Evaluation of Tumor Grade and Proliferation Indices before and after Short-Course Anti-Inflammatory Prednisone Therapy in Canine Cutaneous Mast Cell Tumors: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Assessment of Histologic Parameters
2.4. Assessment of Proliferation Indices
2.5. Statistical Analyses
3. Results
3.1. Study Population and Tumor Details
3.2. Histological Parameters
3.3. Proliferation Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berlato, D.; Bulman-Fleming, J.; Clifford, C.A.; Garrett, L.; Intile, J.; Jones, P.; Kamstock, D.A.; Liptak, J.M.; Pavuk, A.; Powell, R.; et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet. Pathol. 2021, 58, 858–863. [Google Scholar] [CrossRef]
- Freytag, J.O.; Queiroz, M.R.; Govoni, V.M.; Pereira, I.V.A.; Pulz, L.H.; de Francisco Strefezzi, R.; Queiroga, F.L.; Cogliati, B. Prognostic value of immunohistochemical markers in canine cutaneous mast cell tumours: A systematic review and meta-analysis. Vet. Comp. Oncol. 2021, 19, 529–540. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Thamm, D.H. 21–Mast Cell Tumors. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; W.B. Saunders: St. Louis, MO, USA, 2019; pp. 382–403. [Google Scholar]
- De Nardi, A.B.; Dos Santos Horta, R.; Fonseca-Alves, C.E.; de Paiva, F.N.; Linhares, L.C.M.; Firmo, B.F.; Ruiz Sueiro, F.A.; de Oliveira, K.D.; Lourenço, S.V.; De Francisco Strefezzi, R.; et al. Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors. Cells 2022, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine cutaneous mast cell tumor: Morphologic grading and survival time in 83 dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet. Pathol. 2011, 48, 147–155. [Google Scholar] [CrossRef]
- Meuten, D.J.; Moore, F.M.; George, J.W. Mitotic Count and the Field of View Area:Time to Standardize. Vet. Pathol. 2016, 53, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Bertram, C.A.; Aubreville, M.; Gurtner, C.; Bartel, A.; Corner, S.M.; Dettwiler, M.; Kershaw, O.; Noland, E.L.; Schmidt, A.; Sledge, D.G.; et al. Computerized Calculation of Mitotic Count Distribution in Canine Cutaneous Mast Cell Tumor Sections: Mitotic Count Is Area Dependent. Vet. Pathol. 2020, 57, 214–226. [Google Scholar] [CrossRef]
- Reynolds, B.D.; Thomson, M.J.; O’Connell, K.; Morgan, E.J.; Gummow, B. Patient and tumour factors influencing canine mast cell tumour histological grade and mitotic index. Vet. Comp. Oncol. 2019, 17, 338–344. [Google Scholar] [CrossRef]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Miller, R.A.; Kaneene, J.B.; Kiupel, M. Cellular proliferation in canine cutaneous mast cell tumors: Associations with c-KIT and its role in prognostication. Vet. Pathol. 2007, 44, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- McStay, B. Nucleolar organizer regions: Genomic ‘dark matter’ requiring illumination. Genes Dev. 2016, 30, 1598–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, J.G. The human nucleolus organizer regions. Genes Dev. 2019, 33, 1617–1618. [Google Scholar] [CrossRef]
- McCaw, D.L.; Miller, M.A.; Ogilvie, G.K.; Withrow, S.J.; Brewer, W.G., Jr.; Klein, M.K.; Bell, F.W.; Anderson, S.K. Response of canine mast cell tumors to treatment with oral prednisone. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 1994, 8, 406–408. [Google Scholar] [CrossRef]
- Takahashi, T.; Kadosawa, T.; Nagase, M.; Mochizuki, M.; Matsunaga, S.; Nishimura, R.; Sasaki, N. Inhibitory effects of glucocorticoids on proliferation of canine mast cell tumor. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 1997, 59, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Thamm, D.H.; Mauldin, E.A.; Vail, D.M. Prednisone and vinblastine chemotherapy for canine mast cell tumor—41 cases (1992–1997). J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 1999, 13, 491–497. [Google Scholar] [CrossRef]
