Pro-Inflammatory and Immunological Profile of Dogs with Myxomatous Mitral Valve Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Statement
2.2. Electrocardiography, Echocardiography, and MMVD Classification
2.3. Blood Sample Collection
2.4. CBC and Serum Biochemistry
2.5. Leptin and Inflammatory Cytokines Assay
2.6. Monoclonar Antibodies, Immunofluorescence, and Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Clinical Classification, Echocardiographic Parameters, CBC, and Serum Biochemistry
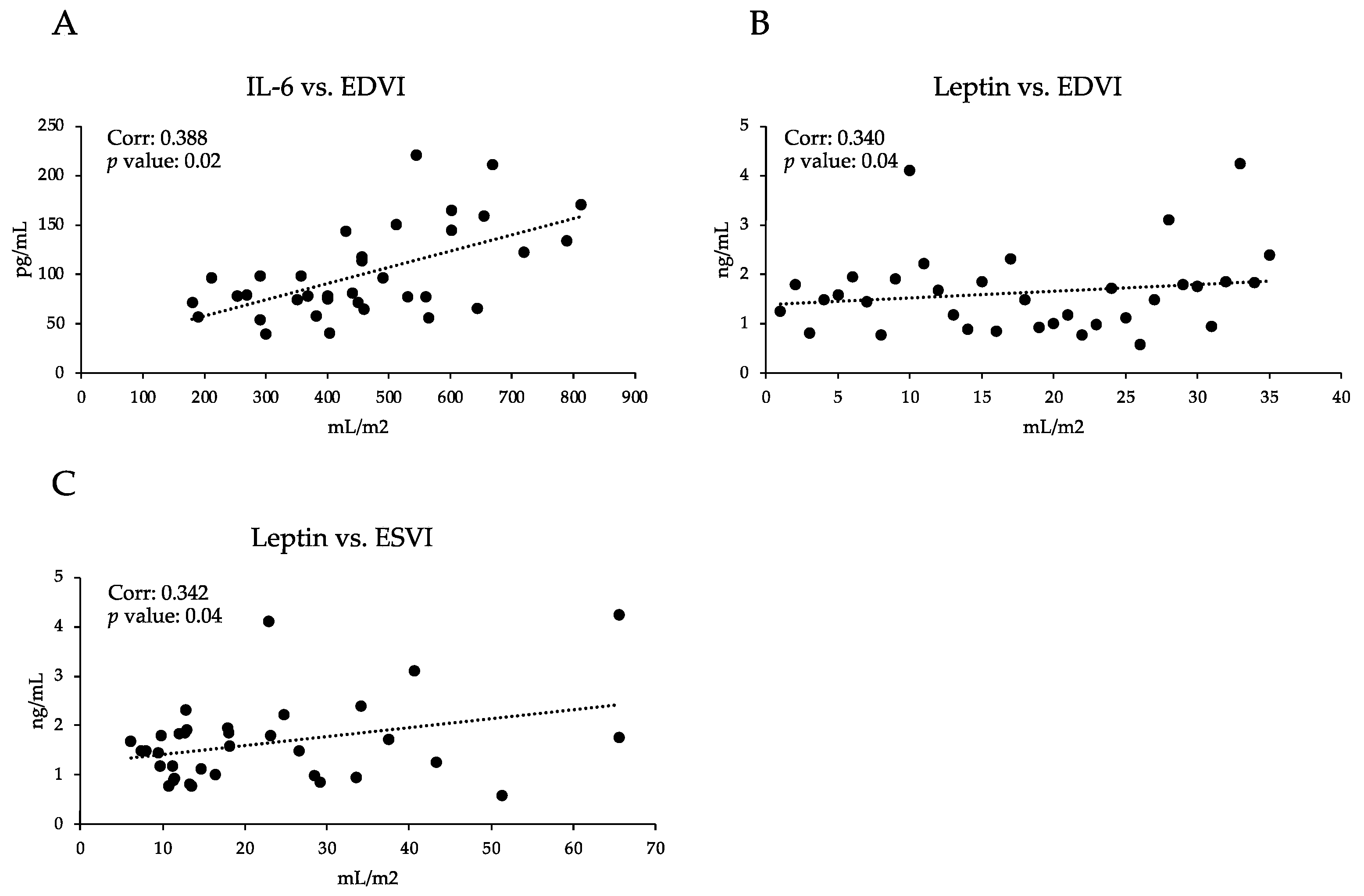
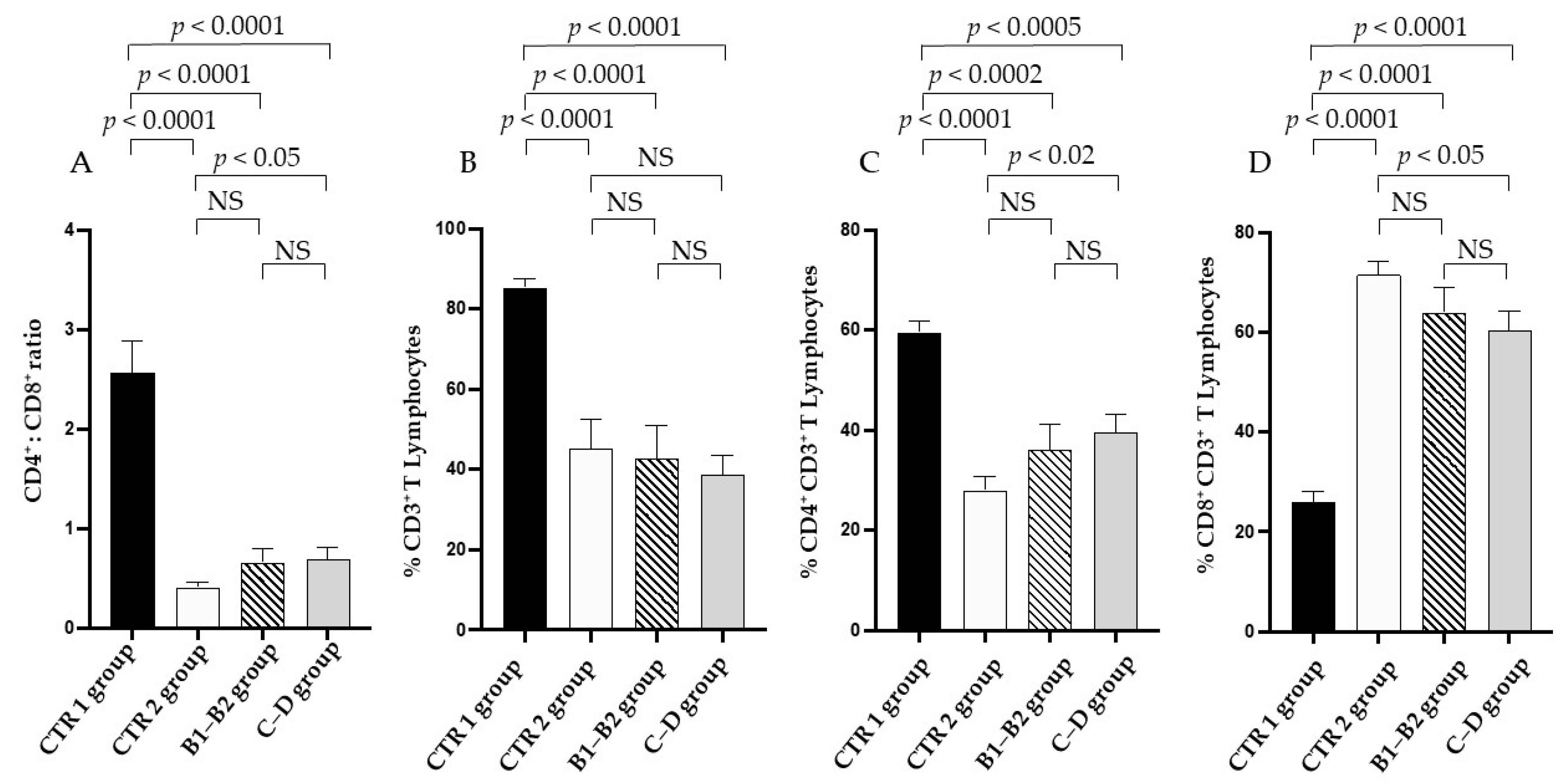
3.2. Immune Profile
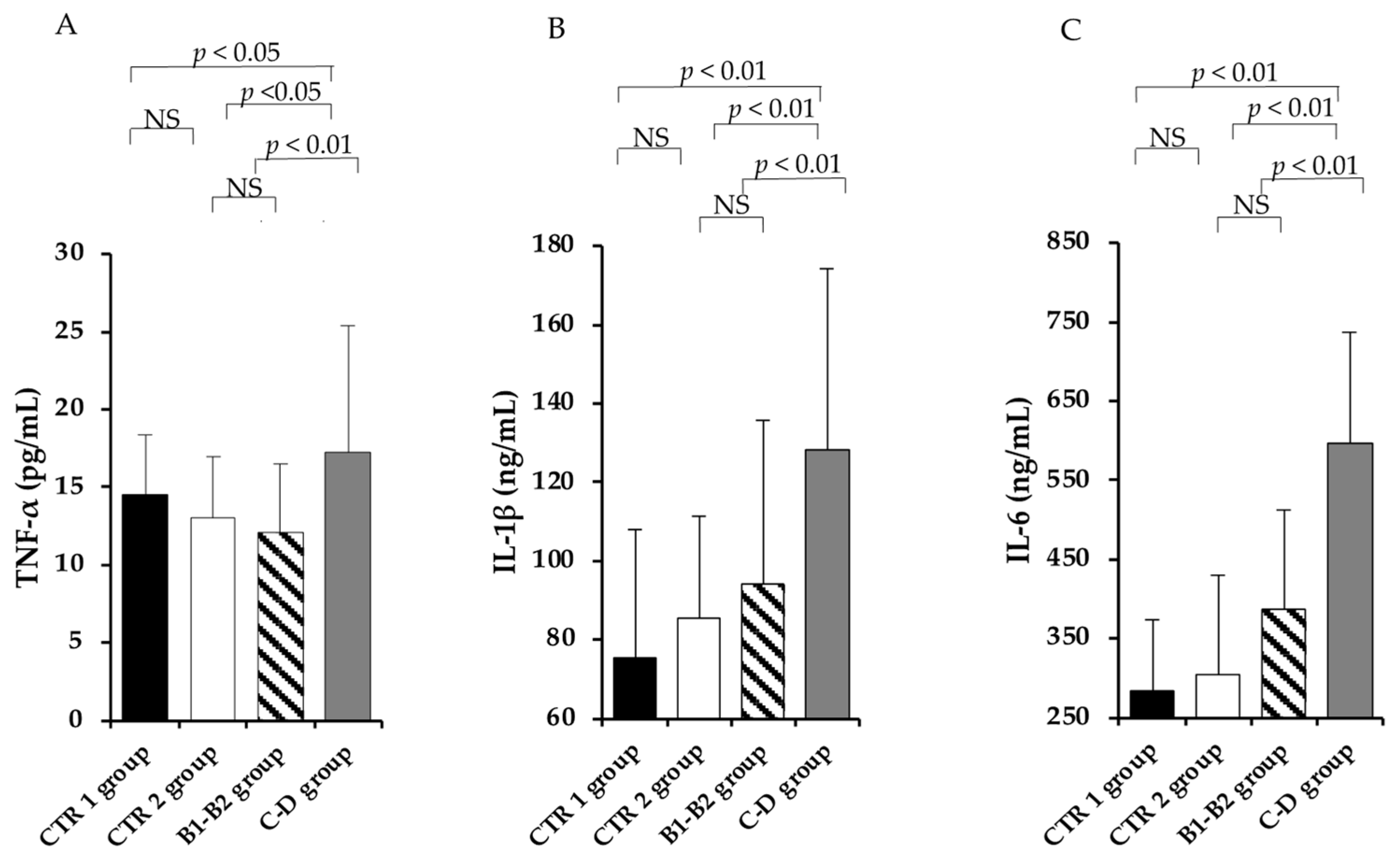
3.3. Pro-Inflammatory Profile
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2020, 34, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; Kim, S.K.; Infante, M.; Mammi, C.; La Vignera, S.; Fabbri, A.; Jaffe, I.Z.; Caprio, M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Front. Endocrinol. 2019, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Sakaguchi, S. Regulatory T cells exert checks and balances on self-tolerance and autoimmunity. Nat. Immunol. 2010, 11, 7–13. [Google Scholar] [CrossRef]
- Pinheiro, D.; Singh, Y.; Grant, C.R.; Appleton, R.C.; Sacchini, F.; Walker, K.R.; Chadbourne, A.H.; Palmer, C.A.; Armitage-Chan, E.; Thompson, I.; et al. Phenotypic and functional characterization of a CD4(+) CD25(high) FOXP3(high) regulatory T-cell population in the dog. Immunology 2011, 132, 111–122. [Google Scholar] [CrossRef]
- Platten, M.; Youssef, S.; Hur, H.M.; Ho, P.P.; Han, M.H.; Lanz, T.V.; Phillips, L.K.; Goldstein, M.J.; Bhat, R.; Raine, C.S.; et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc. Natl. Acad. Sci. USA 2009, 106, 14948–14953. [Google Scholar] [CrossRef] [Green Version]
- Atkins, C.; Bonagura, J.; Ettinger, S.; Fox, P.; Gordon, S.; Haggstrom, J.; Hamlin, R.; Keene, B.; Luis-Fuentes, V.; Stepien, R. Guidelines for the Diagnosis and Treatment of Canine Chronic Valvular Heart Disease. J. Vet. Intern. Med. 2009, 23, 1142–1150. [Google Scholar] [CrossRef]
