Mammary Fibroadenoma in Cats: A Matter of Classification
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
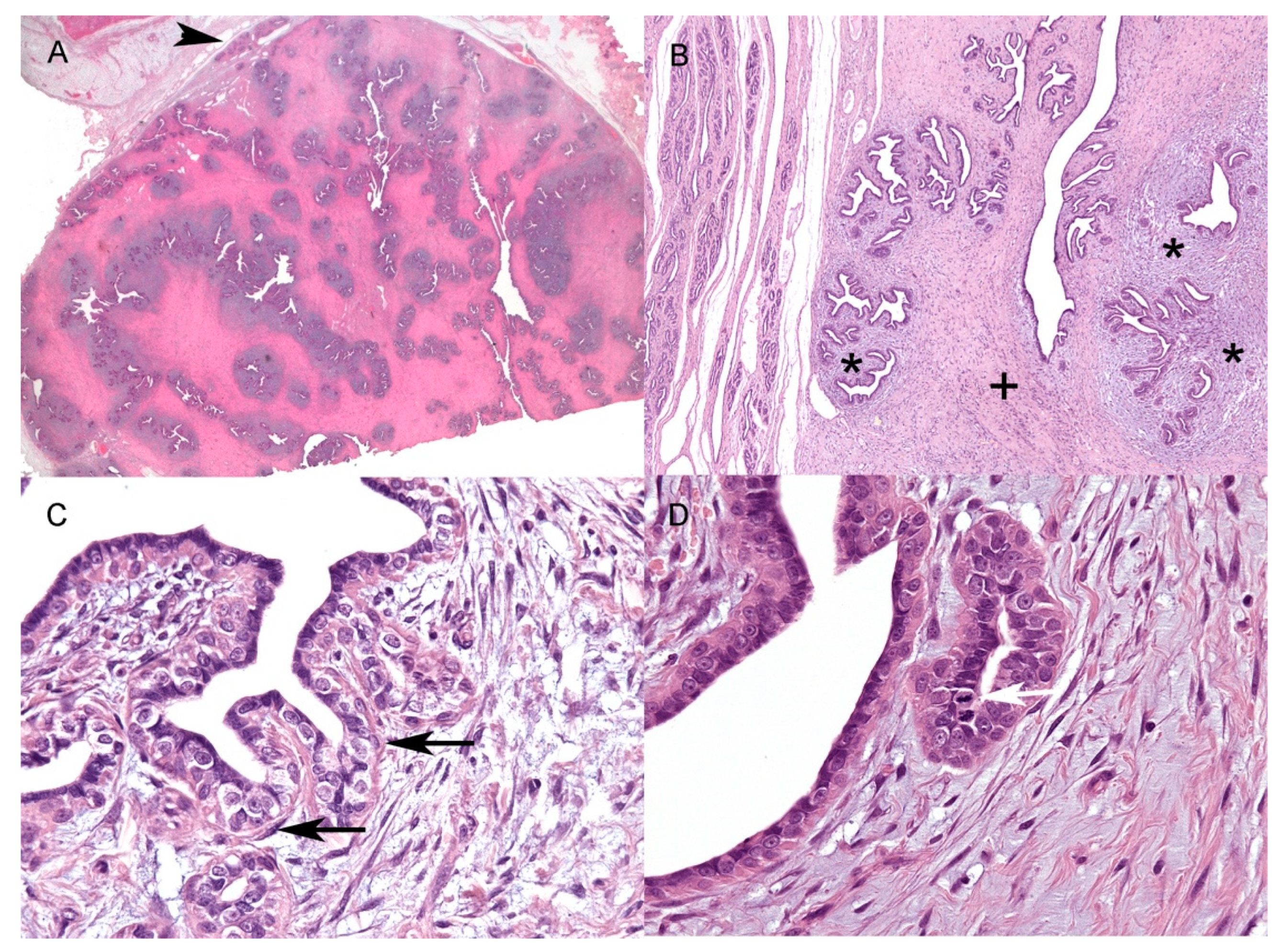
3.2. Histology
3.3. Immunohistochemistry
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vascellari, M.; Baioni, E.; Ru, G.; Carminato, A.; Mutinelli, F. Animal tumour registry of two provinces in northern Italy: Incidence of spontaneous tumours in dogs and cats. BMC Vet. Res. 2009, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappulli, V.; Rasotto, R.; Caliari, D.; Mainenti, M.; Peña, L.; Goldschmidt, M.H.; Kiupel, M. Prognostic Evaluation of Feline Mammary Carcinomas: A Review of the Literature. Vet. Pathol. 2015, 52, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Soremno, K.U.; Worley, D.R.; Zappulli, V. Tumors of the Mammary Gland. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 604–625. [Google Scholar]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Mammary tumors. In Surgical Pathology of Tumors in Domestic Animals; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Washington, DC, USA, 2019; Volume 2, pp. 86–89, 206–210. [Google Scholar]
- Payan-Carreira, R. Feline Mammary Fibroepithelial Hyperplasia: A Clinical Approach. In Insights from Veterinary Medicine; IntechOpen: London, UK, 2013. [Google Scholar]
- Mandel, M. Spontaneous remission of feline benign mammary hypertrophy. Vet. Med. Small Anim. Clin. 1975, 70, 846–847. [Google Scholar] [PubMed]
- Hayden, D.W.; Johnston, S.D.; Kiang, D.T.; Johnson, K.H.; Barnes, D.M. Feline mammary hypertrophy/fibroadenoma complex: Clinical and hormonal aspects. Am. J. Vet. Res. 1981, 42, 1699–1703. [Google Scholar]
- Hayden, D.W.; Barnes, D.M.; Johnson, K.H. Morphologic Changes in the Mammary Gland of Megestrol Acetate-treated and Untreated Cats: A Retrospective Study. Vet. Pathol. 1989, 26, 104–113. [Google Scholar] [CrossRef]
- MacDougall, L.D. Mammary fibroadenomatous hyperplasia in a young cat attributed to treatment with megestrol acetate. Can. Vet. J. 2003, 44, 227–229. [Google Scholar]
- Loretti, A.P.; Ilha, M.R.S.; Breitsameter, I.; Faraco, C.S. Clinical and pathological study of feline mammary fibroadenomatous change associated with depot medroxyprogesterone acetate therapy. Arq. Bras. Med. Vet. Zootec. 2004, 56, 270–274. [Google Scholar] [CrossRef]
- Mayayo, S.L.; Bo, S.; Pisu, M.C. Mammary fibroadenomatous hyperplasia in a male cat. J. Feline Med. Surg. Open Rep. 2018, 4, 2055116918760155. [Google Scholar] [CrossRef]
- De Las Mulas, M.J.; Millán, Y.; Bautista, M.J.; Pérez, J.; Carrasco, L. Oestrogen and progesterone receptors in feline fibroadenomatous change: An immunohistochemical study. Res. Vet. Sci. 2000, 68, 15–21. [Google Scholar] [CrossRef]
- Misdorp, E.; Else, R.W.; Hellmén, E.; Lipscomb, T.P. Histological Classification of Mammary Tumours of the Dog and Cat; Second Series; WHO International Histological Classification of Tumors of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 1999; Volume VII. [Google Scholar]
