Urinary Cytology: Potential Role in Canine Urinary Tract Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Preparation
- -
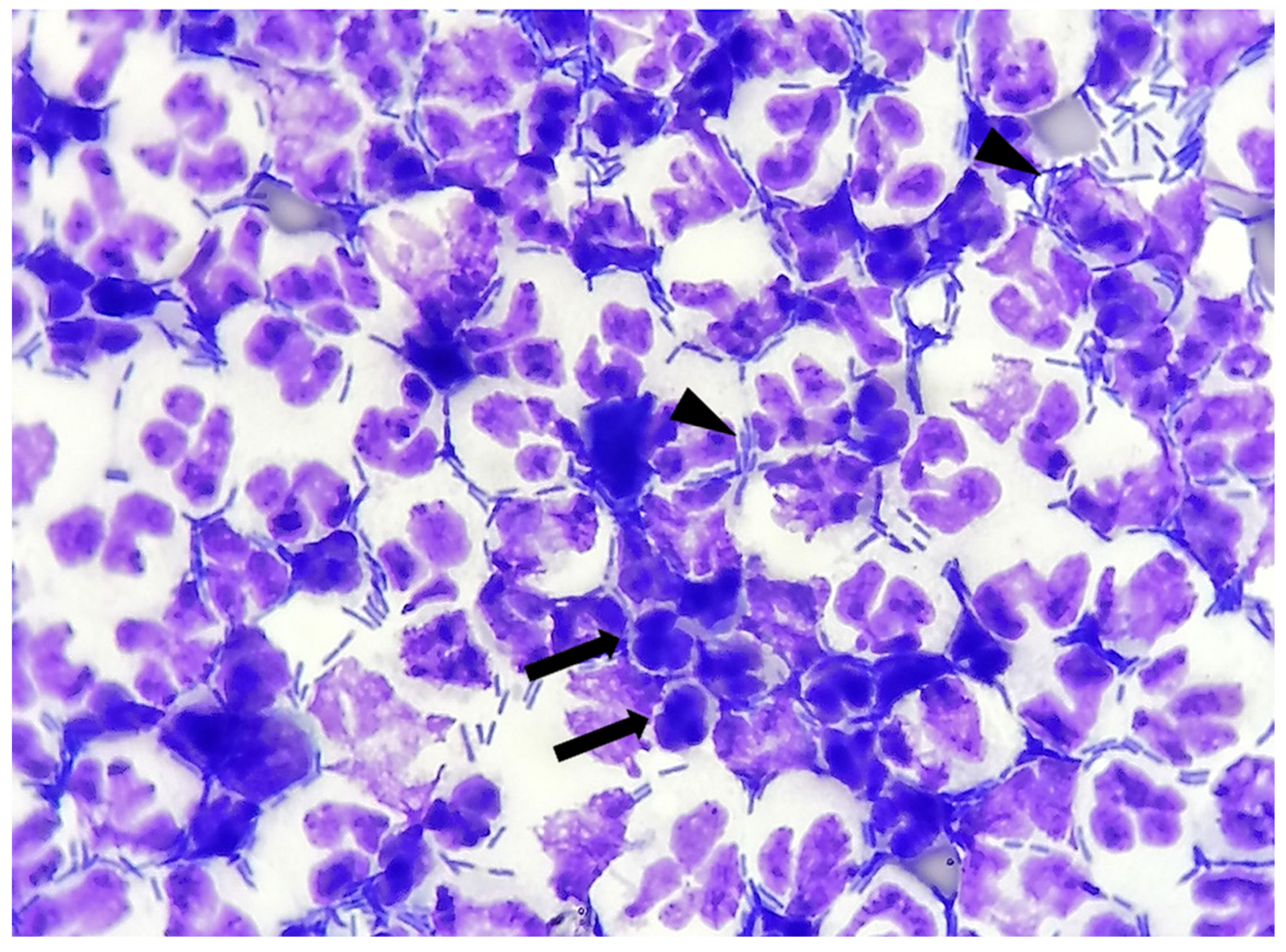
- Bacteria: indicated as present/absent, morphologically identified as rods/cocci/filamentous, and quantified with an estimate of 10 fields at 100× magnification in occasional <3 bacteria/100×, few 3–10 bacteria/100×, moderate 11–40 bacteria/100×, and many >40 bacteria/100× [12]. When detected, bacteria were also classified as intracellular and/or extracellular (Figure 1).
