Histopathological Findings and Metagenomic Analysis of Esophageal Papillary Proliferation Identified in Laying Broiler Breeders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Histopathology
2.3. PCR Primers and Amplification of Papillomavirus
2.4. High-Throughput Sequencing
2.5. Bioinformatic Analysis
3. Results
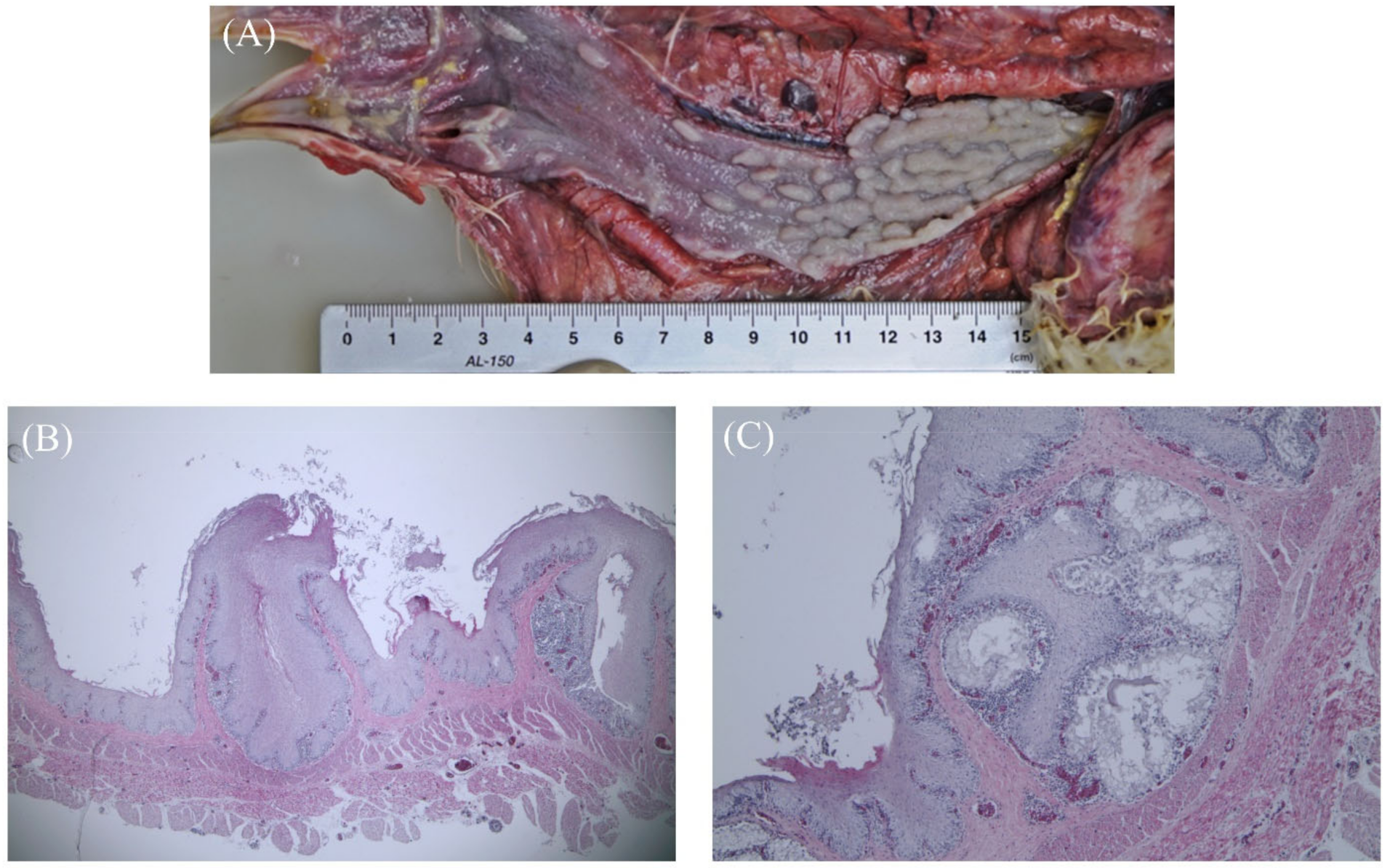
3.1. Gross Findings and Histopathology
3.2. Metagenomics Analysis
3.3. Definitive Diagnosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APQA | Animal and Plant Quarantine Agency |
| nt | Non-redundant nucleotide |
| PCR | Polymerase Chain Reaction |
| SCC | Squamous Cell Carcinoma |
References
- Bang, B.G.; Bang, F.B. Replacement of virus-destroyed epithelium by keratinized squamous cells in vitamin A-deprived chickens. Proc. Soc. Exp. Biol. Med. 1969, 132, 50–54. [Google Scholar] [CrossRef]
- Jones, A.; Suárez-Bonnet, A.; Mitchell, J.; Ramirez, G.; Stidworthy, M.; Priestnall, S. Avian papilloma and squamous cell carcinoma: A histopathological, immunohistochemical and virological study. J. Comp. Pathol. 2020, 175, 13–23. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Reavill, D.R.; David, N. Phalen Gastrointestinal System and Pancreas. In Pathology of Pet and Aviary Birds, 2nd ed.; Iowa State Press: Ames, IA, USA, 2015; pp. 55–94. [Google Scholar]
- Susan, M.; Williams, R.L.R. Scott Hafner. Neoplastic Disease: Other Tumors. In Disease of Poultry, 14th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 637–657. [Google Scholar]
- Munday, J.S.; Kiupel, M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet. Pathol. 2010, 47, 254–264. [Google Scholar] [CrossRef] [Green Version]
- David, E.; Swayne, M.B.; Catherine, M.; Logue Larry, R.; McDougald, V.N.; David, L.S. Diseases of Poultry, 14th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; Volume 1. [Google Scholar]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin. Infect. Dis. 2017, 64, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin. Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef]
- Pérez-Tris, J.; Williams, R.; Abel-Fernández, E.; Barreiro, J.; Conesa, J.; Figuerola, J.; Martinez-Martinez, M.; Ramirez, A.; Benitez, L. A multiplex PCR for detection of poxvirus and papillomavirus in cutaneous warts from live birds and museum skins. Avian Dis. 2011, 55, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, D.W.; Zagordi, O.; Zbinden, A.; Schuurmans, M.M.; Schreiber, P.; Geissberger, F.-D.; Huder, J.B.; Böni, J.; Benden, C.; Mueller, N.J. Unbiased metagenomic sequencing complements specific routine diagnostic methods and increases chances to detect rare viral strains. Diagn. Microbiol. Infect. Dis. 2015, 83, 133–138. [Google Scholar] [CrossRef]