- Goldschmidt, M.; Peña, L.; Zappulli, V. Tumors of the mammary gland. In Tumors in Domestic Animals, 5th ed.; Meuten, D., Ed.; John Wiley and Sons: Ames, IA, USA, 2017; pp. 723–765. [Google Scholar]
- Meuten, D.J.; Moore, F.M.; George, J.W. Mitotic Count and the Field of View Area: Time to Standardize. Vet. Pathol. 2016, 53, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Caliari, D.; Zappulli, V.; Rasotto, R.; Cardazzo, B.; Frassineti, F.; Goldschmidt, M.H.; Castagnaro, M. Triple-negative vimentin-positive heterogeneous feline mammary carcinomas as a potential comparative model for breast cancer. BMC Vet. Res. 2014, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Muscatello, L.V.; Papa, V.; Millanta, F.; Sarli, G.; Bacci, B.; Cenacchi, G.; Poli, A.; Giudice, C.; Brunetti, B. Canine Mammary Carcinoma with Vacuolated Cytoplasm: Glycogen-Rich Carcinoma, a Histological Type Distinct from Lipid-Rich Carcinoma. Vet. Pathol. 2021, 58, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dias Pereira, P.; Carvalheira, J.; Gärtner, F. Cell proliferation in feline normal, hyperplastic and neoplastic mammary tissue—An immunohistochemical study. Vet. J. 2004, 168, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, L.; Panarese, S.; Brunetti, B.; Zambelli, D.; Benazzi, C.; Sarli, G. Quantitative analysis of telomerase in feline mammary tissues. J. Vet. Diagn. Investig. 2009, 21, 369–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasotto, R.; Goldschmidt, M.H.; Castagnaro, M.; Carnier, P.; Caliari, D.; Zappulli, V. The dog as a natural animal model for study of the mammary myoepithelial basal cell lineage and its role in mammary carcinogenesis. J. Comp. Pathol. 2014, 151, 166–180. [Google Scholar] [CrossRef]
- Łopuszyński, W.; Szczubiał, M.; Millán, Y.; Guil-Luna, S.; Sánchez-Céspedes, R.; Martin de las Mulas, J.; Śmiech, A.; Bulak, K. Immunohistochemical expression of p63 protein and calponin in canine mammary tumours. Res. Vet. Sci. 2019, 123, 232–238. [Google Scholar] [CrossRef]
- De Las Mulas, J.; Espinosa De Los Monteros, A.; Bautista, M.J.; Gómez-Villamandos, J.C.; Morales, C. Immunohistochemical distribution pattern of intermediate filament proteins and muscle actin in feline and human mammary carcinomas. J. Comp. Pathol. 1994, 111, 365–381. [Google Scholar] [CrossRef]
- Sarli, G.; Brunetti, B.; Benazzi, C. Mammary mucinous carcinoma in the cat. Vet. Pathol. 2006, 43, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Korsching, E.; Packeisen, J.; Liedtke, C.; Hungermann, D.; Wülfing, P.; van Diest, P.J.; Brandt, B.; Boecker, W.; Buerger, H. The origin of vimentin expression in invasive breast cancer: Epithelial-mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J. Pathol. 2005, 206, 451–457. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, N.; Tokunaga, E.; Kitao, H.; Hisamatsu, Y.; Taketani, K.; Akiyoshi, S.; Okada, S.; Aishima, S.; Morita, M.; Maehara, Y. Vimentin as a poor prognostic factor for triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Shiozaki, H.; Shibamoto, S.; Oka, H.; Kimura, Y.; Tamura, S.; Inoue, M.; Monden, T.; Ito, F.; Monden, M. β-Catenin expression in human cancers. Am. J. Pathol. 1996, 148, 39–46. [Google Scholar] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, E.J.; Hanby, A.M.; Rowan, A.J.; Gillett, C.E.; Thomas, R.E.; Poulsom, R.; Lakhani, S.R.; Ellis, I.O.; Ellis, P.; Tomlinson, I.P.M. The Wnt pathway, epithelial-stromal interactions, and malignant progression in phyllodes tumours. J. Pathol. 2002, 196, 437–444. [Google Scholar] [CrossRef]
