. 2017, 30, 267–277. [Google Scholar] [CrossRef]
- El-Zaiat, H.M.; Kholif, A.E.; Khattab, I.M.; Sallam, S.M. Slow-Release Urea Partially Replace Soybean in the Diet of Holstein Dairy Cows: Intake, Blood Parameters, Nutrients Digestibility, Energy Utilization, and Milk Production. Ann. Anim. Sci. 2021. Available online: https://squ.pure.elsevier.com/en/publications/slow-release-urea-partially-replace-soybean-in-the-diet-of-holste (accessed on 9 March 2022). [CrossRef]
- Liu, X.; Zhang, Y.; Liu, L.; Pan, Y.; Hu, Y.; Yang, P.; Liao, M. Protective and therapeutic effects of nanoliposomal quercetin on acute liver injury in rats. BMC Pharmacol. Toxicol. 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Nam, I.; Lee, S.; Choi, C.; Kim, W.; Kwon, E.; Lee, K.; Lee, M.; Oh, Y. Effect of indigenous herbs on growth, blood metabolites and carcass characteristics in the late fattening period of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2013, 26, 1562. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, K.H.; Nam, I.S.; Kim, W.Y.; Yeo, J.M.; Lee, S.S.; Ju, J.C.; Oh, Y.K. Comparison of blood metabolites and enzyme activities at different slaughter ages of Hanwoo cattle. J. Anim. Sci. Technol. 2012, 54, 443–448. [Google Scholar] [CrossRef]
- Kim, S.H.; Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Park, K.K.; Cho, Y.I.; Son, A.; Lee, S.-S. Growth performance and blood profiles of Hanwoo steers at fattening stage fed Korean rice wine residue. J. Anim. Sci. Technol. 2020, 62, 812. [Google Scholar] [CrossRef]
- Cheng, K.; Yan, E.; Song, Z.; Li, S.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Protective effect of resveratrol against hepatic damage induced by heat stress in a rat model is associated with the regulation of oxidative stress and inflammation. J. Therm. Biol. 2019, 82, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Jahan, N.; Bertoni, G.; Ferrari, A.; Minuti, A. Pro-inflammatory cytokine profile in dairy cows: Consequences for new lactation. Ital. J. Anim. Sci. 2015, 14, 3862. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Cha, K.-M.; Hwang, S.-Y.; Jeong, M.-S.; Kim, S.-K. Korean Red Ginseng (Panax ginseng Meyer) with enriched Rg3 ameliorates chronic intermittent heat stress–induced testicular damage in rats via multifunctional approach. J. Ginseng. Res. 2019, 43, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Hong, H.-D.; Lee, B.-Y. Effect of Panax ginseng and Acanthopanax senticosus on the Expression of HSPs during Heat Stress. Korean Ginseng. Res. Ind. 2009, 3, 3–10. [Google Scholar]
- Liu, W.; Wang, Z.; Leng, J.; Heng, W.; Shen, R.; Gong, X.; Chen, C.; Wang, Y.; Zhang, R.; Li, W. 20 (R)-ginsenoside Rg3, a product of high-efficiency thermal deglycosylation of ginsenoside Rd, exerts protective effects against scrotal heat-induced spermatogenic damage in mice. Biocell 2020, 44, 655. [Google Scholar] [CrossRef]
- Yun, S.-H.; Moon, Y.-S.; SoHn, S.-H.; Jang, I.-S. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp. Anim. 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Sajjanar, B.; Singh, U.; Kumar, S.; Singh, R.; Sengar, G.; Sharma, A. Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus× Bos taurus) breed of cattle: A comparative study. Gene 2014, 536, 435–440. [Google Scholar] [CrossRef]
