Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020–2021 Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. HPAI Surveillance
2.2. Sampling
2.3. PCR and Whole Genome Sequencing
2.4. Epidemiological Investigation
3. Results
3.1. Outbreaks
3.2. Virology and Phylogeny
3.3. Epidemiological Investigation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Available online: https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=202103956 (accessed on 14 May 2022).
- Alarcon, P.; Brouwer, A.; Venkatesh, D.; Duncan, D.; Dovas, C.I.; Georgiades, G.; Monne, I.; Fusaro, A.; Dan, A.; Śmietanka, K.; et al. Comparison of 2016–17 and previous epizootics of highly pathogenic avian influenza H5 Guangdong lineage in Europe. Emerg. Infect. Dis. 2018, 24, 2270–2283. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly pathogenic avian influenza viruses at the wild–domestic bird interface in Europe: Future directions for research and surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Lycett, S.J.; Duchatel, F.; Digard, P. A brief history of bird flu. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180257. [Google Scholar] [CrossRef]
- The Global Consortium for H5N8 and Related Influenza Viruses. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Bodewes, R.; Kuiken, T. Changing role of wild birds in the epidemiology of avian influenza A viruses. Adv. Virus Res. 2018, 100, 279–307. [Google Scholar] [CrossRef]
- Zohari, S.; Gyarmati, P.; Thorén, P.; Czifra, G.; Bröjer, C.; Belák, S.; Berg, M. Genetic characterization of the NS gene indicates co-circulation of two sub-lineages of highly pathogenic avian influenza virus of H5N1 subtype in Northern Europe in 2006. Virus Genes 2008, 36, 117–125. [Google Scholar] [CrossRef]
- National Veterinary Institute. Surveillance of Infectious Diseases in Animals and Humans in Sweden in 2015. Avian Influenza; National Veterinary Institute: Uppsala, Sweden, 2016; pp. 44–48. ISSN 1654-7098. Available online: https://www.sva.se/media/bfcezuvg/surveillance-2015.pdf (accessed on 1 May 2022).
- Jansson, D.; Bröjer, C.; Zohari, S.; Otman, F.; Jeremiasson, M.; Hestvik, G.; Uhlhorn, H. Pathology findings in poultry and free-living wild birds naturally infected with HPAIV H5N8. In Proceedings of the XX World Veterinary Poultry Association Congress (WVPAC), Edinburgh, UK, 3–8 September 2017. WVPA 2017-451. [Google Scholar] [CrossRef]
- Jansson, D.S.; Otman, F.; Bagge, E.; Lindgren, Y.; Engelsen Etterlin, P.; Eriksson, H. Retrospective analysis of post-mortem findings in domestic ducks and geese from non-commercial flocks in Sweden, 2011–2020. Acta Vet. Scand. 2021, 63, 47. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control European Reference Laboratory for Avian Influenza. Scientific report: Avian influenza overview September–December 2021. EFSA J. 2021, 19, 7108. [Google Scholar] [CrossRef]
- Swedish Board of Agriculture. Lantbrukets djur i Sverige i Juni 2020. Available online: https://web.archive.org/web/20220608122644; https://jordbruksverket.se/om-jordbruksverket/jordbruksverkets-officiella-statistik/jordbruksverkets-statistikrapporter/statistik/2021-01-29-lantbrukets-djur-i-juni-2020-slutlig-statistik (accessed on 8 June 2022).
- European Commission, Agriculture and Rural Development. 2021. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/eggs-dashboard_en.pdf (accessed on 15 May 2022).
- Swedish Board of Agriculture. Slakt av Fjäderfä vid Slakteri 1995–2021. Available online: https://web.archive.org/web/20220608131229; https://statistik.sjv.se/PXWeb/pxweb/sv/Jordbruksverkets%20statistikdatabas/Jordbruksverkets%20statistikdatabas__Animalieproduktion__Slakt/JO0604A5.px/?rxid=5adf4929-f548-4f27-9bc9-78e127837625 (accessed on 8 June 2022).
- European Commission, Agriculture, Forestry and Fishery Statistics. 2020. Available online: https://ec.europa.eu/eurostat/documents/3217494/12069644/KS-FK-20-001-EN-N.pdf/a7439b01-671b-80ce-85e4-4d803c44340a?t=1608139005821 (accessed on 2 June 2022).
