The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Examination Methods
2.3. Assessment of Examination Results
2.4. Statistical Analyses
3. Results
3.1. Proportional Frequency of Deficiencies
3.1.1. Dry Matter Content
3.1.2. Sensory Control
3.1.3. Microbiological Quality of Feed Types
3.2. Relationships between Parameters
3.2.1. Dry Matter Content and Microbiological Quality
3.2.2. Association of Sensory and Microbiological Deviations
3.2.3. Relations between Reported Disease Symptoms and Microbiological Deviations
4. Discussion
4.1. Sensory Quality
4.2. Microbiological Quality of Different Forage Types
4.3. Associations between Dry Matter Content and Microbiological Deviations
4.4. Association of Sensory and Microbiological Deviations
4.5. Association of Microbiological Deviations and Pre-Reported Disease Symptoms
4.5.1. Coughing
4.5.2. Gastrointestinal Disorder
4.5.3. Liver Enzyme Alteration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, P.A.; Ellis, M.; Fradinho, J.; Jansson, J.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal 2017, 11, 958–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.; Udén, P. Preference of horses for grass conserved as hay, haylage or silage. Anim. Feed Sci. Technol. 2007, 132, 66–78. [Google Scholar] [CrossRef]
- Müller, C.E. Silage and haylage for horses. Grass Forage Sci. 2018, 73, 815–827. [Google Scholar] [CrossRef]
- Thompson, F. Horses and Hay in Britain. In Horses in European Economic History: A Preliminary Canter; British Agricultural History Society: London, UK, 1983; pp. 1830–1918. [Google Scholar]
- Särkijärvi, S.; Saastamoinen, M. Silage Digestibility in Equine Diets. In Proceedings of the NFJ-Seminar, Production and Utilization of Silage, with Emphasis on New Techniques, Lillehammer, Norway, 27 September 2001; pp. 119–124. [Google Scholar]
- Holmquist, S.; Müller, C. Problems Related to Feeding Forages to Horses. In Proceedings of the XIIIth International Silage Conference, South Ayrshire Council, Auchincruive, Scotland, 11–13 September 2002; pp. 11–13. [Google Scholar]
- Besier, J.; Strickler, B.; Niederhäusern, R.V.; Wyss, U. Hay or haylage for horses: A comparison. Rech. Agron. Suisse 2013, 4, 264–271. [Google Scholar]
- Kamphues, J.; Wolf, P.; Coenen, M.; Eder, K.; Iben, C.; Kienzle, E.; Liesegang, A.; Männer, K.; Zebeli, Q.; Zentek, J. Beurteilung von Futtermitteln. In Supplemente zur Tierernährung für Studium und Praxis; M. & H. Schaper: Hannover, Germany, 2014; pp. 181–188. (In German) [Google Scholar]
- Jansson, A.; Harris, P.; Davey, S.L.; Luthersson, N.; Ragnarsson, S.; Ringmark, S. Straw as an Alternative to Grass Forage in Horses—Effects on Post-Prandial Metabolic Profile, Energy Intake, Behaviour and Gastric Ulceration. Animals 2021, 11, 2197. [Google Scholar] [CrossRef]
- Hansen, D.; Webb, G.W.; Webb, S.P. Digestibility of wheat straw or ammoniated wheat straw in equine diets. J. Equine Vet. Sci. 1992, 12, 223–226. [Google Scholar] [CrossRef]
- Kamphues, J. A systematic approach to evaluate the hygienic quality of feedstuffs for horses. Pferdeheilkunde 2005, 21, 15–18. [Google Scholar] [CrossRef]
- Kamphues, J. Feed Hygiene and Related Disorders in Horses. In Equine Applied and Clinical Nutrition; Geor, R.J., Harris, P.A., Coenen, M., Eds.; Saunders: Philadelphia, PA, USA, 2013; pp. 367–380. [Google Scholar]
- Stickdorn, T.; Ellis, A.; Kienzle, E. Horse Feed Hygiene Evaluation with Microbial and Sensory Examination. In Forages and Grazing in Horse Nutrition; Wageningin Academic Publishers: Wageningen, The Netherlands, 2012; pp. 255–262. [Google Scholar]
- Wolf, P.; Kloetzer, P.; Paulus, C.; Kamphues, J. A Survey on the Hygienic Standard of Feeds for Horses and Its Implication for Environmental Conditions and Animal Health. In Proceedings of the 14th International Congress of the International Society for Animal Hygiene (ISAH), Vechta, Germany, 19–23 July 2009; pp. 1085–1088. [Google Scholar]
- Kamphues, J. Futtermittelhygiene: Charakterisierung, Einflüsse und Bedeutung. Landbauforsch. Völkenrode Sonderh. 2007, 306, 41–55. [Google Scholar]
- EU. Regulation (EC) No 183/2005 of the European Parliament and of the Council Laying down Requirements for Feed Hy-giene. 2005. L35/31-L35/22. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32005R0183 (accessed on 12 January 2021).