- -
- Neutrophils: indicated as present/absent
- -
2.2. Microbiological Analysis
2.3. Statistical Analysis
3. Results
3.1. Signalment
3.2. Physical and Chemical Urinalysis
3.3. Microbiological Evaluation
3.4. Wet-Mount and Cytological Stained Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Isolate | Bacteria | TBC (CFU/mL) | AM | A\C | CV | CZ | CX | EF | MF | AZ | AK | CL | DX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Other Enterobacteriaceae | 1.2 × 102 | I | S | NT | NT | NT | S | NT | S | S | R | I |
| 2 | Other Enterobacteriaceae | 2.5 × 108 | R | R | NT | NT | NT | R | NT | NT | NT | R | R |
| 3 | Other Enterobacteriaceae | 2.5 × 108 | R | R | NT | NT | NT | I | NT | R | S | R | R |
| 4 | Other Enterobacteriaceae | 5.1 × 107 | R | R | NT | NT | NT | S | NT | S | S | R | R |
| 5 | Other Enterobacteriaceae | 3 × 102 | R | R | S | R | S | S | S | S | S | R | S |
| 6 | Escherichia coli | 1.7 × 107 | R | I | NT | NT | NT | S | NT | S | S | R | S |
| 7 | Escherichia coli | 6 × 105 | S | S | S | NT | NT | S | S | NT | S | NT | S |
| 8 | Escherichia coli | 3.3 × 108 | R | I | I | S | S | S | S | R | I | R | S |
| 9 | Escherichia coli | 6 × 105 | R | S | S | NT | NT | S | S | NT | S | NT | S |
| 10 | Escherichia coli | 6.5 × 107 | R | I | NT | NT | NT | R | NT | R | I | R | I |
| 11 | Escherichia coli | 2.9 × 104 | R | I | I | S | I | S | S | R | R | R | S |
| 12 | Escherichia coli | 2.7 × 107 | R | S | NT | NT | NT | S | NT | S | S | R | S |
| 13 | Escherichia coli | 1.9 × 108 | R | R | NT | NT | NT | S | NT | NT | NT | R | I |
| 14 | Escherichia coli | 2.5 × 108 | R | R | NT | NT | NT | R | NT | S | S | R | S |
| 15 | Escherichia coli | 8.8 × 107 | R | I | I | S | S | I | I | R | S | R | S |
| 16 | Escherichia coli | 1 × 108 | R | I | NT | NT | NT | S | NT | S | S | R | S |
| 17 | Escherichia coli | 2.9 × 106 | I | S | S | S | S | S | S | R | I | R | S |
| 18 | Escherichia coli | 4.2 × 105 | R | S | S | S | S | S | S | R | I | R | I |
| 19 | Escherichia coli | 6 × 105 | S | S | S | NT | NT | S | S | NT | S | NT | S |
| 20 | Escherichia coli | 2.3 × 106 | R | I | S | S | S | I | S | I | R | R | S |
| 21 | Escherichia coli | 1.56 × 104 | R | I | R | R | R | R | R | S | S | R | S |
| 22 | Escherichia coli | 1.23 × 108 | I | I | I | S | S | S | I | I | I | R | S |
| 23 | Escherichia coli | 1.03 × 103 | S | I | R | S | S | S | S | S | S | R | S |
| 24 | Escherichia coli | 3.7 × 107 | R | I | I | S | S | S | S | R | S | R | R |
| 25 | Klebsiella spp. | 6 × 105 | R | S | R | R | R | R | R | NT | S | R | S |
| 26 | Klebsiella spp. | 1.3 × 105 | R | R | R | NT | R | R | R | S | R | R | R |
| 27 | Klebsiella spp. | 6 × 105 | R | R | R | R | R | R | R | R | S | R | S |
| 28 | Klebsiella spp. | 4.5 × 108 | R | I | R | R | R | R | R | S | S | R | S |
| 29 | Proteus spp. | 6 × 105 | S | S | S | S | S | S | S | S | S | S | R |
| 30 | Pseudomonas spp. | 2.9 × 105 | R | R | NT | NT | NT | R | NT | NT | S | R | R |
| 31 | Pseudomonas spp. | 4.6 × 105 | R | R | R | R | R | R | R | R | S | R | S |
| 32 | Pseudomonas spp. | 5.4 × 102 | R | R | NT | NT | NT | I | NT | R | S | R | I |
| 33 | Pseudomonas spp. | 3.7 × 107 | R | R | NT | NT | NT | I | NT | I | S | R | R |
| 34 | Staphylococcus spp. | 6 × 105 | S | S | S | NT | NT | S | S | NT | NT | S | S |
| 35 | Staphylococcus spp. | 6.5 × 104 | R | R | NT | NT | NT | R | NT | R | S | R | R |
| 36 | Staphylococcus spp. | 5.8 × 104 | R | S | R | S | R | R | R | R | R | R | R |
| 37 | Staphylococcus spp. | 3.2 × 106 | R | S | S | S | S | S | NT | S | S | I | S |