- Borgarelli, M.; Ferasin, L.; Lamb, K.; Bussadori, C.; Chiavegato, D.; D’Agnolo, G.; Migliorini, F.; Poggi, M.; Santilli, R.A.; Guillot, E.; et al. DELay of Appearance of symptoms of Canine Degenerative Mitral Valve Disease Treated with Spironolactone and Benazepril: The DELAY Study. J. Vet. Cardiol. 2020, 27, 34–53. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Weston, S.A.; Redfield, M.M.; Killian, J.M.; Roger, V.L. Tumor Necrosis Factor-alfa and Mortality in Heart Failure A Community Study. Circulation 2008, 118, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Haudek, S.B.; Taffet, G.E.; Schneider, M.D.; Mann, D.L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Investig. 2007, 117, 2692–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, T.; Gu, Y.; Ortines, R.V.; Bhattacharya, C.; Wang, G.; Xuan, Y.T.; Prabhu, S.D. Divergent TNF receptor-related remodeling responses in heart failure: Role of NF-κB and inflammatory activation. Circulation 2009, 119, 1386–1397. [Google Scholar] [CrossRef]
- Bageghni, S.A.; Hemmings, K.E.; Yuldasheva, N.Y.; Maqbool, A.; Gamboa-Esteves, F.O.; Humphreys, N.E.; Jackson, M.S.; Denton, C.P.; Francis, S.; Porter, K.E.; et al. Fibroblast-specific deletion of IL-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight 2019, 4, e125074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meléndez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin-6 Mediates Myocardial Fibrosis, Concentric Hypertrophy and Diastolic Dysfunction in Rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.H.; Hung, C.S.; Liao, C.W.; Wei, L.H.; Chen, C.W.; Shun, C.T.; Wen, W.F.; Wan, C.H.; Wu, X.M.; Chang, Y.Y.; et al. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc. Res. 2018, 114, 690–702. [Google Scholar] [CrossRef]