- Meyer, H.; Coenen, M. Schadwirkungen durch Futtermittel. In Pferdefütterung; Meyer, H., Coenen, M., Eds.; Enke Verlag: Hannover, Germany, 2014. [Google Scholar]
- DeNotta, S.L.; Divers, T.J. Clinical pathology in the adult sick horse: The gastrointestinal system and liver. Vet. Clin. Equine Pract. 2020, 36, 105–120. [Google Scholar] [CrossRef]
- Divers, T.J. The Equine Liver in Health and Disease. In Proceedings of the AAEP Proceedings, Las Vegas, NV, USA, 5 December 2015; pp. 66–103. [Google Scholar]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.P. Heaves, an asthma-like disease of horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef]
- Bracher, V.; Von Fellenberg, R.; Winder, C.N.; Gruenig, G.; Hermann, M.; Kraehenmann, A. An investigation of the incidence of chronic obstructive pulmonary disease (COPD) in random populations of Swiss horses. Equine Vet. J. 1991, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Beeler-Marfisi, J. Induction and Mechanisms of Recurrent Airway Obstruction in Horses. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2010. [Google Scholar]
- Pirie, R.S.; Couëtil, L.L.; Robinson, N.E.; Lavoie, J.-P. Equine asthma: An appropriate, translational and comprehendible terminology? Equine Vet. J. 2016, 48, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A. Environmental monitoring in relation to equine respiratory disease. Curr. Ther. Equine Med. 1992, 3, 310–315. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Ward, M.P. Analysis of risk factors for recurrent airway obstruction in North American horses: 1444 cases (1990–1999). J. Am. Vet. Med. Assoc. 2003, 223, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Eder, C.; Crameri, R.; Mayer, C.; Eicher, R.; Straub, R.; Gerber, H.; Lazary, S.; Marti, E. Allergen-specific IgE levels against crude mould and storage mite extracts and recombinant mould allergens in sera from horses affected with chronic bronchitis. Vet. Immunol. Immunopathol. 2000, 73, 241–253. [Google Scholar] [CrossRef]
- Pirie, R.; Collie, D.; Dixon, P.; McGorum, B. Inhaled endotoxin and organic dust particulates have synergistic proinflammatory effects in equine heaves (organic dust-induced asthma). Clin. Exp. Allergy 2003, 33, 676–683. [Google Scholar] [CrossRef]
- Franchini, M.; Gill, U.; Fellenberg, R.v.; Bracher, V.D. Interleukin-8 concentration and neutrophil chemotactic activity in bronchoalveolar lavage fluid of horses with chronic obstructive pulmonary disease following exposure to hay. Am. J. Vet. Res. 2000, 61, 1369–1374. [Google Scholar] [CrossRef]