- Sawyer, E.J.; Hanby, A.M.; Poulsom, R.; Jeffery, R.; Gillett, C.E.; Ellis, I.O.; Ellis, P.; Tomlinson, I.P.M. Β-Catenin Abnormalities and Associated Insulin-Like Growth Factor Overexpression Are Important in Phyllodes Tumours and Fibroadenomas of the Breast. J. Pathol. 2003, 200, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Triki, M.; Geyer, F.C.; Lambros, M.B.; Savage, K.; Ellis, I.O.; Lee, A.H.S.; Reis-Filho, J.S. β-Catenin/Wnt signalling pathway in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast. Mod. Pathol. 2010, 23, 1438–1448. [Google Scholar] [CrossRef]
- Thike, A.A.; Brogi, E.; Harada, O.; Oyama, T.; Tse, G. Fibroadenoma. In WHO Classification of Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; Breast Tumours; IARC Press: Lyon, France, 2019; pp. 82–101. [Google Scholar]
- Goodwin, A.M.; D’Amore, P.A. Wnt signaling in the vasculature. Angiogenesis 2002, 5, 1–9. [Google Scholar] [CrossRef]
- Reis, M.; Liebner, S. Wnt signaling in the vasculature. Exp. Cell Res. 2013, 319, 1317–1323. [Google Scholar] [CrossRef]
- Eberhart, C.G.; Tihan, T.; Burger, P.C. Nuclear localization and mutation of beta-catenin in medulloblastomas. J. Neuropathol. Exp. Neurol. 2000, 59, 333–337. [Google Scholar] [CrossRef]
- Yano, H.; Hara, A.; Shinoda, J.; Takenaka, K.; Yoshimi, N.; Mori, H.; Sakai, N. Immunohistochemical analysis of beta-catenin in N-ethyl-N-nitrosourea-induced rat gliomas: Implications in regulation of angiogenesis. Neurol. Res. 2000, 22, 527–532. [Google Scholar] [PubMed]
- Yano, H.; Hara, A.; Takenaka, K.; Nakatani, K.; Shinoda, J.; Shimokawa, K.; Yoshimi, N.; Mori, H.; Sakai, N. Differential expression of beta-catenin in human glioblastoma multiforme and normal brain tissue. Neurol. Res. 2000, 22, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, M.H.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- De Las Mulas, J.M.; van Niel, M.; Millán, Y.; Blankenstein, M.A.; van Mil, F.; Misdorp, W. Immunohistochemical analysis of estrogen receptors in feline mammary gland benign and malignant lesions: Comparison with biochemical assay. Domest. Anim. Endocrinol. 2000, 18, 111–125. [Google Scholar] [CrossRef]
- Millanta, F.; Calandrella, M.; Bari, G.; Niccolini, M.; Vannozzi, I.; Poli, A. Comparison of steroid receptor expression in normal, dysplastic, and neoplastic canine and feline mammary tissues. Res. Vet. Sci. 2005, 79, 225–232. [Google Scholar] [CrossRef]