- Cahalane, A.K.; Payne, S.; Barber, L.G.; Duda, L.E.; Henry, C.J.; Mauldin, G.E.; Frimberger, A.E.; Cotter, S.M.; Moore, A.S. Prognostic factors for survival of dogs with inguinal and perineal mast cell tumors treated surgically with or without adjunctive treatment: 68 cases (1994–2002). J. Am. Vet. Med. Assoc. 2004, 225, 401–408. [Google Scholar] [CrossRef]
- Davies, D.R.; Wyatt, K.M.; Jardine, J.E.; Robertson, I.D.; Irwin, P.J. Vinblastine and prednisolone as adjunctive therapy for canine cutaneous mast cell tumors. J. Am. Anim. Hosp. Assoc. 2004, 40, 124–130. [Google Scholar] [CrossRef]
- Dobson, J.; Cohen, S.; Gould, S. Treatment of canine mast cell tumours with prednisolone and radiotherapy. Vet. Comp. Oncol. 2004, 2, 132–141. [Google Scholar] [CrossRef]
- Camps-Palau, M.A.; Leibman, N.F.; Elmslie, R.; Lana, S.E.; Plaza, S.; McKnight, J.A.; Risbon, R.; Bergman, P.J. Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997–2004). Vet. Comp. Oncol. 2007, 5, 156–167. [Google Scholar] [CrossRef]
- Hayes, A.; Adams, V.; Smith, K.; Maglennon, G.; Murphy, S. Vinblastine and prednisolone chemotherapy for surgically excised grade III canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2007, 5, 168–176. [Google Scholar] [CrossRef]
- Stanclift, R.M.; Gilson, S.D. Evaluation of neoadjuvant prednisone administration and surgical excision in treatment of cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 53–62. [Google Scholar] [CrossRef]
- Vickery, K.R.; Wilson, H.; Vail, D.M.; Thamm, D.H. Dose-escalating vinblastine for the treatment of canine mast cell tumour. Vet. Comp. Oncol. 2008, 6, 111–119. [Google Scholar] [CrossRef]
- Cooper, M.; Tsai, X.; Bennett, P. Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Vet. Comp. Oncol. 2009, 7, 196–206. [Google Scholar] [CrossRef]
- Hosoya, K.; Kisseberth, W.C.; Alvarez, F.J.; Lara-Garcia, A.; Beamer, G.; Stromberg, P.C.; Couto, C.G. Adjuvant CCNU (lomustine) and prednisone chemotherapy for dogs with incompletely excised grade 2 mast cell tumors. J. Am. Anim. Hosp. Assoc. 2009, 45, 14–18. [Google Scholar] [CrossRef]
- Taylor, F.; Gear, R.; Hoather, T.; Dobson, J. Chlorambucil and prednisolone chemotherapy for dogs with inoperable mast cell tumours: 21 cases. J. Small Anim. Pract. 2009, 50, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.A.; Legendre, A.M.; Shaw, N.G.; Phillips, B.; Ogilvie, G.K.; Prescott, D.M.; Atwater, S.W.; Carreras, J.K.; Lana, S.E.; Ladue, T.; et al. Evaluation of 12- and 24-month survival rates after treatment with masitinib in dogs with nonresectable mast cell tumors. Am. J. Vet. Res. 2010, 71, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Rassnick, K.M.; Bailey, D.B.; Russell, D.S.; Flory, A.B.; Kiselow, M.A.; Intile, J.L.; Malone, E.K.; Balkman, C.E.; Barnard, S.M. A phase II study to evaluate the toxicity and efficacy of alternating CCNU and high-dose vinblastine and prednisone (CVP) for treatment of dogs with high-grade, metastatic or nonresectable mast cell tumours. Vet. Comp. Oncol. 2010, 8, 138–152. [Google Scholar] [CrossRef]