- Vandenput, S.; Duvivier, D.H.; Votion, D.; Art, T.; Lekeux, P. Environmental control to maintain stabled COPD horses in clinical remission: Effects on pulmonary function. Equine Vet. J. 1998, 30, 93–96. [Google Scholar] [CrossRef]
- Dixon, P.; Railton, D.; McGorum, B.; Tothill, S. Equine pulmonary disease: A case control study of 300 referred cases. Part 4: Treatments and re-examination findings. Equine Vet. J. 1995, 27, 436–439. [Google Scholar] [CrossRef]
- Leblond, A.; Villard, I.; Leblond, L.; Sabatier, P.; Sasco, A. A retrospective evaluation of the causes of death of 448 insured French horses in 1995. Vet. Res. Commun. 2000, 24, 85–102. [Google Scholar] [CrossRef]
- Tinker, M.K.; White, N.; Lessard, P.; Thatcher, C.; Pelzer, K.; Davis, B.; Carmel, D. Prospective study of equine colic risk factors. Equine Vet. J. 1997, 29, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Pilliner, S.; Davies, Z. Equine Science; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Feige, K.; Fürst, A.; Wehrli Eser, M. Auswirkungen von Haltung, Fütterung und Nutzung auf die Pferdegesundheit unter besonderer Berücksichtigung respiratorischer und gastrointestinaler Krankheiten. Schweiz. Arch. Für Tierheilkd. 2002, 144, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Gieselmann, A. Nutritive Anamnese bei Kolikfällen des Pferdes; University of Vetreinary Medicine Foundation: Hannover, Germany, 1994. [Google Scholar]
- White, A.; Lessard, P. Evaluation of the Horse Farm for a Problem with Colic. In Equine Acute Abdomen; Lea & Febiger: Philadelphia, PA, USA, 1990; pp. 148–151. [Google Scholar]
- Cohen, N.; Gibbs, P.; Woods, A. Dietary and Other Management Factors Associated with Equine Colic. In Proceedings of the AAEP, Albuquerque, NM, USA, 8 December 1999; pp. 96–98. [Google Scholar]
- Cohen, N.; Matejka, P.; Honnas, C.; Hooper, R. Case-control study of the association between various management factors and development of colic in horses. Texas Equine Colic Study Group. J. Am. Vet. Med. Assoc. 1995, 206, 667–673. [Google Scholar] [PubMed]
- Reeves, M. What really causes colic in horses? Epidemiology’s role in elucidating the ultimate multi-factorial disease. Equine Vet. J. 1997, 29, 413–414. [Google Scholar] [CrossRef]
- Hudson, J.M.; Cohen, N.D.; Gibbs, P.G.; Thompson, J.A. Feeding practices associated with colic in horses. J. Am. Vet. Med. Assoc. 2001, 219, 1419–1425. [Google Scholar] [CrossRef]
