Characterization of Equine Chronic Tendon Lesions in Low- and High-Field Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tendon Recovery and Selection for MRI
2.2. Magnetic Resonance Imaging
2.3. Histology
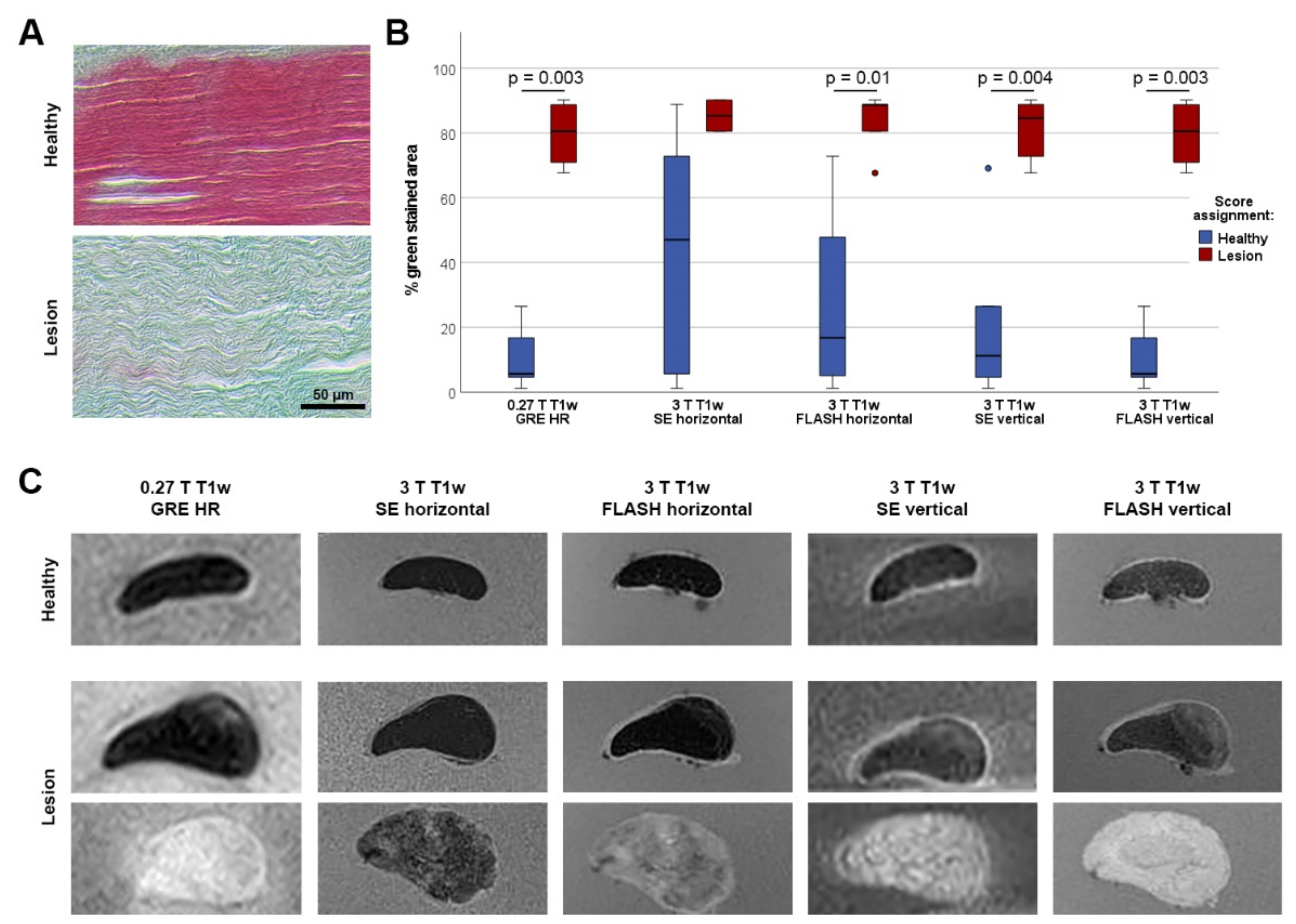
2.4. Qualitative and Quantitative MR Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Agreement of MRI Findings in T1w Low-Field and High-Field Sequences with Histology
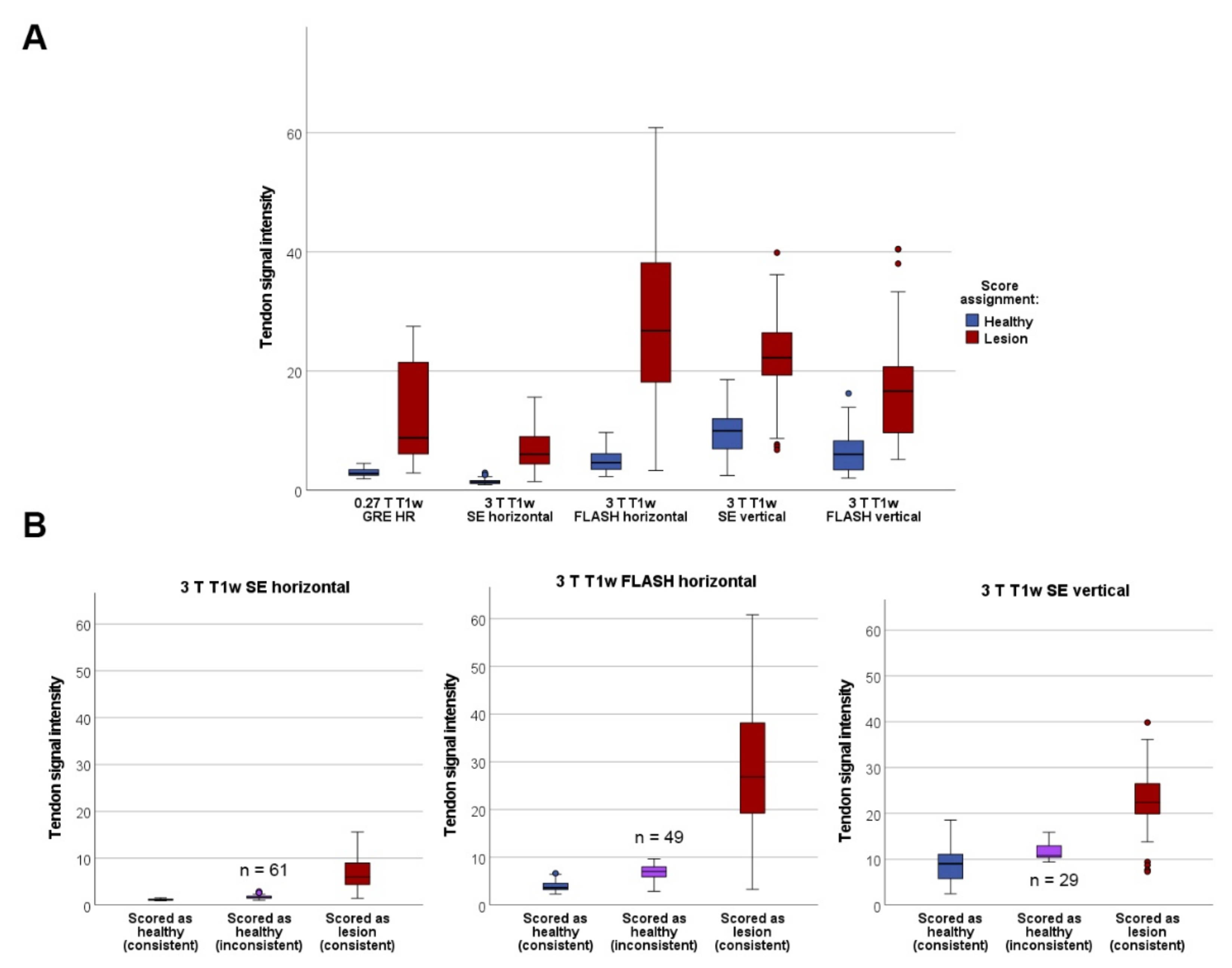
3.2. Agreement of Qualitative Scoring between T1w Sequences and Measured Signal Intensities
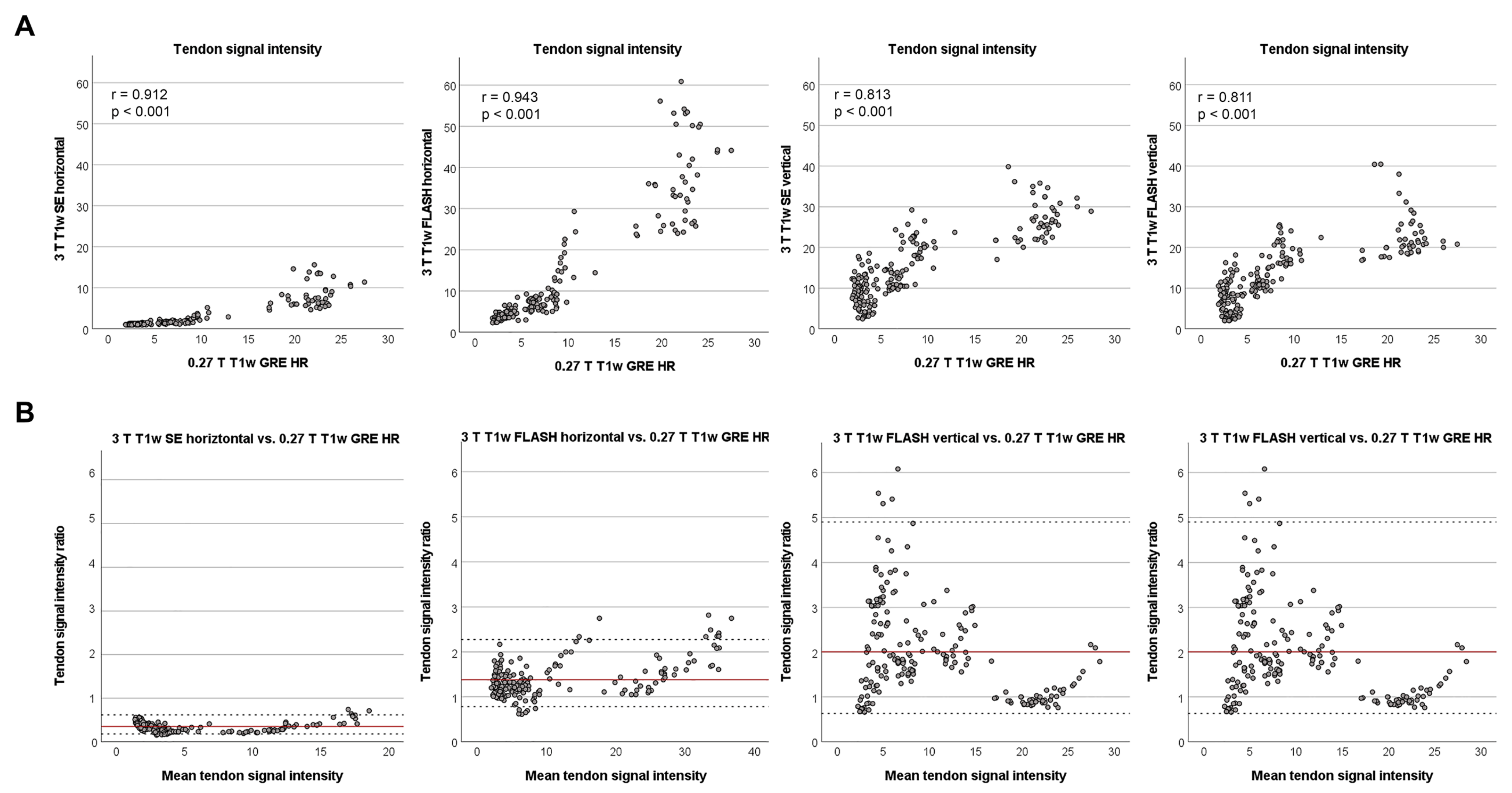
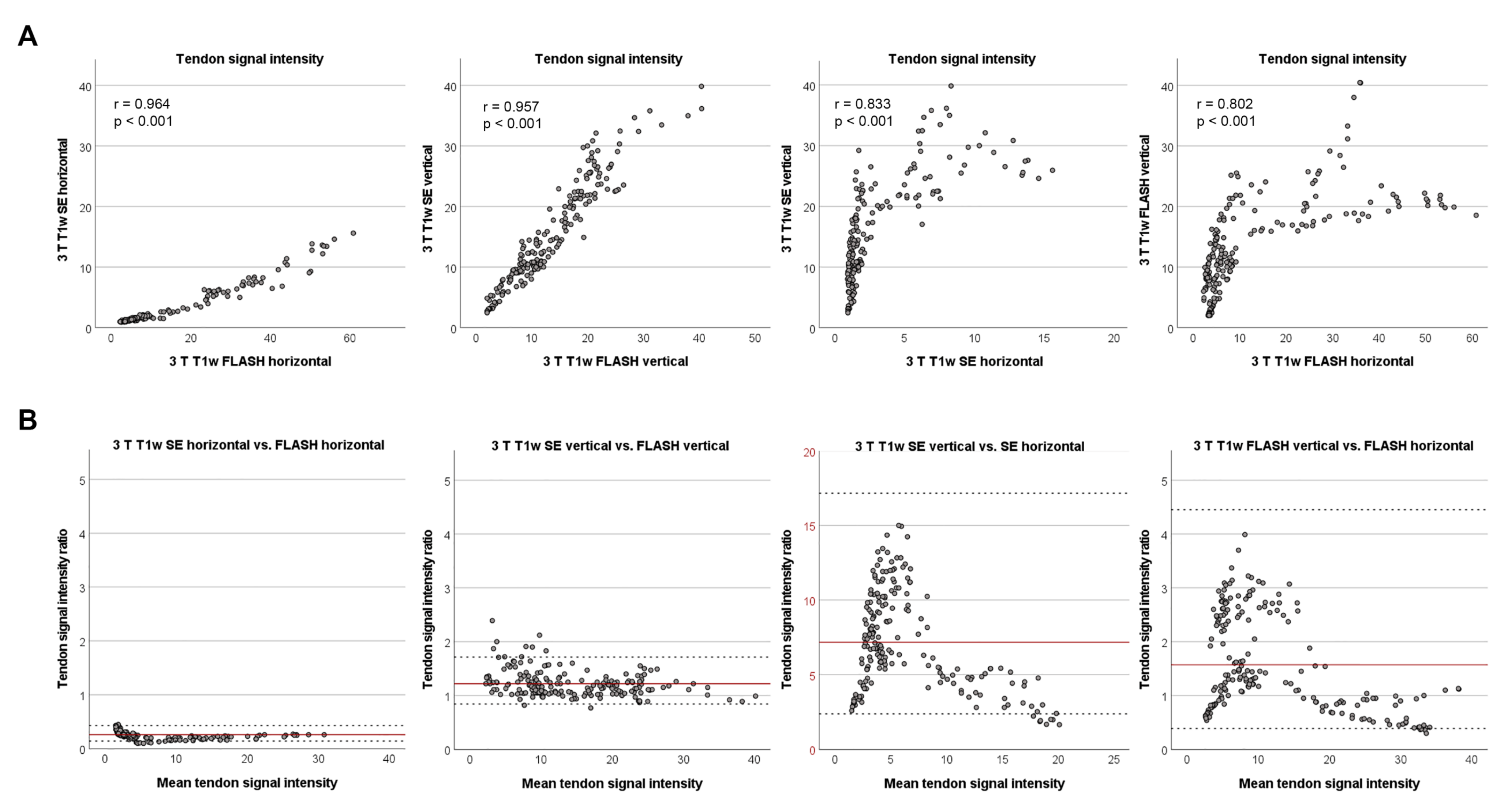
3.3. Agreement of Signal Intensities between Low- and High-Field Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.B.; Harkins, L.S.; Hammond, C.J.; Wood, J.L. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet. J. 2001, 33, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.; Avella, C.; Price, J.; Smith, R.; Wood, J.; Verheyen, K. Descriptive epidemiology of fracture, tendon and suspensory ligament injuries in National Hunt racehorses in training. Equine Vet. J. 2009, 41, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Parkin, T.D.; Riggs, C.M.; Morgan, K.L. Descriptive analysis of retirement of Thoroughbred racehorses due to tendon injuries at the Hong Kong Jockey Club (1992–2004). Equine Vet. J. 2007, 39, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, Y.; Takahashi, T.; Smith, R.K.; Goodship, A.E.; Kuwano, A.; Ueno, T.; Hirano, S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 2004, 36, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Swagemakers, J.H.; Vente, C.; Mahmoud, M. Equine Foot MRI: Past, Present and Future. J. Vet. Sci. Anim. Husb. 2016, 1, 104–105. [Google Scholar]
- Karlin, W.M.; Stewart, A.A.; Durgam, S.S.; Naughton, J.F.; O’Dell-Anderson, K.J.; Stewart, M.C. Evaluation of experimentally induced injury to the superficial digital flexor tendon in horses by use of low-field magnetic resonance imaging and ultrasonography. Am. J. Vet. Res. 2011, 72, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Valdés-Martínez, A.; Turk, P.J.; Wayne McIlwraith, C.; Barrett, M.F.; McGilvray, K.C.; Frisby, D.D. Longitudinal tendon healing assessed with multi-modality advanced imaging and tissue analysis. Equine Vet. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, A.; Svensson, L.; Kristoffersen-Wiberg, M.; Aspelin, P.; Movin, T. Tendon injury and repair after core biopsies in chronic Achilles tendinosis evaluated by serial magnetic resonance imaging. Br. J. Sports Med. 2004, 38, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Pierre-Jerome, C.; Moncayo, V.; Terk, M.R. MRI of the Achilles tendon: A comprehensive review of the anatomy, biomechanics, and imaging of overuse tendinopathies. Acta Radiol. 2010, 51, 438–454. [Google Scholar] [CrossRef]