| 38 | Streptococcus spp. | 1.2 × 102 | S | S | S | S | S | I | I | R | R | R | R |
References
- Swenson, C.L.; Boisvert, A.M.; Kruger, J.M.; Gibbons-Burgener, S.N. Evaluation of modified Wright-staining of urine sediment as a method for accurate detection of bacteriuria in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Way, L.I.; Sullivan, L.A.; Johnson, V.; Morley, P.S. Comparison of routine urinalysis and urine Gram stain for detection of bacteriuria in dogs. J. Vet. Emerg. Crit. Care 2013, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Raskin, R.E.; Meyer, D.J. “Microscopic Examination of the Urinary Sediment” in Canine and Feline Cytology—A Color Atlas and Interpretation Guide, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bartges, J.W. Diagnosis of urinary tract infections. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Connally, H.E. Cytology and fluid analysis of the acute abdomen. Clin. Tech. Small Anim. Pract. 2003, 18, 39–44. [Google Scholar] [CrossRef]
- Johnson, L.; Queen, E.; Vernau, W.; Sykes, J.; Byrne, B. Microbiologic and Cytologic Assessment of Bronchoalveolar Lavage Fluid from Dogs with Lower Respiratory Tract Infection: 105 Cases (2001–2011). J. Vet. Intern. Med. 2013, 27, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Byron, J.K. Urinary Tract Infection. Vet. Clin. N. Am. Small Anim. Pract. 2018, 49, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Albasan, H.; Lulich, J.; Osborne, C.A.; Lekcharoensuk, C.; Ulrich, L.K.; Carpenter, K.A. Effects of storage time and temperature on pH, specific gravity, and crystal formation in urine samples from dogs and cats. J. Am. Vet. Med. Assoc. 2003, 222, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, T.E.; Valenciano, A.; Bowles, M.; Cowell, R.; Tyler, R.; De Nicola, D.B. Atlas of Canine and Feline Urinalysis, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Qamar, I.; Rehman, S.; Mehdi, G.; Maheshwari, V.; Ansari, H.A.; Chauhan, S. Utility of Cytospin and Cell block Technology in Evaluation of Body Fluids and Urine Samples: A Comparative Study. J. Cytol. 2018, 35, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Swenson, C.L.; Boisvert, A.M.; Gibbons-Burgener, S.N.; Kruger, J.M. Evaluation of modified Wright-staining of dried urinary sediment as a method for accurate detection of bacteriuria in cats. Vet. Clin. Pathol. 2011, 40, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis: An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Papini, R.; Ebani, V.V.; Cerri, D.; Guidi, G. Survey on bacterial isolates from dogs with urinary tract infections and their in vitro sensitivity. Rev. De Med. Vet. 2006, 157, 35–41. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute). M02-A12—Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; pp. 1–96. [Google Scholar]
- EUCAST (The European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0; The European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020; p. 2020. [Google Scholar]
- Marques, C.; Gama, L.T.; Belas, A.; Bergström, K.; Beurlet, S.; Briend-Marchal, A.; Broens, E.M.; Costa, M.; Criel, D.; Damborg, P.; et al. European multicenter study on antimicrobial resistance in bacteria isolated from companion animal urinary tract infections. BMC Vet. Res. 2016, 12, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzi, T.E. Urinalysis in Companion Animal Part 1: Collection, Sample Handling, and Initial Evaluation. 2014. Available online: https://www.todaysveterinarypractice.com/todays-technician-urinalysis-in-companion-ani-mals-part-1-collection-sample-handling-initial-evaluation. (accessed on 31 August 2020).