- Meyer, H. Krampfkolik beim Pferd–Vorstellungen zu einer alimentären Genese. Pferdeheilkunde 2001, 17, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Heckotter, E.; Merkt, M.; Bernoth, E.; Kienzle, E.; Kamphues, J. Current problems in veterinary advice on feeding. 6. Adverse effects of feeds in horses. Dtsch. Tierarztl. Wochenschr. 1986, 93, 486–490. [Google Scholar]
- Kaya, G.; Sommerfeld-Stur, I.; Iben, C. Risk factors of colic in horses in Austria. J. Anim. Physiol. Anim. Nutr. 2009, 93, 339–349. [Google Scholar] [CrossRef]
- Coenen, M. Fütterung und Kolik. Pferdeheilkunde 2013, 29, 176–182. [Google Scholar]
- Frape, D. Equine Nutrition and Feeding; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kamphues, J.; Böhm, K. Krampfkoliken bei Pferden nach Fütterung von verdorbenem Hafer. Dtsch. Tierärztl. Wschr. 1990, 97, 367–368. [Google Scholar]
- Anacker, G. Mikrobiologische Futterqualität−Ursache für Gesundheitsprobleme in Milchviehherden? Viehwirtsch. Fachtag. 2010, 40, 93–100. [Google Scholar]
- Kellerman, T.S.; Marasas, W.F.O.; Pienaar, J.; Naudé, T. A mycotoxicosis of equidae caused by Fusarium moniliforme sheldon. A preliminary communication. Onderstepoort J. Vet. Res. 1972, 39, 205–208. [Google Scholar] [PubMed]
- Visscher, C.; Mischok, J.; Sander, S.; Schmicke, M.; Peitzmeier, E.-U.; von dem Busche, I.; Rohn, K.; Kamphues, J. Nutrient digestibility, organ morphometry and performance in vaccinated or non-vaccinated Lawsonia intracellularis infected piglets. BMC Vet. Res. 2018, 14, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphues, J. Lipopolysaccharide in Futtermitteln–mögliche Bedeutung, Bestimmung und Gehalte. Übers Tierernährg 1986, 14, 131–156. [Google Scholar]
- VDLUFA. VDLUFA Methodenbuch, Band III—Die Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2017. [Google Scholar]
- Sander, S.; Verspohl, J.; Wolf, P. Organic rooting materials like straw in pig husbandry-a hazard for animal health? Prakt. Tierarzt 2016, 97, 140–146. [Google Scholar]
- Müller, C.; Hultén, C.; Gröndahl, G. Assessment of hygienic quality of haylage fed to healthy horses. Grass Forage Sci. 2011, 66, 453–463. [Google Scholar] [CrossRef]
- Roussel, S.; Reboux, G.; Dalphin, J.; Bardonnet, K.; Millon, L.; Piarroux, R. Microbiological evolution of hay and relapse in patients with farmer’s lung. Occup. Environ. Med. 2004, 61, e3-e3. [Google Scholar]
- O’brien, M.; O’kiely, P.; Forristal, P.; Fuller, H. Fungal contamination of big-bale grass silage on Irish farms: Predominant mould and yeast species and features of bales and silage. Grass Forage Sci. 2008, 63, 121–137. [Google Scholar] [CrossRef]