- Camacho, R.R.; Carvalho, E.R.; Zacché Pereira, E.; Nelson Gava, F.; Camacho, A.A.; Gonçalves Sousa, M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Arch. Vet. Sci. 2017, 22, 1–8. [Google Scholar]
- Fonfara, S.; Hetzel, Y.; Tew, S.R.; Dukes-Mcewan, J.; Cripps, P.; Clegg, P.D. Leptin Expression in Dogs with Cardiac Disease and Congestive Heart Failure. J. Vet. Intern. Med. 2011, 25, 1017–1024. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, J.H.; Jeung, E.B.; Yang, M.P. Serum Concentrations of Leptin and Adiponectin in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2016, 30, 1589–1600. [Google Scholar] [CrossRef]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef]
- Hansson, K.; Häggström, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in Cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef]
- Bonagura, J.D.; Luis Fuentes, V. Echocardiography. In Small Animal Diagnostic Ultrasound, 3rd ed.; Mattoon, J.S., Nyland, T.G., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2015; pp. 217–331. [Google Scholar]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Haggstrom, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Menciotti, G.; Borgarelli, M. Review of Diagnostic and Therapeutic Approach to Canine Myxomatous Mitral Valve Disease. Vet. Sci. 2017, 4, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M.J. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Rubino, V.; Piantedosi, D.; Di Loria, A.; Ruggiero, G.; Ciaramella, P.; Terrazzano, G. Regulatory T cells, Cytotoxic T lymphocytes and a T(H)1 cytokine profile in dogs naturally infected by Leishmania infantum. Res. Vet. Sci. 2013, 95, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Lanzilli, S.; Rubino, V.; Di Cerbo, A.; Centenaro, S.; Guidetti, G.; Canello, S.; Terrazzano, G. An immune-modulating diet increases the regulatory T cells and reduces T helper 1 inflammatory response in Leishmaniosis affected dogs treated with standard therapy. BMC Vet. Res. 