- Chew, D.J.; Dibartola, S.P. Sample handling, preparation, analysis and urinalysis interpretation. In Interpretation of Canine and Feline Urinalysis, 1st ed.; The Gloyd Group Inc.: Wilmington, DE, USA, 1998; pp. 9–21. [Google Scholar]
- Lulich, J.P.; Osborne, C.A. Urine culture as a test for cure: Why, when, and how? Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Horl, W.H. Common errors in diagnosis and management of urinary tract infection: Pathophisiology and diagnostic techniques. Nephrol. Dial. Transplant. 1999, 14, 2746–2753. [Google Scholar] [CrossRef]
- Meuten, D. Laboratory evaluation and interpretation of the urinary system. In Veterinary Hematology and Clinical Chemistry, 2nd ed.; Thrall, M.A., Weiser, G., Allison, R.W., Campbell, T.W., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 323–377. [Google Scholar]
- DeHeer, H.L.; Parry, B.W.; Grindem, C.B. Pleural fluid. In Diagnostic Cytology and Hematology of the Horse, 2nd ed.; Cowell, R.L., Tyler, R.D., Eds.; Mosby: St. Louis, MO, USA, 2002; pp. 119–126. [Google Scholar]
- Valenciano, A.C.; Arndt, T.P.; Rizzi, T.E. Effusions: Abdominal, thoracic, and pericardial. In Diagnostic Cytology and Hematology of the Dog and Cat, 4th ed.; Valenciano, A.C., Cowell, R.L., Eds.; Mosby: St. Louis, MO, USA, 2014; pp. 244–265. [Google Scholar]
- Forrester, S.D.; Troy, G.C.; Dalton, M.N.; Huffman, J.W.; Holtzman, G. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. J. Vet. Intern. Med. 1999, 13, 557–560. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Stevenson, M.; Malik, R.; Snow, D.; Norris, J.M. Urinary tract infections in cats with chronic kidney disease. J. Feline Med. Surg. 2012, 15, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Y.; Hartmann, F.A.; Jooss, M.K.; Viviano, K.R. Prevalence and clinical outcome of subclinical bacteriuria in female dogs. J. Am. Vet. Med. Assoc. 2014, 245, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, R.; von Vopelius-Feldt, C.; Wolf, G.; Mueller, R.S.; Straubinger, R.K.; Hartmann, K. Urinary tract infections in cats. Prevalence of comorbidities and bacterial species, and determination of antimicrobial susceptibility to commonly used antimicrobial agents. Tierarztl Prax Ausg K Kleintiere Heimtiere 2016, 44, 227–236. [Google Scholar] [CrossRef] [PubMed]