- Hodgson, R.J.; Grainger, A.J.; O’Connor, P.J.; Evans, R.; Coates, L.; Marzo-Ortega, H.; Helliwell, P.; McGonagle, D.; Emery, P.; Robson, M.D. Imaging of the Achilles tendon in spondyloarthritis: A comparison of ultrasound and conventional, short and ultrashort echo time MRI with and without intravenous contrast. Eur. Radiol. 2011, 21, 1144–1152. [Google Scholar] [CrossRef]
- Schramme, M.; Kerekes, Z.; Hunter, S.; Labens, R. MR imaging features of surgically induced core lesions in the equine superficial digital flexor tendon. Vet. Radiol. Ultrasound 2010, 51, 280–287. [Google Scholar] [CrossRef]
- Vallance, S.A.; Vidal, M.A.; Whitcomb, M.B.; Murphy, B.G.; Spriet, M.; Galuppo, L.D. Evaluation of a diode laser for use in induction of tendinopathy in the superficial digital flexor tendon of horses. Am. J. Vet. Res. 2012, 73, 1435–1444. [Google Scholar] [CrossRef]
- Spriet, M.; Murphy, B.; Vallance, S.A.; Vidal, M.A.; Whitcomb, M.B.; Wisner, E.R. Magic angle magnetic resonance imaging of diode laser induced and naturally occurring lesions in equine tendons. Vet. Radiol. Ultrasound 2012, 53, 394–401. [Google Scholar] [CrossRef]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Davis, W.; Daniel, T.M.; Griffith, E.; Seiler, G.S. Elastographic evaluation of naturally occurring tendon and ligament injuries of the equine distal limb. Vet. Radiol. Ultrasound 2015, 56, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Crass, J.R.; Genovese, R.L.; Render, J.A.; Bellon, E.M. Magnetic resonance, ultrasound and histopathologic correlation of acute and healing equine tendon injuries. Vet. Radiol. Ultrasound 1992, 33, 206–216. [Google Scholar] [CrossRef]