- Ottosson, U.; Ottvall, R.; Elmberg, J.; Green, M.; Gustafsson, R.; Haas, F.; Holmqvist, N.; Lindström, Å.; Nilsson, L.; Svensson, M.; et al. Fåglarna i Sverige–Antal och Förekomst; Sveriges Ornitologiska Förening: Halmstad, Sweden, 2012; pp. 25–28. ISBN 978-91-88124-00-5. [Google Scholar]
- Green, M.; Haas, F.; Lindström, Å.; Nilsson, L. Monitoring Population Changes of Birds in Sweden. Annual Report for 2020; Department of Biology, Lund University: Lund, Sweden, 2021; pp. 1–93. Available online: https://www.fageltaxering.lu.se/sites/default/files/files/Rapporter/arsrapportfor2020kf.pdf (accessed on 8 May 2022).
- EUR-Lex. Council Directive 2005/94/EC of 20 December 2005 on Community Measures for the Control of Avian Influenza and Repealing Directive 92/40/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32005L0094 (accessed on 5 June 2022).
- Spackman, E.; Senne, D.A.; Bulaga, L.L.; Myers, T.J.; Perdue, M.L.; Garber, L.P.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of real-time RT-PCR for the detection of avian influenza virus. Avian Dis. 2003, 47, 1079–1082. [Google Scholar] [CrossRef]
- Slomka, M.J.; Pavlidis, T.; Coward, V.J.; Voermans, J.; Koch, G.; Hanna, A.; Banks, J.; Brown, I.H. Validated RealTime reverse transcriptase PCR methods for the diagnosis and pathotyping of Eurasian H7 avian influenza viruses. Influenza Other Respir. Viruses 2009, 3, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; To, T.L.; Tong, H.H.; Coward, V.J.; Hanna, A.; Shell, W.; Pavlidis, T.; Densham, A.L.E.; Kargiolakis, G.; Arnold, M.E.; et al. Challenges for accurate and prompt molecular diagnosis of clades of highly pathogenic avian influenza H5N1 viruses emerging in Vietnam. Avian Pathol. 2012, 41, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Hoffmann, D.; Henritzi, D.; Beer, M.; Harder, T.C. Riems influenza a typing array (RITA): An RT-qPCR-based low density array for subtyping avian and mammalian influenza A viruses. Sci. Rep. 2016, 6, 27211. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Coward, V.J.; Banks, J.; Londt, B.Z.; Brown, I.H.; Voermans, J.; Koch, G.; Handberg, K.J.; Jorgensen, P.H.; Cherbonnel-Pansart, M.; et al. Identification of sensitive and specific avian influenza polymerase chain reaction methods through blind ring trials organized in the European Union. Avian Dis. 2007, 51, 227–234. [Google Scholar] [CrossRef]
- Naguib, M.M.; Graaf, A.; Fortin, A.; Luttermann, C.; Wernery, U.; Amarin, N.; Hussein, H.A.; Sultan, H.; Al Adhadh, B.; Hassan, M.K.; et al. Novel real-time PCR-based patho- and phylotyping of potentially zoonotic avian influenza A subtype H5 viruses at risk of incursion into Europe in 2017. Euro Surveill. 2017, 22, 30435. [Google Scholar] [CrossRef]
- Zhou, B.; Donnelly, M.E.; Scholes, D.T.; George, K.S.; Hatta, M.; Kawaoka, Y.; Wentworth, D.E. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza a viruses. J. Virol. 2009, 83, 10309–10313. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- European Commission. Animal Disease Information System (ADIS). Available online: https://ec.europa.eu/food/animals/animal-diseases/animal-disease-information-system-adis_en#animal-disease-information (accessed on 3 June 2022).
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Reference Laboratory for Avian Influenza. Scientific report: Avian influenza overview May–September 2021. EFSA J. 2022, 20, 7122. [Google Scholar] [CrossRef]
- Swedish Board of Agriculture. Lantbrukets Djur i Juni 2021. Available online: https://web.archive.org/web/20220609145024/; https://jordbruksverket.se/om-jordbruksverket/jordbruksverkets-officiella-statistik/jordbruksverkets-statistikrapporter/statistik/2021-10-14-lantbrukets-djur-i-juni-2021 (accessed on 9 June 2022).