- Hunt, D.E.; Klepac-Ceraj, V.; Acinas, S.G.; Gautier, C.; Bertilsson, S.; Polz, M.F. Evaluation of 23S rRNA PCR primers for use in phylogenetic studies of bacterial diversity. Appl. Environ. Microbiol. 2006, 72, 2221–2225. [Google Scholar] [CrossRef] [Green Version]
- Sabat, A.J.; van Zanten, E.; Akkerboom, V.; Wisselink, G.; van Slochteren, K.; de Boer, R.F.; Hendrix, R.; Friedrich, A.W.; Rossen, J.W.; Kooistra-Smid, A.M.M. Targeted next-generation sequencing of the 16S-23S rRNA region for culture-independent bacterial identification-increased discrimination of closely related species. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.; Penn, C.W.; Pallen, M.J. High-throughput sequencing of 16S rRNA gene amplicons: Effects of extraction procedure, primer length and annealing temperature. PLoS ONE 2012, 7, e38094. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. bioRxiv 2018, 274100. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI Reference Sequence (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2005, 33, D501–D504. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Freeman, S.S.; Herrera, A.F.; Pedamallu, C.S.; Gevers, D.; Duke, F.; Jung, J.; Michaud, M.; Walker, B.J.; Young, S. Sequence-based discovery of Bradyrhizobium enterica in cord colitis syndrome. N. Engl. J. Med. 2013, 369, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.K.; Masia, R. Cord colitis syndrome: A cause of granulomatous inflammation in the upper and lower gastrointestinal tract. Am. J. Surg. Pathol. 2013, 37, 1109. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.F.; Soriano, G.; Bellizzi, A.M.; Hornick, J.L.; Ho, V.T.; Ballen, K.K.; Baden, L.R.; Cutler, C.S.; Antin, J.H.; Soiffer, R.J. Cord colitis syndrome in cord-blood stem-cell transplantation. N. Engl. J. Med. 2011, 365, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Hatkin, J.; Styer, E.; Miller, D. Ingluvial squamous cell carcinoma in a game chicken. Avian Dis. 2002, 46, 1070–1075. [Google Scholar] [CrossRef]
- Ramis, A.; Gibert, X.; Majo, N.; Grifols, J. Metastatic oral squamous cell carcinoma in a Montagu’s harrier (Circus pigargus). J. Vet. Diagn. Investig. 1999, 11, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.I.; Steinberg, H. Primary glossal squamous-cell carcinoma in a Spanish Cochin hen. Avian Dis. 1989, 33, 827–828. [Google Scholar] [CrossRef]
- Murtaugh, R.; Ringler, D.; Petrak, M. Squamous cell carcinoma of the esophagus in an Amazon parrot. J. Am. Vet. Med. Assoc. 1986, 188, 872–873. [Google Scholar]
- Vázquez, S.; Quiroga, M.; Aleman, N.; García, J.; López-Peña, M.; Nieto, J. Squamous cell carcinoma of the oropharynx and esophagus in a Japanese bantam rooster. Avian Dis. 2003, 47, 215–217. [Google Scholar] [CrossRef]
- Laura, N.; Marta, V.; Giacomo, B.; Luca, B. Squamous cell carcinoma of the oropharynx and esophagus with pulmonary metastasis in a backyard laying hen. Avian Dis. 2016, 60, 694–697. [Google Scholar]