2015, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Palatucci, A.; Piantedosi, D.T.; Rubino, V.; Giovazzino, A.; Guccione, J.; Pernice, V.; Ruggiero, G.; Cortese, L.; Terrazzano, G. Circulating regulatory T cells (Treg), leptin and induction of proinflammatory activity in obese Labrador Retriever dogs. Vet. Immunol. Immunopathol. 2018, 202, 122–129. [Google Scholar] [CrossRef]
- Lombardi, P.; Palatucci, A.T.; Giovazzino, A.; Mastellone, V.; Ruggiero, G.; Rubino, V.; Musco, N.; Crupi, R.; Cutrignelli, M.I.; Britti, D.; et al. Clinical and Immunological Response in Dogs Naturally Infected by L. infantum Treated with a Nutritional Supplement. Animals 2019, 9, 501. [Google Scholar] [CrossRef] [Green Version]
- Piantedosi, D.; Palatucci, A.T.; Giovazzino, A.; Ruggiero, G.; Rubino, V.; Musco, N.; Carriero, F.; Farina, F.; Attia, Y.A.E.W.; Terrazzano, G.; et al. Effect of a Weight Loss Program on Biochemical and Immunological Profile, Serum Leptin Levels, and Cardiovascular Parameters in Obese Dogs. Front. Vet. Sci. 2020, 7, 398. [Google Scholar] [CrossRef]
- Felsburg, P.J. Overview of immune system development in the dog: Comparison with humans. Hum. Exp. Toxicol. 2002, 21, 487–492. [Google Scholar] [CrossRef]
- Pereira, M.; Valério-Bolas, A.; Saraiva-Marques, C.; Alexandre-Pires, G.; Pereira da Fonseca, I.; Santos-Gomes, G. Development of Dog Immune System: From in Uterus to Elderly. Vet. Sci. 2019, 21, 83. [Google Scholar] [CrossRef] [Green Version]
- Greeley, E.; Kealy, R.; Ballam, J.; Lawler, D.; Segre, M. The influence of age on the canine immune system. Vet. Immunol. Immunopathol. 1996, 55, 1–10. [Google Scholar] [CrossRef]
- Withers, S.S.; Moore, P.F.; Chang, H.; Choi, J.W.; McSorley, S.J.; Kent, M.S.; Monjazeb, A.M.; Canter, R.J.; Murphy, W.J.; Sparger, E.E.; et al. Multi-color flow cytometry for evaluating age-related changes in memory lymphocyte subsets in dogs. Dev. Comp. Immunol. 