- Sherlock, C.E.; Mair, T.S.; Ireland, J.; Blunden, T. Do low field magnetic resonance imaging abnormalities correlate with macroscopical and histological changes within the equine deep digital flexor tendon? Res. Vet. Sci. 2015, 98, 92–97. [Google Scholar] [CrossRef]
- Murray, R.C.; Mair, T.S.; Sherlock, C.E.; Blunden, A.S. Comparison of high-field and low-field magnetic resonance images of cadaver limbs of horses. Vet. Rec. 2009, 165, 281–288. [Google Scholar] [CrossRef]
- Berner, D.; Brehm, W.; Gerlach, K.; Offhaus, J.; Scharner, D.; Burk, J. Variation in the MRI signal intensity of naturally occurring equine superficial digital flexor tendinopathies over a 12-month period. Vet. Rec. 2020, 187, e53. [Google Scholar] [CrossRef]
- Busoni, V.; Snaps, F. Effect of deep digital flexor tendon orientation on magnetic resonance imaging signal intensity in isolated equine limbs-the magic angle effect. Vet. Radiol. Ultrasound 2002, 43, 428–430. [Google Scholar] [CrossRef]
- Peh, W.C.; Chan, J.H. The magic angle phenomenon in tendons: Effect of varying the MR echo time. Br. J. Radiol. 1998, 71, 31–36. [Google Scholar] [CrossRef]
- Karantanas, A.H.; Zibis, A.H.; Papanikolaou, N. Increased signal intensity on fat-suppressed three-dimensional T1-weighted pulse sequences in patellar tendon: Magic angle effect? Skelet. Radiol. 2001, 30, 67–71. [Google Scholar] [CrossRef]