| Dipstick Chemical Analysis | |||||
|---|---|---|---|---|---|
| Parameter | Quantitative Estimation | Number of Samples (%) | N Symptomatic (%) | N Asymptomatic (%) | p Value * |
| Leucocytes | Absent | 49 (55) | 11 (22.5) | 38 (77.5) | 0.0027 |
| 25 leucocytes/μL | 13 (14.6) | 9 (69) | 4 (31) | ||
| 100 leucocytes/μL | 7 (7.9) | 5 (71.4) | 2 (28.6) | ||
| 500 leucocytes/μL | 20 (22.5) | 9 (45) | 11 (55) | ||
| Proteins | Absent | 44 (49.4) | 18 (41) | 26 (59) | 0.9307 |
| 30 mg/dL | 24 (27) | 11 (46) | 13 (54) | ||
| 100 mg/dL | 8 (9) | 4 (50) | 4 (50) | ||
| 500 mg/dL | 13 (14.6) | 4 (31) | 9 (69) | ||
| Glucose | Absent | 79 (88.8) | 32 (40.5) | 47 (59.5) | 0.6736 |
| 50 mg/dL | 2 (2.2) | 0 (0) | 2 (100) | ||
| 300 mg/dL | 3 (3.4) | 1 (33.3) | 2 (66.7) | ||
| 1000 mg/dL | 5 (5.6) | 1 (20) | 4 (80) | ||
| Ketones | Absent | 80 (89.9) | 30 (37.5) | 50 (62.5) | 0.7271 |
| 15 mg/dL | 7 (7.9) | 4 (57) | 3 (43) | ||
| 50 mg/dL | 2 (2.2) | 0 (0) | 2 (100) | ||
| Urobilinogen | Normal | 76 (85.4) | 25 (33) | 51 (67) | 0.0187 |
| 1 mg/dL | 7 (7.9) | 6 (85.7) | 1 (14.3) | ||
| 4 mg/dL | 5 (5.6) | 2 (40) | 3 (60) | ||
| 8 mg/dL | 1 (1.1) | 1 (100) | 0 (0) | ||
| Bilirubin | Absent | 76 (85.5) | 26 (34.2) | 50 (65.8) | 0.1624 |
| 1 mg/dL | 6 (6.7) | 4 (66.7) | 2 (33.3) | ||
| 3 mg/dL | 6 (6.7) | 3 (50) | 3 (50) | ||
| 6 mg/dL | 1 (1.1) | 1 (100) | 0 (0) | ||
| Blood | Absent | 28 (31.5) | 9 (32) | 19 (68) | 0.2669 |
| 10 erythrocytes/μL | 9 (10.1) | 5 (55.5) | 4 (44.5) | ||
| 25 erythrocytes/μL | 15 (16.8) | 6 (40) | 9 (60) | ||
| 50 erythrocytes/μL | 9 (10.1) | 1 (11.1) | 8 (88.9) | ||
| 250 erythrocytes/μL | 28 (31.5) | 13 (46.4) | 15 (53.6) | ||
| Parameter | Bacteria with Wet-Mount Evaluation | Bacteria with Stained Cytology (Diff-Quick) | ||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| Sensibility | 61% | 0.45–0.74 | 76% | 0.61–0.87 |
| Specificity | 83% | 0.70–0.91 | 83% | 0.70–0.91 |
| Positive Predictive Value | 72% | 0.65–0.79 | 76% | 0.69–0.83 |