- Carlsten, K.S.; London, C.A.; Haney, S.; Burnett, R.; Avery, A.C.; Thamm, D.H. Multicenter prospective trial of hypofractionated radiation treatment, toceranib, and prednisone for measurable canine mast cell tumors. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2012, 26, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.H.; Venable, R.O.; Vail, D.M.; Williams, L.E.; Clifford, C.A.; Axiak-Bechtel, S.M.; Avery, A.C.; Thamm, D.H. Pulse-Administered Toceranib Phosphate Plus Lomustine for Treatment of Unresectable Mast Cell Tumors in Dogs. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2015, 29, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Smrkovski, O.A.; Essick, L.; Rohrbach, B.W.; Legendre, A.M. Masitinib mesylate for metastatic and non-resectable canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2015, 13, 314–321. [Google Scholar] [CrossRef]
- Serra Varela, J.C.; Pecceu, E.; Handel, I.; Lawrence, J. Tolerability of a rapid-escalation vinblastine-prednisolone protocol in dogs with mast cell tumours. Vet. Med. Sci. 2016, 2, 266–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavcar, S.; de Vos, J.; Kessler, M.; de Fornel, P.; Buracco, P.; Murphy, S.; Hirschberger, J.; Argyle, D.J. Combination toceranib and lomustine shows frequent high grade toxicities when used for treatment of non-resectable or recurrent mast cell tumours in dogs: A European multicentre study. Vet. J. 2017, 224, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Case, A.; Burgess, K. Safety and efficacy of intralesional triamcinolone administration for treatment of mast cell tumors in dogs: 23 cases (2005–2011). J. Am. Vet. Med. Assoc. 2018, 252, 84–91. [Google Scholar] [CrossRef]
- Hay, J.K.; Larson, V.S. Lomustine (CCNU) and prednisone chemotherapy for high-grade completely excised canine mast cell tumors. Can. Vet. J. La Rev. Vet. Can. 2019, 60, 1326–1330. [Google Scholar]
- Mendez, S.E.; Drobatz, K.J.; Duda, L.E.; White, P.; Kubicek, L.; Sorenmo, K.U. Treating the locoregional lymph nodes with radiation and/or surgery significantly improves outcome in dogs with high-grade mast cell tumours. Vet. Comp. Oncol. 2010, 18, 239–246. [Google Scholar] [CrossRef]
- Todd, J.E.; Nguyen, S.M.; White, J.; Langova, V.; Thomas, P.M.; Tzannes, S. Combination vinblastine and palladia for high-grade and metastatic mast cell tumors in dogs. Can. Vet. J. La Rev. Vet. Can. 2021, 62, 1335–1340. [Google Scholar]
- Marconato, L.; Stefanello, D.; Kiupel, M.; Finotello, R.; Polton, G.; Massari, F.; Ferrari, R.; Agnoli, C.; Capitani, O.; Giudice, C.; et al. Adjuvant medical therapy provides no therapeutic benefit in the treatment of dogs with low-grade mast cell tumours and early nodal metastasis undergoing surgery. Vet. Comp. Oncol. 2020, 18, 409–415. [Google Scholar] [CrossRef]
- Horta, R.S.; Lavalle, G.E.; Monteiro, L.N.; Souza, M.C.C.; Cassali, G.D.; Araújo, R.B. Assessment of Canine Mast Cell Tumor Mortality Risk Based on Clinical, Histologic, Immunohistochemical, and Molecular Features. Vet. Pathol. 2018, 55, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Linde, K.J.; Stockdale, S.L.; Mison, M.B.; Perry, J.A. The effect of prednisone on histologic and gross characteristics in canine mast cell tumors. Can. Vet. J. La Rev. Vet. Can. 2021, 62, 45–50. [Google Scholar]
- Cockburn, E.; Janovec, J.; Solano, M.A.; L’Eplattenier, H. Marginal excision of cutaneous mast cell tumors in dogs was not associated with a higher rate of complications or prolonged wound healing than marginal excision of soft tissue sarcomas. J. Am. Vet. Med. Assoc. 2022, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.M.; Knapp, D.W.; DeNicola, D.B.; Glickman, N.; Bonney, P. Prognosis following surgical excision of canine cutaneous mast cell tumors with histopathologically tumor-free versus nontumor-free margins: A retrospective study of 31 cases. J. Am. Anim. Hosp. Assoc. 2002, 38, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Weisse, C.; Shofer, F.S.; Sorenmo, K. Recurrence rates and sites for grade II canine cutaneous mast cell tumors following complete surgical excision. J. Am. Anim. Hosp. Assoc. 