- Li, T.; Mirowitz, S.A. Manifestation of magic angle phenomenon: Comparative study on effects of varying echo time and tendon orientation among various MR sequences. Magn. Reson. Imaging 2003, 21, 741–744. [Google Scholar] [CrossRef]
- Berner, D.; Brehm, W.; Gerlach, K.; Gittel, C.; Offhaus, J.; Paebst, F.; Scharner, D.; Burk, J. Longitudinal Cell Tracking and Simultaneous Monitoring of Tissue Regeneration after Cell Treatment of Natural Tendon Disease by Low-Field Magnetic Resonance Imaging. Stem Cells Int. 2016, 2016, 1207190. [Google Scholar] [CrossRef] [Green Version]
- Sherlock, C.E.; Mair, T.S. Magic angle effect on low field magnetic resonance images in the superficial digital flexor tendon in the equine proximal pastern region. Vet. J. 2016, 217, 126–131. [Google Scholar] [CrossRef]
- Doll, C.U.; Niebert, S.; Burk, J. Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling. Int. J. Mol. Sci. 2021, 22, 12798. [Google Scholar] [CrossRef]
- Martinello, T.; Pascoli, F.; Caporale, G.; Perazzi, A.; Iacopetti, I.; Patruno, M. Might the Masson trichrome stain be considered a useful method for categorizing experimental tendon lesions? Histol. Histopathol. 