| Negative Predictive Value | 74% | 0.68–0.80 | 83% | 0.78–0.88 |
| p value | <0.0001 | <0.0001 | ||
| Finding | Positive UC (n = 38) | Negative UC (n= 52) | Total | OR | CI 95% | p Value |
|---|---|---|---|---|---|---|
| Bacteria (WM) | 23 (60.5%) | 9 (17.3%) | 32 (28.8%) | 7.1 | 2.77–19.31 | <0.001 |
| Extracellular bacteria (SC) | 30 (78.9%) | 8 (15.4%) | 38 (42.2%) | 20.6 | 6.97–60.99 | <0.001 |
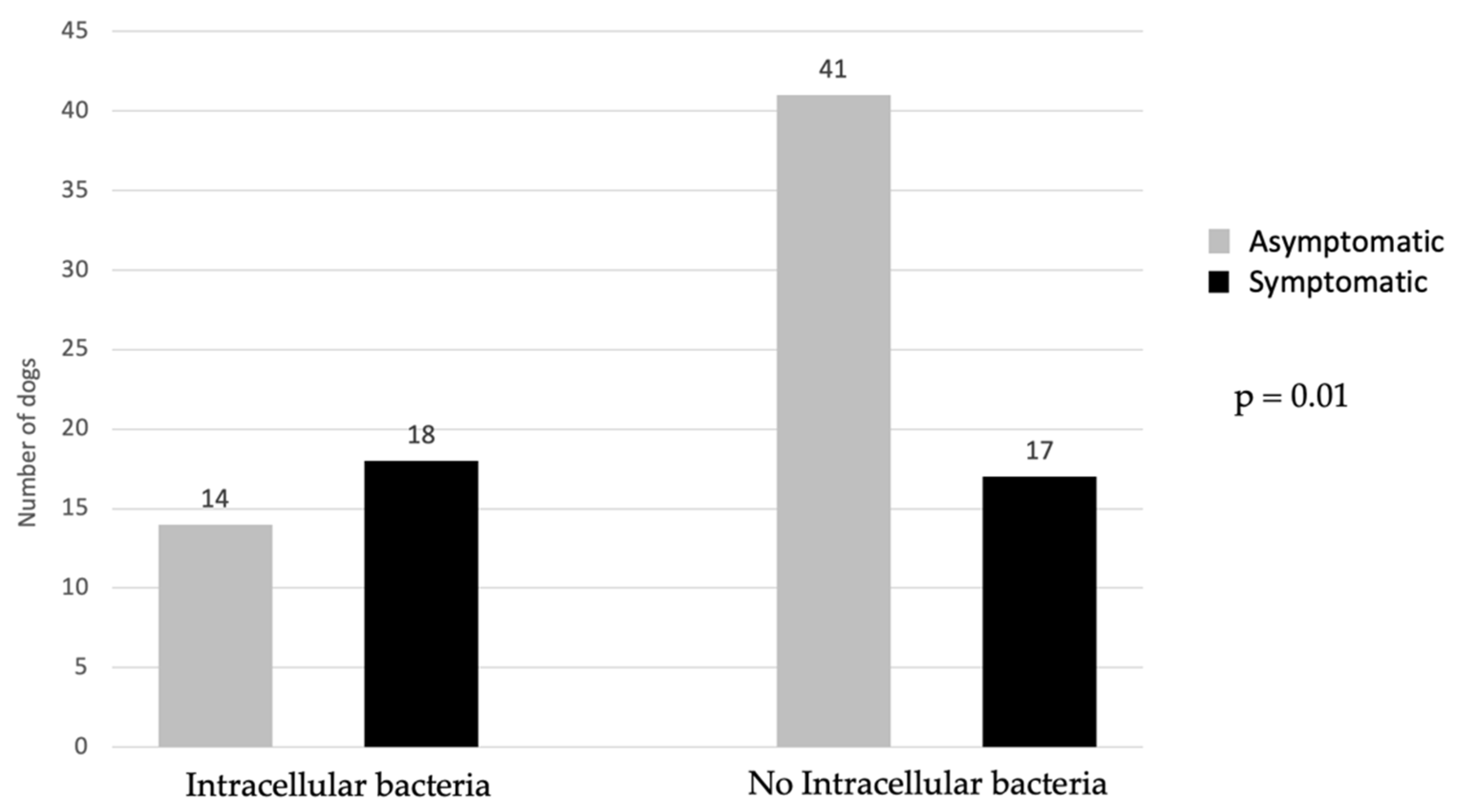
| Intracellular bacteria (SC) | 27 (71%) | 5 (9.6%) | 32 (35.6%) | 23.1 | 7.24–73.47 | <0.001 |
| Neutrophils (SC) | 29 (76.31%) | 17 (32.7%) | 46 (51.1%) | 6.6 | 2.57–17.08 | <0.001 |
| Degenerated neutrophils (SC) | 29 (76.31%) | 11 (21.1%) | 40 (44.4%) | 12 | 4.41–32.68 | <0.001 |
| Intracellular bacteria and degenerated neutrophils (SC) | 27 (71%) | 5 (9.6%) | 32 (35.6%) | 23.1 | 7.24–73.47 | <0.001 |
| Phase | Sig. | OR | Lower 95% OR | Upper 96% OR | |
|---|---|---|---|---|---|
| Phase 1 | SCS | 0.647 | 1.32 | 0.406 | 4.26 |
| IB | 0.008 | 13.97 | 1.969 | 99.18 | |
| DN | 0.576 | 1.67 | 0.274 | 10.26 | |
| Phase 2 | IB | 0.003 | 16.20 | −2.514 | 104.4 |
| DN | 0.641 | 1.52 | 0.262 | 8.78 | |
| Phase 3 | IB | 0.000 | 23.07 | 7.246 | 73.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, I.; Habermaass, V.; Gori, E.; Ebani, V.V.; Pierini, A.; Marchetti, V. Urinary Cytology: Potential Role in Canine Urinary Tract Infections. Vet. Sci. 2022, 9, 304. https://doi.org/10.3390/vetsci9060304
Lippi I, Habermaass V, Gori E, Ebani VV, Pierini A, Marchetti V. Urinary Cytology: Potential Role in Canine Urinary Tract Infections. Veterinary Sciences. 2022; 9(6):304. https://doi.org/10.3390/vetsci9060304
Chicago/Turabian StyleLippi, Ilaria, Verena Habermaass, Eleonora Gori, Valentina Virginia Ebani, Alessio Pierini, and Veronica Marchetti. 2022. "Urinary Cytology: Potential Role in Canine Urinary Tract Infections" Veterinary Sciences 9, no. 6: 304. https://doi.org/10.3390/vetsci9060304
APA StyleLippi, I., Habermaass, V., Gori, E., Ebani, V. V., Pierini, A., & Marchetti, V. (2022). Urinary Cytology: Potential Role in Canine Urinary Tract Infections. Veterinary Sciences, 9(6), 304. https://doi.org/10.3390/vetsci9060304