- O’Brien, M.; O’Kiely, P.; Forristal, P.D.; Fuller, H.T. Visible fungal growth on baled grass silage during the winter feeding season in Ireland and silage characteristics associated with the occurrence of fungi. Anim. Feed Sci. Technol. 2007, 139, 234–256. [Google Scholar] [CrossRef]
- Müller, C.E. Fermentation patterns of small-bale silage and haylage produced as a feed for horses. Grass Forage Sci. 2005, 60, 109–118. [Google Scholar] [CrossRef]
- McGorum, B.; Dixon, P.; Halliwell, R. Responses of horses affected with chronic obstructive pulmonary disease to inhalation challenges with mould antigens. Equine Vet. J. 1993, 25, 261–267. [Google Scholar] [CrossRef]
- Ward, M.P.; Couëtil, L.L. Climatic and aeroallergen risk factors for chronic obstructive pulmonary disease in horses. Am. J. Vet. Res. 2005, 66, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Pirie, R.; Dixon, P.; Collie, D.; McGorum, B. Pulmonary and systemic effects of inhaled endotoxin in control and heaves horses. Equine Vet. J. 2001, 33, 311–318. [Google Scholar] [CrossRef] [PubMed]
- CLARKE, A.F.; MADELIN, T. Technique for assessing respiratory health hazards from hay and other source materials. Equine Vet. J. 1987, 19, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Burrell, M.H. Endoscopic and virological observations on respiratory disease in a group of young Thoroughbred horses in training. Equine Vet. J. 1985, 17, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Anacker, G. Mikrobiologische Belastung von Hauptfutterkomponenten—Ursache für Gesundheitsprobleme in Milchviehherden; Thüringer Landesanstalt für Landwirtschaft: Jena, Germany, 2007. [Google Scholar]
- ADLER, A. Orientierungswerte für Keimzahlen in Heu. In Proceedings of the ALVA-Tagung St. Virgil, Salzburg, Austria, 18–19 May 2009; p. 193. [Google Scholar]
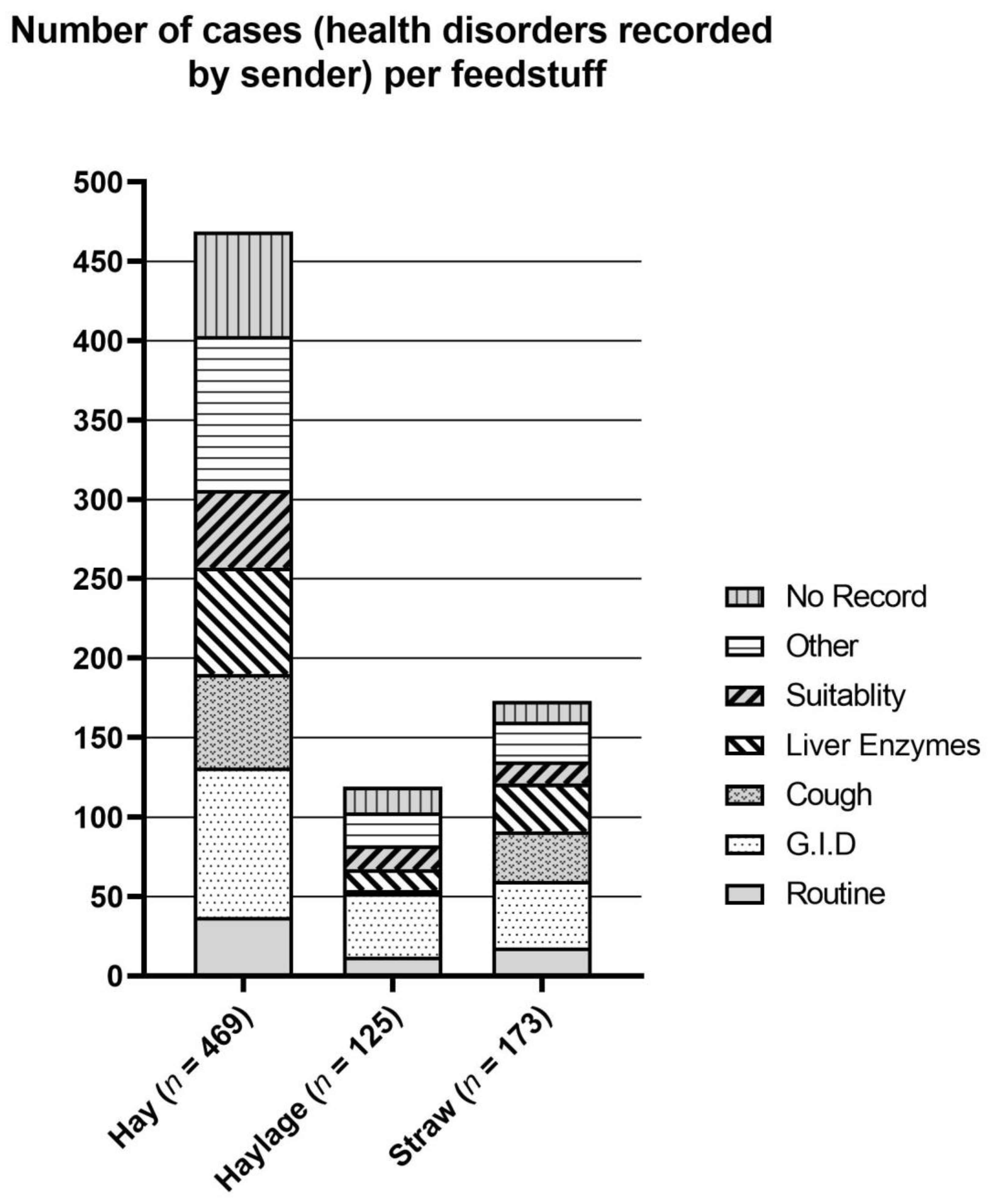
| Parameter | Hay and Straw | Silage | ||
|---|---|---|---|---|
| Findings | Points Deducted | Findings | Points Deducted | |
| Odor | Unremarkable | 0 | Unremarkable | 0 |
| Yeasty nuances | –2 | |||
| Distinct yeasty, alcoholic | –4 | |||
| Musty nuances | –5 | Slightly moldy, musty | –6 | |
| Moldy/putrid | –10 | Moldy, rotten, or fecal odor | –10 | |
| Texture | Dry | 0 | Slight to distinct heating | –2 to –4 |
| Sightly clammy | –2 | Slight to distinct loss of structure | –2 to –10 | |
| Clammy, moist | –5 | Above average contamination with sand/soil | –2 to –6 | |
| Color | Product—typical | 0 | Unremarkable | 0 |