2002, 38, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Gieger, T.L.; Theon, A.P.; Werner, J.A.; McEntee, M.C.; Rassnick, K.M.; DeCock, H.E. Biologic behavior and prognostic factors for mast cell tumors of the canine muzzle: 24 cases (1990–2001). J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2003, 17, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.A.; King, G.K.; Carreras, J.K. Efficacy of radiation therapy for incompletely resected grade-III mast cell tumors in dogs: 31 cases (1987–1998). J. Am. Vet. Med. Assoc. 2004, 224, 79–82. [Google Scholar] [CrossRef]
- Sfiligoi, G.; Rassnick, K.M.; Scarlett, J.M.; Northrup, N.C.; Gieger, T.L. Outcome of dogs with mast cell tumors in the inguinal or perineal region versus other cutaneous locations: 124 cases (1990–2001). J. Am. Vet. Med. Assoc. 2005, 226, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.N.; Dernell, W.S.; Withrow, S.J.; Ehrhart, E.J.; Thamm, D.H.; Lana, S.E. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004). J. Am. Vet. Med. Assoc. 2006, 228, 91–95. [Google Scholar] [CrossRef]
- Hillman, L.A.; Garrett, L.D.; de Lorimier, L.P.; Charney, S.C.; Borst, L.B.; Fan, T.M. Biological behavior of oral and perioral mast cell tumors in dogs: 44 cases (1996–2006). J. Am. Vet. Med. Assoc. 2010, 237, 936–942. [Google Scholar] [CrossRef]
- Elliott, J.W.; Cripps, P.; Blackwood, L.; Berlato, D.; Murphy, S.; Grant, I.A. Canine oral mucosal mast cell tumours. Vet. Comp. Oncol. 2016, 14, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Schwab, T.M.; Popovitch, C.; DeBiasio, J.; Goldschmidt, M. Clinical outcome for MCTs of canine pinnae treated with surgical excision (2004–2008). J. Am. Anim. Hosp. Assoc. 2014, 50, 187–191. [Google Scholar] [CrossRef]
- Trappler, M.C.; Popovitch, C.A.; Goldschmidt, M.H.; Goldschmidt, K.H.; Risbon, R.E. Scrotal tumors in dogs: A retrospective study of 676 cases (1986–2010). Can. Vet. J. La Rev. Vet. Can. 2014, 55, 1229–1233. [Google Scholar]
- Lejeune, A.; Skorupski, K.; Frazier, S.; Vanhaezebrouck, I.; Rebhun, R.B.; Reilly, C.M.; Rodriguez, C.O., Jr. Aggressive local therapy combined with systemic chemotherapy provides long-term control in grade II stage 2 canine mast cell tumour: 21 cases (1999-2012). Vet. Comp. Oncol. 2015, 13, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.L.; Van Lelyveld, S.; Warland, J.; Dobson, J.M.; Foale, R.D. A retrospective review of treatment and response of high-risk mast cell tumours in dogs. Vet. Comp. Oncol. 2016, 14, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Milovancev, M.; Townsend, K.L.; Tuohy, J.L.; Gorman, E.; Bracha, S.; Curran, K.M.; Russell, D.S. Long-term outcomes of dogs undergoing surgical resection of mast cell tumors and soft tissue sarcomas: A prospective 2-year-long study. Vet. Surg. VS 2020, 49, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, S.; Kiupel, M.; Finotello, R.; Stefanello, D.; Faroni, E.; Bertazzolo, W.; Bonfanti, U.; Rigillo, A.; Del Magno, S.; Foglia, A.; et al. A retrospective study on prophylactic regional lymphadenectomy versus nodal observation only in the management of dogs with stage I, completely resected, low-grade cutaneous mast cell tumors. BMC Vet. Res. 2021, 17, 331. [Google Scholar] [CrossRef]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Distelhorst, C.W. Recent insights into the mechanisms of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002, 9, 17. [Google Scholar] [CrossRef]
- Matsuda, A. Long-term in-vitro glucocorticoid treatment induces glucocorticoid resistance in canine mast cell tumors. Can. J. Vet. Res. = Rev. Can. De Rech. Vet. 2021, 85, 302–308. [Google Scholar]