2018, 87, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Yu, X.; Shen, L. Autoimmune thyroid diseases and Th17/Treg lymphocytes. Life Sci. 2018, 192, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Mcnair, A.; Phadwal, K.; MacRae, V.; Corcoran, B. The Role of Transforming Growth Factor Signaling in Myxomatous Mitral Valve Degeneration. Front. Cardiovasc. Med. 2022, 9, 872288. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Patel, J.; Mohamed, E.; Greene, M.; Moskovits, N.; Shani, J. The immunoregulatory role of cytokines in congestive heart failure. Interdiscip. J. Microinflamm. 2014, 1, 2. [Google Scholar]
- Dick, S.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Sanders-Van Wijk, S.; van Empel, V.; Davarzani, N.; Maeder, M.T.; Handschin, R.; Pfisterer, M.E.; Brunner-La Rocca, H.P.; Time-CHF investigators. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur. J. Hearth Fail. 2015, 17, 1006–1014. [Google Scholar] [CrossRef]
- Oral, H.; Sivasubramanian, N.; Dyke, D.B.; Mehta, R.H.; Grossman, P.M.; Briesmiester, K.; Fay, W.P.; Pagani, F.D.; Bolling, S.F.; Mann, D.L.; et al. Myocardial proinflammatory cytokine expression and left ventricular remodeling in patients with chronic mitral regurgitation. Circulation 2003, 107, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J. Vet. Intern. Med. 2009, 19, 537–541. [Google Scholar] [CrossRef]
- Zois, N.E.; Moesgaard, S.G.; Kjelgaard-Hansen, M.; Rasmussen, C.E.; Falk, T.; Fossing, C.; Häggström, J.; Pedersen, H.D.; Olsen, L.H. Circulating cytokine concentrations in dogs with different degrees of myxomatous mitral valve disease. Vet. J. 2012, 192, 106–111. [Google Scholar] [CrossRef]
- Mavropoulou, A.; Guazzetti, S.; Borghetti, P.; De Angelis, E.; Quintavalla, C. Cytokine expression in peripheral blood mononuclear cells of dogs with mitral valve disease. Vet. J. 2016, 2016, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Svete, A.; Verk, B.N.; Čebulj-Kadunc, N.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Inflammation and its association with oxidative stress in dogs with heart failure. BMC Vet. Res. 2021, 17, 176. [Google Scholar]