- SLU Artdatabanken. Rödlistade Arter i Sverige 2020. 2020. ISBN 978-91-87853-55-5 (pdf). Available online: https://www.artdatabanken.se/globalassets/ew/subw/artd/2.-var-verksamhet/publikationer/31.-rodlista-2020 (accessed on 15 May 2022).
- Simeonov, S.; Milchev, B.; Boev, Z. Study of the Eagle Owl (Bubo bubo (L.)) (Aves: Strigiformes) in the Strandzha mountain (Southeast Bulgaria). II. Food spectrum and trophic specialization. Acta Zool. Bulg. 1998, 50, 87–100. [Google Scholar]
- Globig, A.; Staubach, C.; Sauter-Louis, C.; Dietze, K.; Homeier-Bachmann, T.; Probst, C.; Gethmann, J.; Depner, K.R.; Grund, C.; Harder, T.C.; et al. Highly pathogenic avian influenza H5N8 Clade 2.3.4.4b in Germany in 2016/2017. Front. Vet. Sci. 2017, 4, 240. [Google Scholar] [CrossRef]
- Śmietanka, K.; Śweiętoń, E.; Wyrostek, K.; Kozak, E.; Tarasiuk, K.; Styś-Fijoł, N.; Dziadek, K.; Niemczuk, K. Highly pathogenic avian influenza H5Nx in Poland in 2020/2021: A descriptive epidemiological study of a large-scale epidemic. J. Vet. Res. 2022, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.L.; Stegeman, J.A.; Koch, G.; de Wit, S.J.; Elbers, A.R. Rate of introduction of a low pathogenic avian influenza virus infection in different poultry production sectors in the Netherlands. Influenza Other Respir. Viruses 2013, 7, 6–10. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Animal Health and Welfare. Statement on urgent request on avian influenza. EFSA J. 2017, 15, 4687. [Google Scholar] [CrossRef][Green Version]
- Hansson, I.; Engvall, E.O.; Vågsholm, I.; Nyman, A. Risk factors associated with the presence of Campylobacter-positive broiler flocks in Sweden. Prev. Vet. Med. 2010, 96, 114–121. [Google Scholar] [CrossRef]