- Rettenmund, C.L.; Newton, A.L.; Calle, P.P. Uropygial gland squamous cell carcinoma in chinstrap (Pygoscelis antarcticus) and gentoo (Pygoscelis papua) penguins at the Wildlife Conservation Society’s Central Park Zoo. J. Zoo Wildl. Med. 2015, 46, 113–119. [Google Scholar] [CrossRef]
- Shivaprasad, H.; Barnes, H. Integumentary system. Avian Histopathol. 2008, 1, 402–403. [Google Scholar]
- Nesheim, M.C.; Austic, R.E.; Cand, L. Poultry Production, 12th ed.; Lea and Febiger: Philadelphia, PA, USA, 1979. [Google Scholar]
- Friedman, A.; Meidovsky, A.; Leitner, G.; Sklan, D. Decreased resistance and immune response to Escherichia coli infection in chicks with low or high intakes of vitamin A. J. Nutr. 1991, 121, 395–400. [Google Scholar] [CrossRef]


| Bacteria | Reads | |
|---|---|---|
| Esophageal Papillary Proliferation (% Total Reads) | Control (% Total Reads) | |
| Bradyrhizobium sp. | 48,023 (85.1) | - |
| Sphingomonas sp. | 4565 (8.1) | - |
| Gordonia sp. | 3400 (6) | - |
| Ralstonia pickettii | 261 (0.5) | - |
| Clostridium beijerinckii | 3 (<0.1) | - |
| Lactobacillaceae | 2 (<0.1) | 39,355 (49.2) |
| Escherichia coli | 2 (<0.1) | 302 (0.4) |
| Staphylococcus sp. | - | 9303 (11.6) |
| Veillonella sp. | - | 6423 (8) |
| Bifidobacterium sp. | - | 5927 (7.4) |
| Corynebacterium sp. | - | 1693 (2.1) |
| Kurthia sp. | - | 1659 (2.1) |
| Gallibacterium anatis | - | 1409 (1.8) |
| Rothia sp. | - | 152 (0.2) |
| Enterococcus | - | 12 (<0.1) |
| Uncultured bacterium | 156 (0.3) | 13,778 (17.2) |
| Total | 56,412 | 80,013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Song, H.-S.; Kim, C.-H.; Kwon, Y.-K.; Park, C.-K.; Kim, H.-R. Histopathological Findings and Metagenomic Analysis of Esophageal Papillary Proliferation Identified in Laying Broiler Breeders. Vet. Sci. 2022, 9, 332. https://doi.org/10.3390/vetsci9070332
Kim S-H, Song H-S, Kim C-H, Kwon Y-K, Park C-K, Kim H-R. Histopathological Findings and Metagenomic Analysis of Esophageal Papillary Proliferation Identified in Laying Broiler Breeders. Veterinary Sciences. 2022; 9(7):332. https://doi.org/10.3390/vetsci9070332
Chicago/Turabian StyleKim, Si-Hyeon, Hye-Soon Song, Chung-Hyun Kim, Yong-Kuk Kwon, Choi-Kyu Park, and Hye-Ryoung Kim. 2022. "Histopathological Findings and Metagenomic Analysis of Esophageal Papillary Proliferation Identified in Laying Broiler Breeders" Veterinary Sciences 9, no. 7: 332. https://doi.org/10.3390/vetsci9070332
APA StyleKim, S.-H., Song, H.-S., Kim, C.-H., Kwon, Y.-K., Park, C.-K., & Kim, H.-R. (2022). Histopathological Findings and Metagenomic Analysis of Esophageal Papillary Proliferation Identified in Laying Broiler Breeders. Veterinary Sciences, 9(7), 332. https://doi.org/10.3390/vetsci9070332