2015, 30, 963–969. [Google Scholar]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Nevill, A.M.; Atkinson, G. Assessing agreement between measurements recorded on a ratio scale in sports medicine and sports science. Br. J. Sports Med. 1997, 31, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- McKnight, A.L.; Manduca, A.; Felmlee, J.P.; Rossman, P.J.; McGee, K.P.; Ehman, R.L. Motion-correction techniques for standing equine MRI. Vet. Radiol. Ultrasound 2004, 45, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Karjalainen, P.T.; Soila, K.; Aronen, H.J.; Pihlajamäki, H.K.; Tynninen, O.; Paavonen, T.; Tirman, P.F.J. MR imaging of overuse injuries of the Achilles tendon. AJR Am. J. Roentgenol. 2000, 175, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Juras, V.; Welsch, G.; Bär, P.; Kronnerwetter, C.; Fujita, H.; Trattnig, S. Comparison of 3T and 7T MRI clinical sequences for ankle imaging. Eur. J. Radiol. 2012, 81, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Dahlgren, L.A. Pathobiology of Tendon and Ligament Injuries. Clin. Tech. Equine Pract. 2007, 6, 168–173. [Google Scholar] [CrossRef]
| Sequence | Plane | Matrix | FOV 1 (mm) | TE(ms) | TR 2 (ms) | Slice Thickness (mm) | Gap (mm) |
|---|---|---|---|---|---|---|---|
| T1w GRE HR 3 | transverse | 512 × 512 | 169.98 | 8 | 78 | 3.5 | 0.7 |
| STIR FSE 4 HR | transverse | 512 × 512 | 169.98 | 29 | 3968 | 3.5 | 0.7 |
| T2w FSE HR | transverse | 512 × 512 | 169.98 | 87 | 2475 | 3.5 | 0.7 |
| Sequence | Plane | Matrix | FOV 1 (mm) | TE (ms) | TR 2 (ms) | Slice Thickness (mm) | Gap (mm) |
|---|---|---|---|---|---|---|---|
| T1w SE horizontal | transverse | 512 × 512 | 170 | 12 | 449 | 3.5 | 0.7 |