- Eriksson, H.; Jansson, D.; Ågren, E. Associations between housing system, facilities, management and biosecurity routines in layer flocks using Additive Bayesian Networks. In Proceedings of the XXI Veterinary Poultry Association Congress (WVPAC), Bangkok, Thailand, 16–20 September 2019; p. 431. [Google Scholar]
- Guinat, C.; Nicolas, G.; Vergne, T.; Bronner, A.; Durand, B.; Courcoul, A.; Gilbert, M.; Guerin, J.L.; Paul, M.C. Spatio-temporal patterns of highly pathogenic avian influenza virus subtype H5N8 spread, France, 2016 to 2017. Euro Surveill. 2018, 23, 1700791. [Google Scholar] [CrossRef] [PubMed]
- Guinat, C.; Comin, A.; Kratzer, G.; Durand, B.; Delesalle, L.; Delpont, M.; Guerin, J.L.; Paul, M.C. Biosecurity risk factors for highly pathogenic avian influenza (H5N8) virus infection in duck farms, France. Transbound. Emerg. Dis. 2020, 67, 2961–2970. [Google Scholar] [CrossRef]
- Björnham, O.; Sigg, R.; Burman, J. Multilevel model for airborne transmission of foot-and-mouth disease applied to Swedish livestock. PLoS ONE 2020, 15, e0232489. [Google Scholar] [CrossRef]
- Ypma, R.J.; Jonges, M.; Bataille, A.; Stegeman, A.; Koch, G.; van Boven, M.; Koopmans, M.; van Ballegooijen, W.M.; Wallinga, J. Genetic data provide evidence for wind-mediated transmission of highly pathogenic avian influenza. J. Infect. Dis. 2013, 207, 730–735. [Google Scholar] [CrossRef]
- Torremorell, M.; Alonso, C.; Davies, P.R.; Raynor, P.C.; Patnayak, D.; Torchetti, M.; McCluskey, B. Investigation into the airborne dissemination of H5N2 highly pathogenic avian influenza virus during the 2015 spring outbreaks in the Midwestern United States. Avian Dis. 2016, 60, 637–643. [Google Scholar] [CrossRef]