- Hume, C.T.; Kiupel, M.; Rigatti, L.; Shofer, F.S.; Skorupski, K.A.; Sorenmo, K.U. Outcomes of dogs with grade 3 mast cell tumors: 43 cases (1997–2007). J. Am. Anim. Hosp. Assoc. 2011, 47, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Kiupel, M.; Farrelly, J.; Cohen, R.; Olmsted, G.; Kirpensteijn, J.; Brocks, B.; Post, G. Recurrence rates and clinical outcome for dogs with grade II mast cell tumours with a low AgNOR count and Ki67 index treated with surgery alone. Vet. Comp. Oncol. 2017, 15, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincenti, S.; Findji, F. Influence of treatment on the outcome of dogs with incompletely excised grade-2 mast cell tumors. Schweiz. Arch. Fur Tierheilkd. 2017, 159, 171–177. [Google Scholar] [CrossRef]
- Olsen, J.A.; Thomson, M.; O’Connell, K.; Wyatt, K. Combination vinblastine, prednisolone and toceranib phosphate for treatment of grade II and III mast cell tumours in dogs. Vet. Med. Sci. 2018, 4, 237–251. [Google Scholar] [CrossRef]
- Kiupel, M.; Camus, M. Diagnosis and Prognosis of Canine Cutaneous Mast Cell Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 819–836. [Google Scholar] [CrossRef]
- Moore, A.S.; Frimberger, A.E.; Taylor, D.; Sullivan, N. Retrospective outcome evaluation for dogs with surgically excised, solitary Kiupel high-grade, cutaneous mast cell tumours. Vet. Comp. Oncol. 2020, 18, 402–408. [Google Scholar] [CrossRef]
- Karbe, G.T.; Davis, E.; Runge, J.J.; Brown, D.C.; Holt, D.E. Evaluation of scar revision after inadequate primary excision of cutaneous mast cell tumors in 85 dogs (2000–2013). Vet. Surg. VS 2021, 50, 807–815. [Google Scholar] [CrossRef]
- Mason, S.L.; Pittaway, C.; Gil, B.P.; Russak, O.M.; Westlake, K.; Berlato, D.; Benoit, J.; Morris, J.; Dobson, J.M. Outcomes of adjunctive radiation therapy for the treatment of mast cell tumors in dogs and assessment of toxicity: A multicenter observational study of 300 dogs. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2021, 35, 2853–2864. [Google Scholar] [CrossRef]
- Guerra, D.; Faroni, E.; Sabattini, S.; Agnoli, C.; Chalfon, C.; Stefanello, D.; Del Magno, S.; Cola, V.; Grieco, V.; Marconato, L. Histologic grade has a higher-weighted value than nodal status as predictor of outcome in dogs with cutaneous mast cell tumors and overtly metastatic sentinel lymph nodes. Vet. Comp. Oncol. 2022. [Google Scholar] [CrossRef]
- Teng, S.P.; Hsu, W.L.; Chiu, C.Y.; Wong, M.L.; Chang, S.C. Overexpression of P-glycoprotein, STAT3, phospho-STAT3 and KIT in spontaneous canine cutaneous mast cell tumours before and after prednisolone treatment. Vet. J. 2012, 193, 551–556. [Google Scholar] [CrossRef]
- Veterinary Co-operative Oncology Group. Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet. Comp. Oncol. 2004, 2, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Pratschke, K.M.; Atherton, M.J.; Sillito, J.A.; Lamm, C.G. Evaluation of a modified proportional margins approach for surgical resection of mast cell tumors in dogs: 40 cases (2008–2012). J. Am. Vet. Med. Assoc. 2013, 243, 1436–1441. [Google Scholar] [CrossRef]
- Chu, M.L.; Hayes, G.M.; Henry, J.G.; Oblak, M.L. Comparison of lateral surgical margins of up to two centimeters with margins of three centimeters for achieving tumor-free histologic margins following excision of grade I or II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2020, 256, 567–572. [Google Scholar] [CrossRef]