| Items | RGB (1) | Timothy Hay |
|---|---|---|
| Chemical composition | ||
| DM (%as fed) | 90.5 | 93.3 |
| aNDF (%DM) | 54.5 | 64.4 |
| ADF (%DM) | 47.4 | 42.6 |
| Lignin (%DM) | 14.7 | 6.60 |
| Ash (%DM) | 7.51 | 7.69 |
| EE (%DM) | 1.03 | 1.68 |
| CP (%DM) | 17.0 | 11.5 |
| TDN (%DM) | 44.3 | 54.8 |
| Items | CON | GINSENG |
|---|---|---|
| Ingredients (% DM) | ||
| Commercial concentrate mix | 43.5 | 43.5 |
| Corn flake | 26.7 | 26.7 |
| Timothy | 26.2 | 24.2 |
| Red ginseng byproduct | 0 | 2.0 |
| Molasses | 3.1 | 3.1 |
| Vitamin and mineral mix (1) | 0.5 | 0.5 |
| Chemical composition | ||
| DM (%as fed) | 65.0 | 65.0 |
| aNDF (%DM) | 33.1 | 32.7 |
| ADF (%DM) | 20.3 | 20.3 |
| Lignin (%DM) | 3.20 | 3.40 |
| Ash (%DM) | 6.12 | 6.14 |
| EE (%DM) | 4.66 | 4.66 |
| CP (%DM) | 12.3 | 12.5 |
| TDN (%DM) | 70.7 | 71.0 |
| NEm (Mcal/kg of DM) | 1.67 | 1.66 |
| Gene | Primer Sequences | Primer Condition [(initialization) → (Denaturation → Annealing → Elongation)] | Accession Number | Size (bp) | Reference | |
|---|---|---|---|---|---|---|
| IL-1B | F | AGTGCCTACGCACATGTCTTC | one cycle (95 °C, 3 min) → 40 cycles (95 °C, 30 s → 60 °C, 30 s → 72 °C, 30 s) | NM_174093.1 | 114 | [25] |
| R | TGCGTCACACAGAAACTC GTC | |||||
| IL-6 | F | CACCCCAGGCAGACTACTTC | one cycle (95 °C, 3 min) → 40 cycles (95 °C, 30 s → 64 °C, 30 s → 72 °C, 30 s) | NM_173923.2 | 215 | |
| R | AGCAAATCGCCTGATTGAAC | |||||
| IL-10 | F | AAGGTGAAGAGAGTCTTCAGTGAGC | one cycle (95 °C, 3 min) → 40 cycles (95 °C, 30 s → 63 °C, 30 s → 72 °C, 30 s) | NM_174088 | 208 | [26] |
| R | TGCATCTTCGTTGTCATGTAGG | |||||
| TNF-a | F | GCTCCAGAAGTTGCTTGTGC | one cycle (95 °C, 10 min) → 40 cycles (95 °C, 30 s → 60 °C, 30 s → 72 °C, 30 s) | NM_173966.3 | 149 | [27] |
| R | AACCAGAGGGCTGTTGATGG | |||||
| HSP 70 | F | TACGTGGCCTTCACCGATAC | one cycle (95 °C, 3 min) → 40 cycles (95 °C, 30 s → 64 °C, 30 s → 68 °C, 30 s) | U09861 | 171 | [28] |
| R | GTCGTTGATGACGCGGAAAG | |||||
| HSP 90 | F | GGAGGATCACTTGGCTGTCA | one cycle (95 °C, 3 min) → 40 cycles (95 °C, 10 s → 62 °C, 30 s → 72 °C, 30 s) | NM_001012670 | 177 | [28] |
| R | GGGATTAGCTCCTCGCAGTT | |||||
| β-actin (1) | F | AGCAAGCAGGAGTACGATGAGT | NM_173979.3 | 239 | [29] | |
| R | ATCCAACCGACTGCTGTCA | |||||
| β-actin (2) | F | CAGCAGATGTGGATCAGCAAGC | NM_173979.3 | 91 | [27] | |
| R | AAC GCA GCT AAC AGT CCG CC | |||||
| Items | CON | GINSENG | SEM | p-Value |
|---|---|---|---|---|
| Rumen parameters | ||||
| IVDMD (%) | 85.6 | 88.1 | 0.52 | 0.006 |
| IVNDFD (%aNDF) | 66.0 | 72.9 | 1.19 | 0.002 |
| IVCPD (%CP) | 91.3 | 96.8 | 0.50 | <0.001 |
| pH | 6.34 | 6.24 | 0.010 | 0.001 |
| TVFA (mM) | 81.2 | 87.5 | 4.34 | 0.227 |
| Acetate (mmol/mol) | 508.4 | 510.0 | 3.35 | 0.685 |
| Propionate (mmol/mol) | 302.3 | 302.2 | 2.33 | 0.678 |
| Butyrate (mmol/mol) | 138.3 | 135.9 | 1.42 | 0.256 |
| A:P ratio | 1.68 | 1.70 | 0.024 | 0.577 |
| NH3-N (mg/dL) | 35.2 | 37.6 | 0.68 | 0.043 |
| Gas (mL/g DM) | ||||
| 3 h | 26.7 | 26.9 | 0.91 | 0.876 |
| 6 h | 64.3 | 66.3 | 2.36 | 0.472 |
| 12 h | 124.6 | 125.5 | 4.13 | 0.878 |