- Scoizec, A.; Niqueux, E.; Thomas, R.; Daniel, P.; Schmitz, A.; Le Bouquin, S. Airborne detection of H5N8 highly pathogenic avian influenza virus genome in poultry farms, France. Front. Vet. Sci. 2018, 5, 15. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L.; Sims, L.D. Influenza. In Diseases of Poultry, 14th ed.; Swayne, D.E., Bouliannw, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., de Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2020; Volume 1, pp. 210–256. ISBN 9781119371168. [Google Scholar]
- Aldous, E.W.; Seekings, J.M.; McNally, A.; Nili, H.; Fuller, C.M.; Irvine, R.M.; Alexander, D.J.; Brown, I.H. Infection dynamics of highly pathogenic avian influenza and virulent avian paramyxovirus type 1 viruses in chickens, turkeys and ducks. Avian Pathol. 2010, 39, 265–273. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare. Scientific opinion on avian influenza. EFSA J. 2017, 15, 4991. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, R.; Ramis, A.; Nofrarias, M.; Wali, N.; Valle, R.; Perez, M.; Perlas, A.; Majo, N. Pathobiology of the highly pathogenic avian influenza viruses H7N1 and H5N8 in different chicken breeds and role of Mx 2032 G/A polymorphism in infection outcome. Vet. Res. 2020, 51, 113. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, F.; Fornasiero, D.; De Marco, M.A.; Zecchin, B.; Mulatti, P.; Delogu, M.; Terregino, C. Active surveillance for highly pathogenic avian influenza viruses in wintering waterbirds in Northeast Italy, 2020–2021. Microorganisms 2021, 9, 2188. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.E.; Ribble, C.; Stephen, C.; Kelton, D.; Toews, L.; Osterhold, J.; Wheeler, H. Use of observed wild bird activity on poultry farms and a literature review to target species as high priority for avian influenza testing in 2 regions of Canada. Can. Vet. J. 2012, 53, 158–166. [Google Scholar]