- Selmic, L.E.; Ruple, A. A systematic review of surgical margins utilized for removal of cutaneous mast cell tumors in dogs. BMC Vet. Res. 2020, 16, 5. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Kojimoto, A.; Uchida, K.; Chambers, J.; Shii, H. Long-term postsurgical outcomes of mast cell tumors resected with a margin proportional to the tumor diameter in 23 dogs. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 2021, 83, 230–233. [Google Scholar] [CrossRef]
- Saunders, H.; Thomson, M.J.; O’Connell, K.; Bridges, J.P.; Chau, L. Evaluation of a modified proportional margin approach for complete surgical excision of canine cutaneous mast cell tumours and its association with clinical outcome. Vet. Comp. Oncol. 2021, 19, 604–615. [Google Scholar] [CrossRef]
- Shaw, T.; Kudnig, S.T.; Firestone, S.M. Diagnostic accuracy of pre-treatment biopsy for grading cutaneous mast cell tumours in dogs. Vet. Comp. Oncol. 2018, 16, 6. [Google Scholar] [CrossRef]
- Romansik, E.M.; Reilly, C.M.; Kass, P.H.; Moore, P.F.; London, C.A. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet. Pathol. 2007, 44, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, L.B.; Sueiro, F.A.; Cavalcanti, J.N.; Metze, K. The importance of the mitotic index as a prognostic factor for survival of canine cutaneous mast cell tumors: A validation study. Vet. Pathol. 2009, 46, 362–364, author reply 364–365. [Google Scholar] [CrossRef]
- van Lelyveld, S.; Warland, J.; Miller, R.; Maw, H.; Foale, R.; Goodfellow, M.; Dobson, J. Comparison between Ki-67 index and mitotic index for predicting outcome in canine mast cell tumours. J. Small Anim. Pract. 2015, 56, 312–319. [Google Scholar] [CrossRef]
- Vascellari, M.; Giantin, M.; Capello, K.; Carminato, A.; Morello, E.M.; Vercelli, A.; Granato, A.; Buracco, P.; Dacasto, M.; Mutinelli, F. Expression of Ki67, BCL-2, and COX-2 in canine cutaneous mast cell tumors: Association with grading and prognosis. Vet. Pathol. 2013, 50, 110–121. [Google Scholar] [CrossRef]
- Sabattini, S.; Scarpa, F.; Berlato, D.; Bettini, G. Histologic grading of canine mast cell tumor: Is 2 better than 3? Vet. Pathol. 2015, 52, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Northrup, N.C.; Howerth, E.W.; Harmon, B.G.; Brown, C.A.; Carmicheal, K.P.; Garcia, A.P.; Latimer, K.S.; Munday, J.S.; Rakich, P.M.; Richey, L.J.; et al. Variation among Pathologists in the Histologic Grading of Canine Cutaneous Mast Cell Tumors with Uniform Use of a Single Grading Reference. J. Vet. Diagn. Investig. 2005, 17, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Scase, T.J.; Edwards, D.; Miller, J.; Henley, W.; Smith, K.; Blunden, A.; Murphy, S. Canine mast cell tumors: Correlation of apoptosis and proliferation markers with prognosis. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2006, 20, 151–158. [Google Scholar] [CrossRef]
- Seguin, B.; Besancon, M.F.; McCallan, J.L.; Dewe, L.L.; Tenwolde, M.C.; Wong, E.K.; Kent, M.S. Recurrence rate, clinical outcome, and cellular proliferation indices as prognostic indicators after incomplete surgical excision of cutaneous grade II mast cell tumors: 28 dogs (1994–2002). J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2006, 20, 933–940. [Google Scholar]
- Maglennon, G.A.; Murphy, S.; Adams, V.; Miller, J.; Smith, K.; Blunden, A.; Scase, T.J. Association of Ki67 index with prognosis for intermediate-grade canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2008, 6, 268–274. [Google Scholar] [CrossRef]
- Krick, E.L.; Kiupel, M.; Durham, A.C.; Thaiwong, T.; Brown, D.C.; Sorenmo, K.U. Investigating Associations Between Proliferation Indices, C-kit, and Lymph Node Stage in Canine Mast Cell Tumors. J. Am. Anim. Hosp. Assoc. 2017, 53, 258–264. [Google Scholar] [CrossRef]