| 24 h | 209.4 | 212.0 | 2.87 | 0.519 |
| 36 h | 254.2 | 259.0 | 2.68 | 0.208 |
| 48 h | 282.4 | 289.6 | 2.26 | 0.057 |
| Fitted parameters of gas (1) | ||||
| Kg | 0.038 | 0.038 | 0.0003 | 1.000 |
| Vmax | 341.7 | 353.8 | 7.14 | 0.210 |
| Items | CON | GINSENG | SEM | p-Value |
|---|---|---|---|---|
| Initial BW (kg) | 563.0 | 581.1 | 13.96 | 0.310 |
| Final BW (kg) | 582.6 | 599.3 | 14.74 | 0.379 |
| DMI (kg/d) | 7.82 | 7.80 | 0.326 | 0.961 |
| ADG (g/d) | 670.4 | 595.8 | 99.20 | 0.614 |
| FCR | 17.7 | 13.5 | 5.17 | 0.427 |
| Respiration rate (breaths/min) | 52.2 | 51.5 | 2.93 | 0.800 |
| Rectal temperature (°C) | 39.0 | 38.9 | 0.14 | 0.490 |
| Items | CON | GINSENG | SEM | p-Value |
|---|---|---|---|---|
| NH3-N (mg/dL) | 3.56 b | 6.14 a | 0.815 | 0.003 |
| TVFA (mM) | 65.7 | 66.1 | 3.03 | 0.884 |
| Acetate (mmol/mol) | 603.9 | 601.7 | 8.68 | 0.795 |
| Propionate (mmol/mol) | 217.2 | 216.3 | 6.39 | 0.893 |
| Butyrate (mmol/mol) | 141.3 | 144.8 | 6.16 | 0.574 |
| A:P ratio | 2.80 | 2.81 | 0.100 | 0.939 |
| pH | 6.71 | 6.78 | 0.080 | 0.401 |
| Items | CON | GINSENG | SEM | p-Value |
|---|---|---|---|---|
| TP (g/dL) | 6.20 | 6.54 | 0.303 | 0.261 |
| AST (U/L) | 52.4 b | 70.0 a | 4.72 | <0.001 |
| ALT (U/L) | 17.3 | 19.2 | 1.07 | 0.088 |
| BUN (mg/dL) | 9.94 | 11.28 | 0.480 | 0.013 |
| Ca (mg/dL) | 9.40 | 9.52 | 0.337 | 0.718 |
| IP (mg/dL) | 7.15 | 7.28 | 0.203 | 0.522 |
| Mg (mg/dL) | 2.25 | 2.32 | 0.056 | 0.241 |
| T-Chol (mg/dL) | 111.9 | 112.3 | 7.63 | 0.966 |
| TG (mg/dL) | 19.9 | 18.5 | 1.43 | 0.402 |
| Glu (mg/dL) | 69.4 | 67.7 | 2.19 | 0.454 |
| Alb (g/dL) | 3.23 | 3.24 | 0.118 | 0.924 |
| Crea (mg/dL) | 1.34 | 1.34 | 0.064 | 0.987 |
| Items | Maximum | Minimum |
|---|---|---|
| Temperature (°C) | 29.3 ± 3.35 | 20.6 ± 2.03 |
| Relative humidity (%) | 89.0 ± 8.48 | 55.5 ± 12.53 |
| THI (1) | 83.1 ± 4.96 | 66.4 ± 2.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Kim, H.; Moon, J.; Kim, J.; Kim, H.; Seo, J. Effects of Red Ginseng Byproducts on Rumen Fermentation, Growth Performance, Blood Metabolites, and mRNA Expression of Heat Shock Proteins in Heat-Stressed Fattening Hanwoo Steers. Vet. Sci. 2022, 9, 220. https://doi.org/10.3390/vetsci9050220
Yoo D, Kim H, Moon J, Kim J, Kim H, Seo J. Effects of Red Ginseng Byproducts on Rumen Fermentation, Growth Performance, Blood Metabolites, and mRNA Expression of Heat Shock Proteins in Heat-Stressed Fattening Hanwoo Steers. Veterinary Sciences. 2022; 9(5):220. https://doi.org/10.3390/vetsci9050220
Chicago/Turabian StyleYoo, Daekyum, Hanbeen Kim, Joonbeom Moon, Jongnam Kim, Hyeran Kim, and Jakyeom Seo. 2022. "Effects of Red Ginseng Byproducts on Rumen Fermentation, Growth Performance, Blood Metabolites, and mRNA Expression of Heat Shock Proteins in Heat-Stressed Fattening Hanwoo Steers" Veterinary Sciences 9, no. 5: 220. https://doi.org/10.3390/vetsci9050220
APA StyleYoo, D., Kim, H., Moon, J., Kim, J., Kim, H., & Seo, J. (2022). Effects of Red Ginseng Byproducts on Rumen Fermentation, Growth Performance, Blood Metabolites, and mRNA Expression of Heat Shock Proteins in Heat-Stressed Fattening Hanwoo Steers. Veterinary Sciences, 9(5), 220. https://doi.org/10.3390/vetsci9050220