- Peres Rubio, C.; Saril, A.; Kocaturk, M.; Tanaka, R.; Koch, J.; Ceron, J.J.; Yilmaz, Z. Changes of inflammatory and oxidative stress biomarkers in dogs with different stages of heart failure. BMC Vet. Res. 2020, 16, 433. [Google Scholar]
- Lieb, W.; Sullivan, L.M.; Harris, T.B.; Roubenoff, R.; Benjamin, E.J.; Levy, D.; Fox, C.S.; Wang, T.J.; Wilson, P.W.; Kannel, W.B. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care 2009, 32, 612–616. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piantedosi, D.; Musco, N.; Palatucci, A.T.; Carriero, F.; Rubino, V.; Pizzo, F.; Nasir, S.; Molinaro, G.; Ruggiero, G.; Terrazzano, G.; et al. Pro-Inflammatory and Immunological Profile of Dogs with Myxomatous Mitral Valve Disease. Vet. Sci. 2022, 9, 326. https://doi.org/10.3390/vetsci9070326
Piantedosi D, Musco N, Palatucci AT, Carriero F, Rubino V, Pizzo F, Nasir S, Molinaro G, Ruggiero G, Terrazzano G, et al. Pro-Inflammatory and Immunological Profile of Dogs with Myxomatous Mitral Valve Disease. Veterinary Sciences. 2022; 9(7):326. https://doi.org/10.3390/vetsci9070326
Chicago/Turabian StylePiantedosi, Diego, Nadia Musco, Anna Teresa Palatucci, Flavia Carriero, Valentina Rubino, Francesco Pizzo, Saad Nasir, Giuseppe Molinaro, Giuseppina Ruggiero, Giuseppe Terrazzano, and et al. 2022. "Pro-Inflammatory and Immunological Profile of Dogs with Myxomatous Mitral Valve Disease" Veterinary Sciences 9, no. 7: 326. https://doi.org/10.3390/vetsci9070326
APA StylePiantedosi, D., Musco, N., Palatucci, A. T., Carriero, F., Rubino, V., Pizzo, F., Nasir, S., Molinaro, G., Ruggiero, G., Terrazzano, G., Lombardi, P., & Cortese, L. (2022). Pro-Inflammatory and Immunological Profile of Dogs with Myxomatous Mitral Valve Disease. Veterinary Sciences, 9(7), 326. https://doi.org/10.3390/vetsci9070326