- Marouda, C.; Anagnostou, T.; Savvas, I.; Papazoglou, L.G.; Psalla, D. Τhe Effect of Opioid Administration on Cytologic and Histopathologic Diagnosis of Canine Cutaneous Mast Cell Tumors Treated by Surgical Excision. Vet. Sci. 2022, 9, 202. [Google Scholar] [CrossRef]
- Murphy, S.; Sparkes, A.H.; Smith, K.C.; Blunden, A.S.; Brearley, M.J. Relationships between the histological grade of cutaneous mast cell tumours in dogs, their survival and the efficacy of surgical resection. Vet. Rec. 2004, 154, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, R.P.; Ludwig, L.L.; Bergman, P.J.; Newman, S.J.; Simpson, A.M.; Patnaik, A.K. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2006, 228, 210–215. [Google Scholar] [CrossRef]
- Ozaki, K.; Yamagami, T.; Nomura, K.; Narama, I. Prognostic significance of surgical margin, Ki-67 and cyclin D1 protein expression in grade II canine cutaneous mast cell tumor. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 2007, 69, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, B.; Boston, S.; Monteith, G. Factors influencing complete tumor excision of mast cell tumors and soft tissue sarcomas: A retrospective study in 100 dogs. Can. Vet. J. La Rev. Vet. Can. 2011, 52, 1209–1214. [Google Scholar]
- Kry, K.L.; Boston, S.E. Additional local therapy with primary re-excision or radiation therapy improves survival and local control after incomplete or close surgical excision of mast cell tumors in dogs. Vet. Surg. 2014, 43, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.; Mullin, C.; Balko, J.; Goldschmidt, M.; Krick, E.; Hume, C.; Brown, D.C.; Sorenmo, K. Evaluation of histological grade and histologically tumour-free margins as predictors of local recurrence in completely excised canine mast cell tumours. Vet. Comp. Oncol. 2015, 13, 70–76. [Google Scholar] [CrossRef]
- Risselada, M.; Mathews, K.G.; Griffith, E. Surgically planned versus histologically measured lateral tumor margins for resection of cutaneous and subcutaneous mast cell tumors in dogs: 46 cases (2010–2013). J. Am. Vet. Med. Assoc. 2015, 247, 184–189. [Google Scholar] [CrossRef]
- Lowe, R.; Gavazza, A.; Impellizeri, J.A.; Soden, D.M.; Lubas, G. The treatment of canine mast cell tumours with electrochemotherapy with or without surgical excision. Vet. Comp. Oncol. 2017, 15, 775–784. [Google Scholar] [CrossRef]
- Robinson, W.P.; Elliott, J.; Baines, S.J.; Owen, L.; Shales, C.J. Intramuscular mast cell tumors in 7 dogs. Can. Vet. J. La Rev. Vet. Can. 2017, 58, 931–935. [Google Scholar]
- Dores, C.B.; Milovancev, M.; Russell, D.S. Comparison of histologic margin status in low-grade cutaneous and subcutaneous canine mast cell tumours examined by radial and tangential sections. Vet. Comp. Oncol. 2018, 16, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Marconato, L.; Buracco, P.; Boracchi, P.; Giudice, C.; Iussich, S.; Grieco, V.; Chiti, L.E.; Favretto, E.; Stefanello, D. The impact of extirpation of non-palpable/normal-sized regional lymph nodes on staging of canine cutaneous mast cell tumours: A multicentric retrospective study. Vet. Comp. Oncol. 2018, 16, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Milovancev, M.; Bartels, C.; Irvin, V.L.; Tuohy, J.L.; Townsend, K.L.; Leeper, H. Histologically low-grade, yet biologically high-grade, canine cutaneous mast cell tumours: A systematic review and meta-analysis of individual participant data. Vet. Comp. Oncol. 2020, 18, 580–589. [Google Scholar] [CrossRef]
- Iodence, A.E.; Wallace, M.L.; Grimes, J.A.; Schmiedt, C.W. Dogs undergoing surgical excision of mast cell tumors are not at increased risk of incisional complications. J. Am. Vet. Med. Assoc. 2021, 260, S88–S95. [Google Scholar] [CrossRef]