| Hay: focally gray, whitish | –2 | Whitish, grayish, greenish, blackish color deviations/deposits | ||
| Hay: Diffusely discolored Straw: Dirty grayish/brownish/blackish | –5 | Scattered discoloration | –4 | |
| Straw: Whitish gray/red discoloration | –10 | Frequent discoloration | –10 | |
| Impurities (deposits, visible mold growth/infestation with storage mites) | None | 0 | Containing waste material, poisonous plants, plant parts altered by disease | –2 to –10 |
| Medium-grade | –5 | |||
| High-grade | –10 | |||
| Hygienic Status | Total Points Deducted according to Sensory Analysis | ||
|---|---|---|---|
| Hay | Straw | Silage | |
| Adequate | 0 | 0 | 0 |
| Minor deficiencies | –1 to –5 | –1 to –5 | –1 to –5 |
| Major deficiencies | –6 to –10 | –6 to –10 | –6 to –10 |
| Massive deficiencies | –11 to –40 | –11 to –30 | –11 to –46 |
| Type of Micro-Organism | Classification | Group No 1 | Exemplary Species | Orientation Value (cfu/g feed) | ||
|---|---|---|---|---|---|---|
| Hay | Silage | Straw | ||||
| Aerobic bacteria | “Epiphytic” (product-typical) bacteria | 1 | Pantoea agglomerans, Pseudomonas, Enterobacteriaceae | 30 × 106 | 100 × 106 | 0.2 × 106 |
| Spoilage bacteria | 2 | Bacillus, Staphylococcus, Micrococcus | 2 × 106 | 2 × 106 | 0.2 × 106 | |
| 3 | Streptomyces | 0.15 × 106 | 0.15 × 106 | 0.01 × 106 | ||
| Molds | “Epiphytic” (product-typical) molds | 4 | Aspergillus, Penicillium, Scopulariopsis | 200 × 103 | 200 × 103 | 5 × 103 |
| Spoilage molds | 5 | Mucorales | 100 × 103 | 100 × 103 | 5 × 103 | |
| Yeasts | 6 | all species | 5 × 103 | 5 × 103 | 5 × 103 | |
| Feed Type | n | Proportional Frequency (%) of Deviations | ||||||
|---|---|---|---|---|---|---|---|---|
| SC | DM | Aerobic Bacteria 1 | Molds 1 | Yeasts 1 | Micro-Biology (Total) 1 | LPS >50 µg 2 | ||
| Roughage | 767 | 49.5 | 38.0 | 23.5 | 25.2 | 10.1 | 36.9 | 60.0 |
| Hay | 469 | 44.2 | 36.9 | 12.4 | 25.5 | 4.6 | 29.7 | 52.2 |
| Haylage * | 125 | 44.4 | 62.2 | 55.9 | 27.5 | 28.6 | 60.8 | 40.0 |
| Haylage ** | 125 | 20.8 | 20.8 | 29.4 | 53.6 | |||
| Straw | 173 | 67.1 | 22.0 | 27.0 | 22.8 | 9.9 | 38.3 | 73.0 |
| Classification | Group No 1 | Exemplary Species | Detection Frequency (%) in Forage | ||
|---|---|---|---|---|---|
| Hay | Haylage | Straw | |||
| “Epiphytic” (product-typical) bacteria | 1 | Pantoea, Pseudomonas, Enterobacteriaceae | 54.7 | 54.5 | 63.7 |
| Spoilage bacteria | 2 | Bacillus, Staphylococcus, Micrococcus | 38.2 | 44.5 | 36.0 |
| 3 | Streptomyces | 7.1 | 1.0% | 0.3 | |