- Elbers, A.R.W.; Gonzales, J.L. Quantification of visits of wild fauna to a commercial free-range layer farm in the Netherlands located in an avian influenza hot-spot area assessed by video-camera monitoring. Transbound. Emerg. Dis. 2020, 67, 661–677. [Google Scholar] [CrossRef]
- Engström, B.; Jansson, D.; Carlsson, U.; Sternberg Lewerin, S. Questionnaire study among Swedish poultry producers focussing on wild birds as a source of contagious diseases. Svensk VetTidn. 2007, 95, 11–17. [Google Scholar]
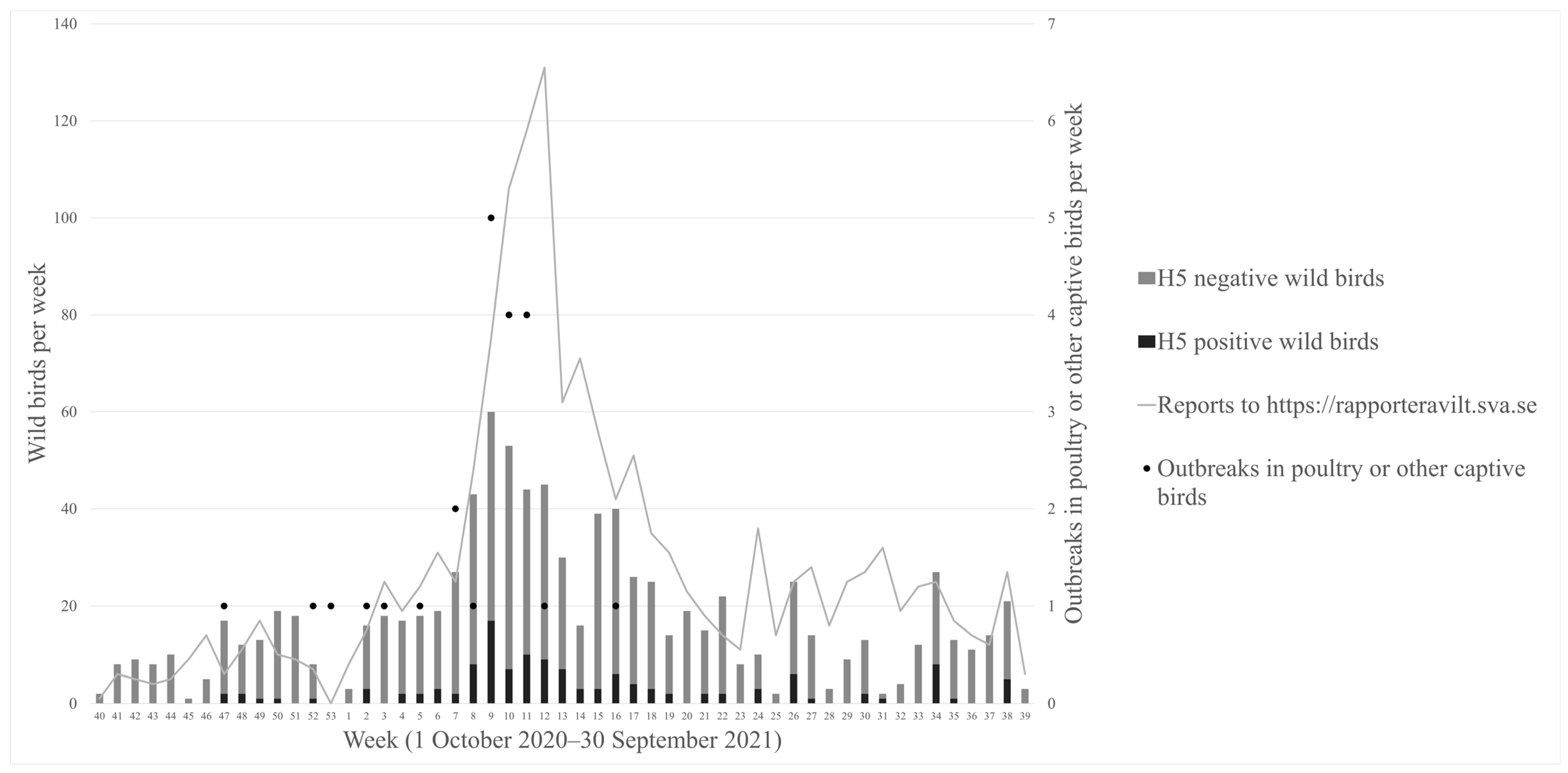

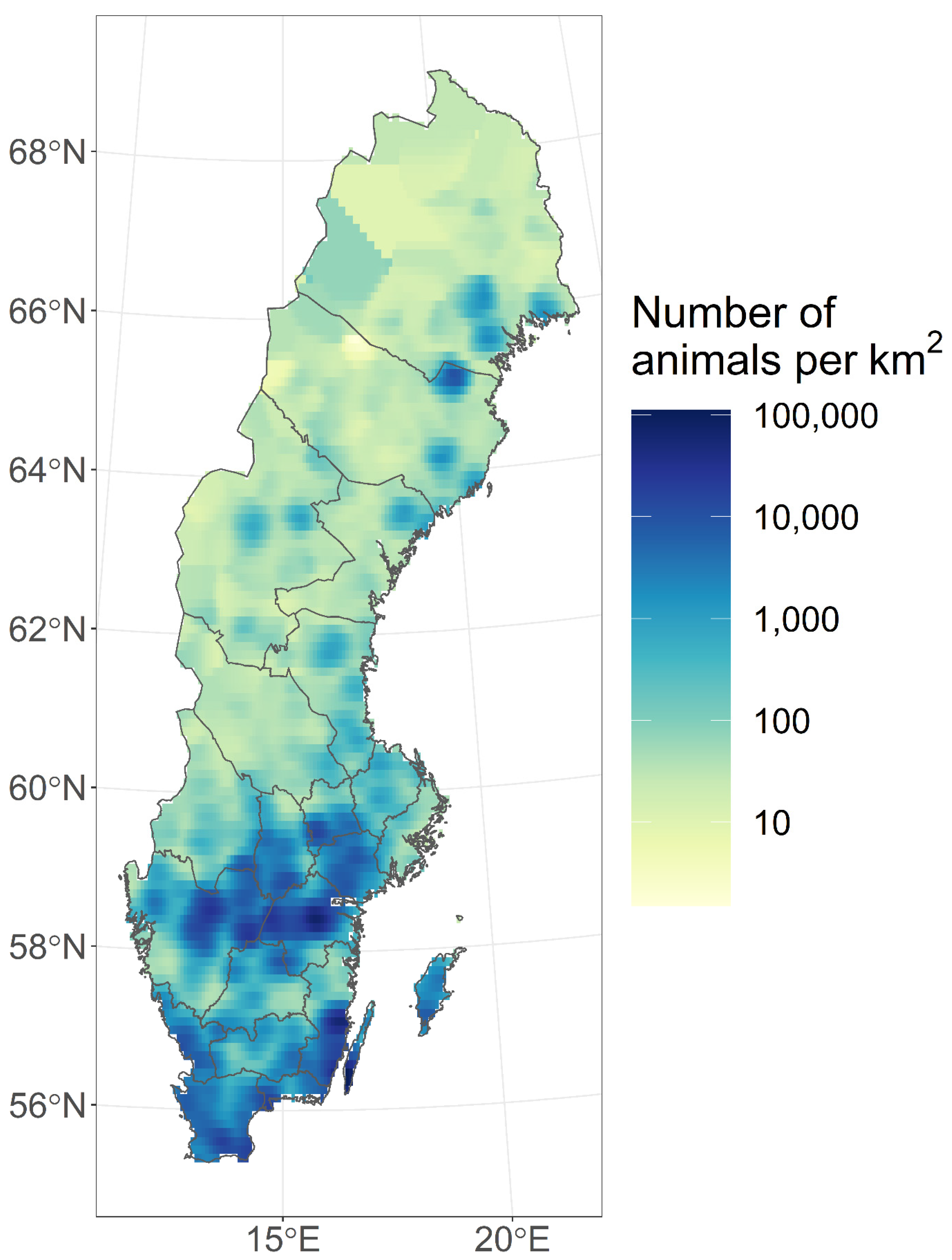

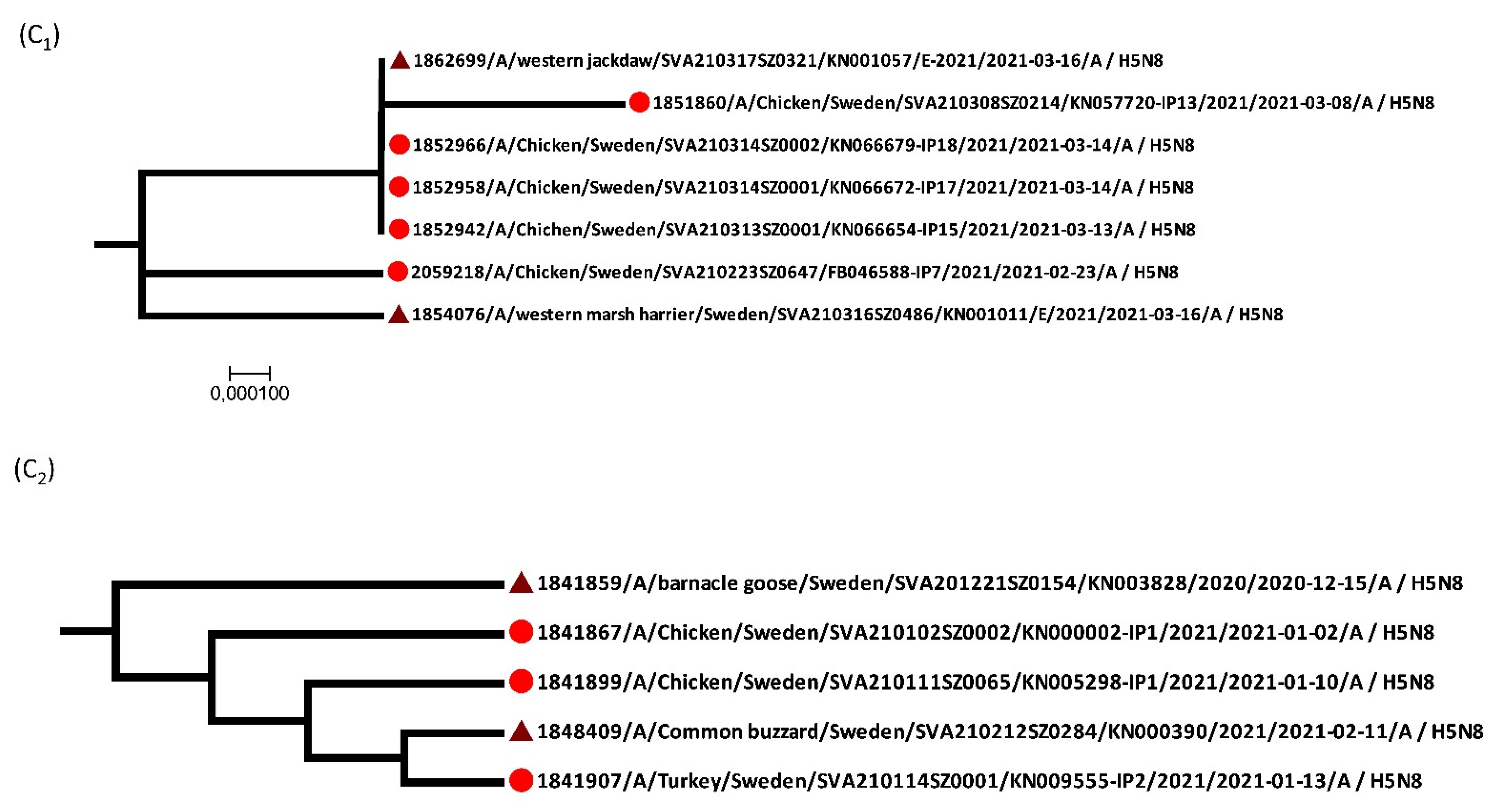


| Bird Species (English) | Bird Species (Latin) | Positive/Tested |
|---|---|---|
| Barnacle goose | Branta leucopsis | 15/19 |
| Peregrine falcon1 | Falco peregrinus | 12/22 |
| Common buzzard | Buteo buteo | 12/36 |
| Eagle owl 1 | Bubo bubo | 11/22 |
| Mute swan | Cygnus olor | 11/35 |
| Northern goshawk | Accipiter gentilis | 9/34 |
| Common eider | Somateria mollissima | 8/10 |
| Whooper swan | Cygnus cygnus | 7/16 |
| Canada goose | Branta canadensis | 7/14 |
| White-tailed eagle 1 | Haliaeetus albicilla | 7/90 |
| Mallard | Anas platyrhynchos | 6/37 |
| Common pheasant | Phasianus colchicus | 5/13 |
| Greylag goose | Anser anser | 4/11 |
| Herring gull | Larus argentatus | 3/18 |
| Tawny owl | Strix aluco | 2/18 |
| Common goldeneye | Bucephala clangula | 2/3 |
| Eurasian oystercatcher | Haematopus ostralegus | 2/2 |
| Great white-fronted goose | Anser albifrons | 1/1 |
| Western marsh harrier1 | Circus aeruginosus | 1/1 |
| Western jackdaw | Corvus monedula | 1/31 |
| Hooded crow | Corvus cornix | 1/16 |
| Black-headed gull | Chroicocephalus ridibundus | 1/12 |