- Kiser, P.K.; Löhr, C.V.; Meritet, D.; Spagnoli, S.T.; Milovancev, M.; Russell, D.S. Histologic processing artifacts and inter-pathologist variation in measurement of inked margins of canine mast cell tumors. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 2018, 30, 377–385. [Google Scholar] [CrossRef] [PubMed]
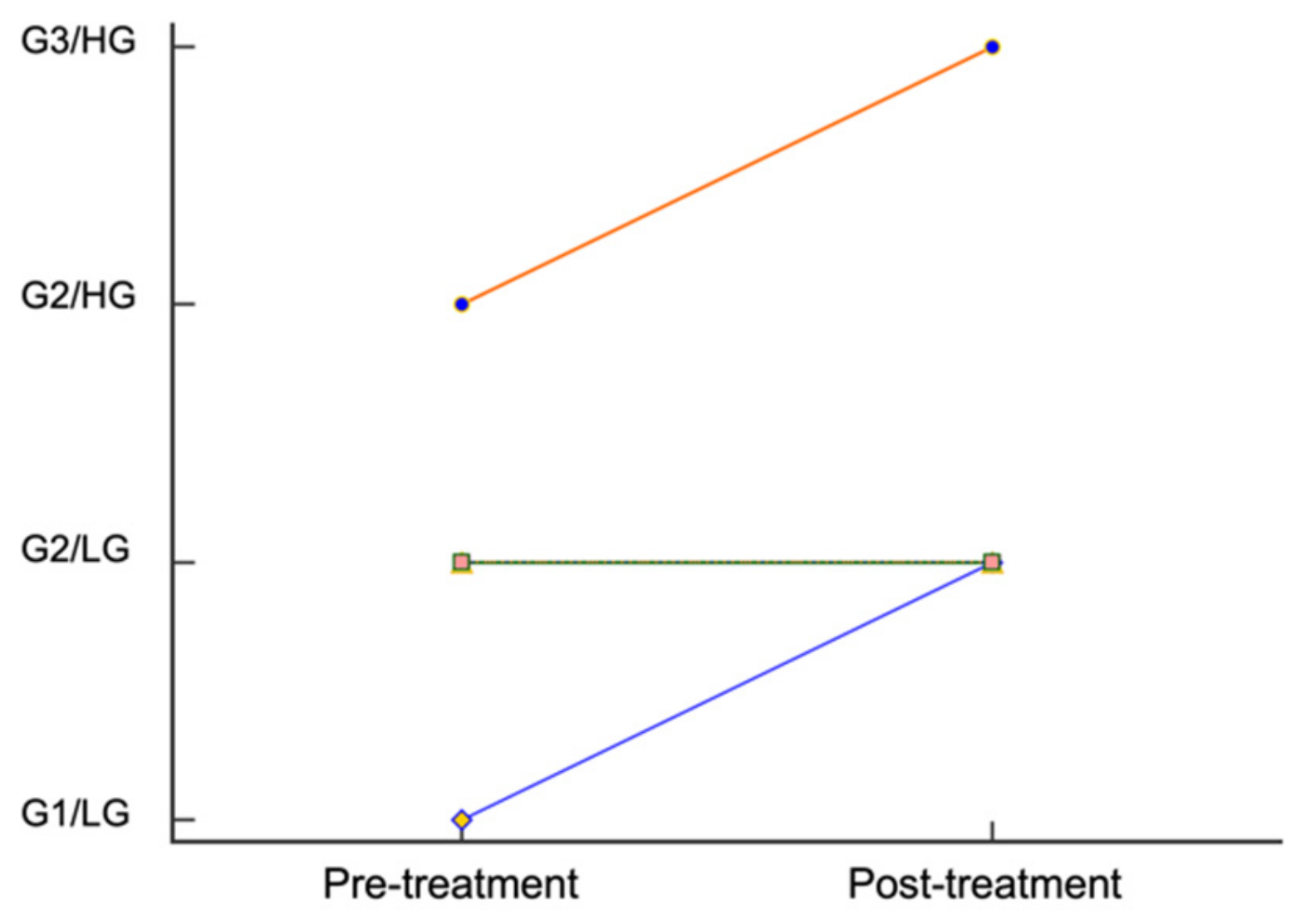




| Patient # | Breed | Age (y) | Sex | Weight (kg) | Recurrent or Novel | Tumor Location | Tumor Volume (mm3) | Tumor Volume (% Change) | Tumor Grade | Surgical Margins 1 and Histologic Margins 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Golden retriever | 7.5 | FS | 34.5 | Novel | Proximal lateral left forelimb | pre: 2205 | −49 | Pre: G2/LG | Wide |
| Post: 1125 | Post: G2/LG | Complete | ||||||||
| 2 | Mixed | 3.2 | FS | 45.3 | Novel | Tail | Pre: 1425 | −64.9 | Pre: G2/LG | Wide |
| Post: 500 | Post: G2/LG | Complete | ||||||||
| 3 | Yorkshire terrier | 5.3 | MC | 4.3 | Novel | Ventral to left eye | Pre: 1078 | 0 | Pre: G2/HG | Marginal |
| Post: 1078 | Post: G3/HG | Incomplete | ||||||||
| 4 | Staffordshire terrier | 6.7 | MC | 27.3 | Novel | Interdigital | Pre: 3300 | −22.7 | Pre: G2/LG | Marginal |
| Post: 2550 | Post: G2/LG | Incomplete | ||||||||
| 5 | Mixed | 11.6 | MC | 30.6 | Recurrent | Left abdomen | Pre: 160,080 | −75.4 | Pre: G2/LG | Wide |
| Post: 39,360 | Post: G2/LG | Complete | ||||||||
| 6 | Miniature Schnauzer | 9.6 | MC | 10.1 | Novel | Right dorsal tarsus | Pre: 588 | 200.7 | Pre: G2/LG | Proportional |
| Post: 1768 | Post: G2/LG | Incomplete | ||||||||
| 7 | Mixed | 8.3 | MC | 12.4 | Novel | Distal medial left hindlimb | Pre: 765 | −60.8 | Pre: G1/LG | Proportional |
| Post: 300 | Post: G2/LG | Incomplete | ||||||||
| 8 | Norwegian elkhound | 9.3 | FS | 24.9 | Novel | Oral cavity | Pre: 9996 | −82.7 | Pre: G2/LG | Marginal |
| Post: 1729 | Post: G2/LG | Complete | ||||||||
| 9 | German shorthair pointer | 3.5 | MC | 27.4 | Novel | Proximal lateral right hindlimb | Pre: 2890 | −56.4 | Pre: G2/LG | Wide |
| Post: 1260 | Post: G2/LG | Incomplete | ||||||||
| 10 | Mixed | 3.9 | MC | 38.9 | Recurrent | Distal lateral right hindlimb | Pre: 58,608 | 0 | Pre: G2/LG | Wide |
| Post: 58,608 | Post: G2/LG | Complete | ||||||||
| 11 | Beagle | 8.2 | FS | 19.8 | Novel | Left inguinal region | Pre: 9620 | −57.3 | Pre: G2/LG | Wide |
| Post: 4104 | Post: G2/LG | Complete |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klahn, S.; Dervisis, N.; Lahmers, K.; Benitez, M. Evaluation of Tumor Grade and Proliferation Indices before and after Short-Course Anti-Inflammatory Prednisone Therapy in Canine Cutaneous Mast Cell Tumors: A Pilot Study. Vet. Sci. 2022, 9, 277. https://doi.org/10.3390/vetsci9060277
Klahn S, Dervisis N, Lahmers K, Benitez M. Evaluation of Tumor Grade and Proliferation Indices before and after Short-Course Anti-Inflammatory Prednisone Therapy in Canine Cutaneous Mast Cell Tumors: A Pilot Study. Veterinary Sciences. 2022; 9(6):277. https://doi.org/10.3390/vetsci9060277
Chicago/Turabian StyleKlahn, Shawna, Nikolaos Dervisis, Kevin Lahmers, and Marian Benitez. 2022. "Evaluation of Tumor Grade and Proliferation Indices before and after Short-Course Anti-Inflammatory Prednisone Therapy in Canine Cutaneous Mast Cell Tumors: A Pilot Study" Veterinary Sciences 9, no. 6: 277. https://doi.org/10.3390/vetsci9060277
APA StyleKlahn, S., Dervisis, N., Lahmers, K., & Benitez, M. (2022). Evaluation of Tumor Grade and Proliferation Indices before and after Short-Course Anti-Inflammatory Prednisone Therapy in Canine Cutaneous Mast Cell Tumors: A Pilot Study. Veterinary Sciences, 9(6), 277. https://doi.org/10.3390/vetsci9060277