| Feedstuff | DM Content | % cfu > n.c. (According to VDLUFA 2017) | Aspergillus Cultivation (% positive) | ||
|---|---|---|---|---|---|
| Aerobic Bacteria | Molds | Yeasts | |||
| Hay | ≥86% (n = 149) | 9.8 | 26.7 | 1.9 | 43.2 |
| <86% (n = 255) | 15.2 | 24.3 | 9.3 | 40.3 | |
| Level of significance (p-value) | |||||
| ≥86% vs. <86% | 0.1393 | 0.6055 | 0.0018 | 0.8615 | |
| Haylage | <50% (n = 18) | 77.8 | 27.8 | 77.8 | 11.1 |
| 50–70% (n = 45) | 58.1 | 11.6 | 79.1 | 25.6 | |
| >70% (n = 56) | 50.0 | 39.6 | 58.5 | 47.2 | |
| Level of significance (p-value) | |||||
| 50–70% vs. <50% | 0.3425 | 0.1202 | 0.7131 | 0.2081 | |
| 50–70% vs. >70% | 0.8726 | 0.0021 | 0.1900 | 0.0298 | |
| < 50% vs. > 70% | 0.0393 | 0.3675 | 0.1422 | 0.0826 | |
| Straw | <86% (n = 255) | 24.3 | 19.1 | 9.4 | 44.8 |
| ≥86% (n = 149) | 41.4 | 24.1 | 16.0 | 30.9 | |
| Level of significance (p-value) | |||||
| ≥86% vs. <86% | 0.1151 | 0.5466 | 0.3407 | 0.0722 | |
| Deviations within the Sensory Control | Exceeding Counts of Aerobic Bacteria (% cfu > n.c.) 1 | Exceeding LPS Content (>50 µg/g) 2 | |||||
|---|---|---|---|---|---|---|---|
| Forage (n = 624) | Hay (n = 368) | Haylage (n = 118) | Straw (n = 138) | Forage (n = 145) | Hay (n = 86) | Straw (n = 49) | |
| Inadequate sensory score | 0.1204 | 0.0963 | 0.2464 | 0.0807 | 0.3324 | 0.3530 | 0.1757 |
| Texture | 0.0862 | 0.1463 | 0.0126 | 0.1560 | 0.0174 | 0.0794 | −0.0834 |
| Odor | 0.1699 | 0.1278 | 0.3341 | 0.1131 | 0.1809 | 0.1891 | 0.0909 |
| Color | 0.2315 | 0.1464 | 0.2167 | 0.1722 | 0.0281 | - | −0.0214 |
| Deposits | 0.1192 | 0.1012 | 0.0746 | −0.0073 | 0.1363 | 0.2091 | 0.0369 |
| Storage mites | −0.0829 | −0.0645 | 0.0268 | −0.0187 | 0.0144 | 0.0736 | −0.0208 |
| Odor + deposits | 0.1378 | 0.1113 | 0.1264 | 0.0564 | 0.1158 | 0.1108 | 0.0541 |
| Smell + texture | 0.0919 | 0.1467 | 0.0197 | 0.1849 | 0.0864 | 0.1131 | 0.0521 |
| Color + odor | 0.2073 | 0.1167 | 0.2112 | 0.1495 | 0.1694 | 0.1131 | 0.1390 |
| Color + deposits | 0.1430 | 0.0474 | 0.1157 | 0.0836 | 0.2444 | 0.1556 | 0.2732 |
| Deviations in Sensory Control | Mold Counts Exceeding Orientation Values (% cfu > n.c.) | Yeast Counts Exceeding Orientation Values (% cfu > n.c.) | ||||||
|---|---|---|---|---|---|---|---|---|
| Forage (n = 704) | Hay (n = 426) | Haylage (n = 119) | Straw (n = 159) | Forage (n = 639) | Hay (n = 381) | Haylage (n = 119) | Straw (n = 139) | |
| Inadequate sensory score | 0.2164 | 0.2097 | 0.4347 | 0.1167 | 0.0656 | 0.0364 | 0.1713 | 0.0625 |
| Texture | 0.1279 | 0.1588 | 0.0121 | 0.1296 | 0.0499 | 0.0661 | 0.0075 | 0.1387 |