| Bean goose | Anser fabalis | 1/2 |
| Common kestrel1 | Falco tinnunculus | 1/16 |
| Bird Category | No. of Farms | No. Susceptible Birds | ||
|---|---|---|---|---|
| H5N8 | H5N5 | Total | ||
| Laying hens (aviary, indoor) | 2 | 1 | 3 | 1,250,000 |
| Laying hens (organic) | 3 | 1 | 4 | 85,000 |
| Layer pullets | 0 | 1 | 1 | 735,000 |
| Broiler parents | 2 | 0 | 2 | 138,000 |
| Broilers (organic) | 1 | 0 | 1 | 14,300 |
| Turkeys (meat-type) | 4 | 0 | 4 | 41,000 |
| Game pheasants | 1 | 0 | 1 | 500 |
| Non-commercial poultry | 4 | 3 | 7 | 450 |
| Zoological collection (mixed poultry species) | 1 | 0 | 1 | 38 |
| Total | 18 | 6 | 24 | 2,264,288 |
| No./Case ID | Poultry Category |
No of Susceptible Animals on Farm | Initially Affected Flock | Date of Diagnosis | Subtype | County | |
|---|---|---|---|---|---|---|---|
| Flock Size | Age | ||||||
| 1 (1/2020) | Turkeys (meat-type) | 5100 | 2000 | 12 weeks | 14 November | H5N8 | Skåne |
| 2 (2/2020) | Hobby chickens | 30 | 30 | Adults | 22 December | H5N8 | Skåne |
| 3 (IP1) | Broiler breeders (parents) | 84,850 | 4300 | 28 weeks | 3 January | H5N8 | Skåne |
| 4 (IP2) | Turkeys (meat-type) | 2350 | 850 | 16/17 weeks | 14 January | H5N8 | Skåne |
| 5 (IP3) 1 | Laying hens (aviary, indoor) | 1,200,000 | 85,000 | 42 weeks | 18 January | H5N5 | Kalmar |
| 6 (IP4) 1 | Layer pullets | 735,000 | 245,000 | 16 weeks | 1 February | H5N5 | Kalmar |
| 7 (IP5) | Turkeys (meat-type) | 3500 | 3500 | 12 weeks | 15 February | H5N8 | Skåne |
| 8 (IP6) | Hobby chickens and ducks | 46 | 46 | Adults | 17 February | H5N8 | Västra Götaland |
| 9 (IP7) | Broiler (organic) 2 | 14,300 | 4880 | 60 d | 24 February | H5N8 | Östergötland |
| 10 (IP8) | Game pheasants | 470 | 470 | Unknown | 24 February | H5N8 | Skåne |