| T1w 3D trans/SE vertical | transverse | 256 × 256 | 170 | 8.9 | 600 | 0.7 | 0 |
| Fl2d/FLASH horizontal | transverse | 512 × 512 | 170 | 4.56 | 590 | 3.5 | 0.7 |
| Fl2d/FLASH vertical | transverse | 512 × 512 | 170 | 4.56 | 590 | 3.5 | 0.7 |
| STIR SE | transverse | 512 × 512 | 170 | 51 | 4100 | 3.5 | 0.7 |
| T2w TSE 3 | transverse | 512 × 512 | 170 | 118 | 2475 | 3.5 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doll, C.U.; von Pueckler, K.; Offhaus, J.; Berner, D.; Burk, J. Characterization of Equine Chronic Tendon Lesions in Low- and High-Field Magnetic Resonance Imaging. Vet. Sci. 2022, 9, 297. https://doi.org/10.3390/vetsci9060297
Doll CU, von Pueckler K, Offhaus J, Berner D, Burk J. Characterization of Equine Chronic Tendon Lesions in Low- and High-Field Magnetic Resonance Imaging. Veterinary Sciences. 2022; 9(6):297. https://doi.org/10.3390/vetsci9060297
Chicago/Turabian StyleDoll, Carla Ulrike, Kerstin von Pueckler, Julia Offhaus, Dagmar Berner, and Janina Burk. 2022. "Characterization of Equine Chronic Tendon Lesions in Low- and High-Field Magnetic Resonance Imaging" Veterinary Sciences 9, no. 6: 297. https://doi.org/10.3390/vetsci9060297
APA StyleDoll, C. U., von Pueckler, K., Offhaus, J., Berner, D., & Burk, J. (2022). Characterization of Equine Chronic Tendon Lesions in Low- and High-Field Magnetic Resonance Imaging. Veterinary Sciences, 9(6), 297. https://doi.org/10.3390/vetsci9060297