| Odor | 0.2091 | 0.2329 | 0.3667 | 0.0310 | 0.0614 | 0.0589 | 0.0364 | 0.0716 |
| Color | 0.1789 | 0.1205 | 0.4018 | 0.1633 | 0.1381 | 0.0962 | 0.1752 | 0.0477 |
| Deposits | 0.1691 | 0.1295 | 0.4062 | 0.0997 | 0.1528 | 0.0589 | 0.2462 | 0.0491 |
| Storage mites | 0.0574 | 0.0686 | 0.0853 | –0.0068 | –0.0739 | –0.0428 | 0.0246 | –0.0560 |
| Odor + deposits | 0.1290 | 0.0897 | 0.3770 | 0.0477 | 0.0972 | 0.0673 | 0.1189 | 0.0099 |
| Smell + texture | 0.1597 | 0.1815 | 0.0749 | 0.1670 | 0.0773 | 0.1076 | 0.0156 | 0.1813 |
| Color + odor | 0.1276 | 0.0951 | 0.4159 | 0.0089 | 0.1484 | 0.1509 | 0.1711 | 0.0175 |
| Color + deposits | 0.1292 | 0.0761 | 0.3952 | 0.1999 | 0.1430 | 0.1050 | 0.1343 | 0.0899 |
| Influencing Variable | Level of Significance (Effect = Pre-Reported Coughing) | |||||
|---|---|---|---|---|---|---|
| Roughage | Hay | Straw | ||||
| n | p-Value | n | p-Value | n | p-Value | |
| Detection of storage mites * | 135 | 0.1255 | 87 | 0.1123 | 48 | 0.9546 |
| Molds ** | 135 | 0.8730 | 87 | 0.9009 | 48 | 0.5201 |
| Yeasts ** | 115 | 0.7159 | 71 | 0.9445 | 44 | 0.1314 |
| Aerobic bacteria ** | 115 | 0.8731 | 74 | 0.9827 | 41 | 0.3124 |
| LPS *** | 29 | 0.2273 | 16 | 0.0625 1 | 13 | 0.5571 1 |
| Cultivation of Aspergillus spp. | 135 | 0.0118 | 87 | 0.0216 | 48 | 0.9298 |
| Preliminary Report | n | Counts of Aspergillus spp. (log10 cfu g−1) | ||||
|---|---|---|---|---|---|---|
| Mean | s.d. | s.e. | Min | Max | ||
| Routine examination | 59 | 1.48 | 2.08 | 0.27 | 0.00 | 6.08 |
| Coughing | 89 | 2.25 | 2.17 | 0.23 | 0.00 | 6.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intemann, S.; Reckels, B.; Schubert, D.; Wolf, P.; Kamphues, J.; Visscher, C. The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports. Vet. Sci. 2022, 9, 226. https://doi.org/10.3390/vetsci9050226
Intemann S, Reckels B, Schubert D, Wolf P, Kamphues J, Visscher C. The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports. Veterinary Sciences. 2022; 9(5):226. https://doi.org/10.3390/vetsci9050226
Chicago/Turabian StyleIntemann, Sandra, Bernd Reckels, Dana Schubert, Petra Wolf, Josef Kamphues, and Christian Visscher. 2022. "The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports" Veterinary Sciences 9, no. 5: 226. https://doi.org/10.3390/vetsci9050226
APA StyleIntemann, S., Reckels, B., Schubert, D., Wolf, P., Kamphues, J., & Visscher, C. (2022). The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports. Veterinary Sciences, 9(5), 226. https://doi.org/10.3390/vetsci9050226