- Millanta, F.; Calandrella, M.; Vannozzi, I.; Poli, A. Steroid hormone receptors in normal, dysplastic and neoplastic feline mammary tissues and their prognostic significance. Vet. Rec. 2006, 158, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.A.; Gracanin, A.; de Gier, J.; Rao, N.; Schaefers-Okkens, A.; Rutteman, G.; Kooistra, H. Molecular genetics and biology of progesterone signaling in mammary neoplasia. In Proceedings of the Joint Meeting of the 7th International Symposium on Canine and Feline Reproduction and the 15th Congress of the European Veterinary Society for Small Animal Reproduction, Whistler, BC, Canada, 26–29 July 2012; pp. 107–108. [Google Scholar]
- De las Mulas, J.M.; Van Niel, M.; Millan, Y.; Ordas, J.; Blankenstein, M.A.; van Mil, F.; Misdorp, W. Progesterone receptors in normal, dysplastic and tumourous feline mammary glands. Comparison with oestrogen receptors status. Res. Vet. Sci. 2002, 72, 153–161. [Google Scholar] [CrossRef]
- Soares, M.; Ribeiro, R.; Najmudin, S.; Gameiro, A.; Rodrigues, R.; Cardoso, F.; Ferreira, F. Serum HER2 levels are increased in cats with mammary carcinomas and predict tissue HER2 status. Oncotarget 2016, 7, 17314–17326. [Google Scholar] [CrossRef] [Green Version]
- Sammarco, A.; Finesso, G.; Zanetti, R.; Ferro, S.; Rasotto, R.; Caliari, D.; Goldschmidt, M.H.; Orvieto, E.; Castagnaro, M.; Cavicchioli, L.; et al. Biphasic Feline Mammary Carcinomas Including Carcinoma and Malignant Myoepithelioma. Vet. Pathol. 2020, 57, 377–387. [Google Scholar] [CrossRef]
- Morris, J.S.; Nixon, C.; Bruck, A.; Nasir, L.; Morgan, I.M.; Philbey, A.W. Immunohistochemical expression of TopBP1 in feline mammary neoplasia in relation to histological grade, Ki67, ERalpha and p53. Vet. J. 2008, 175, 218–226. [Google Scholar] [CrossRef]
- Zedda, M.T.; Bogliolo, L.; Antuofermo, E.; Falchi, L.; Ariu, F.; Burrai, G.P.; Pau, S. Hypoluteoidism in a dog associated with recurrent mammary fibroadenoma stimulated by progestin therapy. Acta Vet. Scand. 2017, 59, 4–9. [Google Scholar]
- Millanta, F.; Lazzeri, G.; Mazzei, M.; Vannozzi, I.; Poli, A. MIB-1 Labeling Index in Feline Dysplastic and Neoplastic Mammary Lesions and Its Relationship with Postsurgical Prognosis. Vet. Pathol. 2002, 39, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Antigen | Clone | Dilution | Host Species | Manufacturer |
|---|---|---|---|---|
| β-catenin | 14/Beta-Catenin | 1/100 | Mouse | BD Biosciences |
| CK5/6 | D5/I6 B4 | 1/50 | Mouse | Invitrogen |
| CK19 | BA 17 | 1/400 | Mouse | Histo-line Laboratories |
| PanCK | AE1/AE3 | 1/100 | Mouse | Dakocytomation |
| ER | ERa NCL-ER-6F11 | 1/40 | Mouse | Novocastra |
| Ki67 | MIB-1 | 1/50 | Mouse | Dakocytomation |
| p63 | 4A4 | 1/200 | Mouse | Santa Cruz Biotechnology |
| PR | PR88 | 1/80 | Mouse | Biogenex |
| PR | 1E2 | prediluted | Rabbit | Roche |
| PR | PR16 | 1/80 | Mouse | BioCare |
| Vimentin | V9 | 1/150 | Mouse | Dakocytomation |
| Case no. | Breed | Age (yy) | Sex | Size * | MC ep | MC st | KI67 ep | Ki67 st | CK19 (C) | p63 (N) | Calponin (C) | ER 6F11 (N) | Vimentin ** (C) | β-catenin (M & C) | PanCK (C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abyssinian | 10 | F | 2.5 × 2.2 | 3 | 0 | 14.5 | 8.3 | 100% ductal and tubular polygonal epithelial cells | 100% ductal and tubular basal elongated cells | <100% ductal and tubular basal elongated cells | 18.3% ductal and tubular polygonal epithelial cells | 30% ductal and tubular polygonal epithelial cells | 100% ductal and tubular cells | 100% ductal and tubular cells |
| 2 | DSH | 10 | FS | 2.7 × 2 | 0 | 0 | 3.1 | 2.3 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | neg | no epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
| 3 | Maine Coon | 3 | FS | 1.8 × 1.1 | 8 | 2 | 18.0 | 8.2 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | neg | no epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
| 4 | DSH | 5 | FS | 1.7 × 1.2 | 5 | 1 | 20.9 | 13.7 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | neg | no epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
| 5 | DSH | 5 | FS | 2 × 1.3 | 1 | 0 | 2.3 | 0.9 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | neg | NA | NA | NA |
| 6 | DSH | 1 | F | 2.8 × 1.9 | 3 | 1 | 34.2 | 14.6 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | neg | ductal and tubular polygonal epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
| 7 | Persian | 10 | F | 1.4 × 0.7 | 5 | 3 | 25.4 | 16.1 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | 17.6% ductal and tubular polygonal epithelial cells | 90% ductal and tubular polygonal epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
| 8 | DSH | 8 | F | 2 × 1 | 0 | 0 | 8,1 | 4.6 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | neg | 50% ductal and tubular polygonal epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
| 9 | DSH | 8 | M | 4.3 × 2.1 | 6 | 1 | 21.5 | 15.7 | 100% ductal and tubular polygonal epithelial cells | <100% ductal and tubular basal elongated cell | <100% ductal and tubular basal elongated AND stromal cells | 28.7% ductal and tubular polygonal epithelial cells | 80% ductal and tubular polygonal epithelial cells | 100% ductal and tubular cells AND stromal cells | 100% ductal and tubular cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrigiani, F.; Moccia, V.; Brunetti, B.; Millanta, F.; Valdivia, G.; Peña, L.; Cavicchioli, L.; Zappulli, V. Mammary Fibroadenoma in Cats: A Matter of Classification. Vet. Sci. 2022, 9, 253. https://doi.org/10.3390/vetsci9060253
Torrigiani F, Moccia V, Brunetti B, Millanta F, Valdivia G, Peña L, Cavicchioli L, Zappulli V. Mammary Fibroadenoma in Cats: A Matter of Classification. Veterinary Sciences. 2022; 9(6):253. https://doi.org/10.3390/vetsci9060253
Chicago/Turabian StyleTorrigiani, Filippo, Valentina Moccia, Barbara Brunetti, Francesca Millanta, Guillermo Valdivia, Laura Peña, Laura Cavicchioli, and Valentina Zappulli. 2022. "Mammary Fibroadenoma in Cats: A Matter of Classification" Veterinary Sciences 9, no. 6: 253. https://doi.org/10.3390/vetsci9060253
APA StyleTorrigiani, F., Moccia, V., Brunetti, B., Millanta, F., Valdivia, G., Peña, L., Cavicchioli, L., & Zappulli, V. (2022). Mammary Fibroadenoma in Cats: A Matter of Classification. Veterinary Sciences, 9(6), 253. https://doi.org/10.3390/vetsci9060253