| 11 (IP9) | Hobby chickens | 11 | 11 | Adults | 28 February | H5N5 | Skåne |
| 12 (IP10) | Hobby mixed species 3 | 263 | 263 | Mixed ages | 1 March | H5N8 | Halland |
| 13 (IP11) | Zoological collection 4 | 38 | 38 | Adults | 2 March | H5N8 | Skåne |
| 14 (IP12) | Laying hens (organic) | 18,000 | 18,000 | 80 weeks | 3 March | H5N5 | Skåne |
| 15 (IP13) | Laying hens (organic) | 24,000 | 24,000 | 64 weeks | 8 March | H5N8 | Östergötland |
| 16 (IP14) | Hobby mixed species 5 | 33 | 33 | Adults | 11 March | H5N5 | Skåne |
| 17 (IP15) | Laying hens (aviary, indoor) | 33,000 | 33,000 | 53 weeks | 13 March | H5N8 | Östergötland |
| 18 (IP16) | Broiler breeder parents | 53,200 | 13,300 | 36 weeks | 14 March | H5N8 | Skåne |
| 19 (IP17) | Laying hens (aviary, indoor) | 21,000 | 21,000 | 22 weeks | 15 March | H5N8 | Östergötland |
| 20 (IP18) | Laying hens (organic) | 26,400 | 18,000/8400 6 | 68 weeks | 15 March | H5N8 | Östergötland |
| 21 (IP19) | Turkeys (meat-type) | 30,000 | 2300/2300 6 | 11/18 weeks | 16 March | H5N8 | Skåne |
| 22 (IP20) | Hobby mixed species 7 | 63 | 63 | Adults | 21 March | H5N8 | Skåne |
| 23 (IP21) | Hobby chickens and ducks | 14 | 14 | Adults | 23 March | H5N5 | Stockholm |
| 24 (IP22) | Laying hens (organic) | 18,000 | 18,000 | 42 weeks | 20 April | H5N8 | Skåne |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, M.; Bröjer, C.; Zohari, S.; Nöremark, M.; Uhlhorn, H.; Jansson, D.S. Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020–2021 Season. Vet. Sci. 2022, 9, 344. https://doi.org/10.3390/vetsci9070344
Grant M, Bröjer C, Zohari S, Nöremark M, Uhlhorn H, Jansson DS. Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020–2021 Season. Veterinary Sciences. 2022; 9(7):344. https://doi.org/10.3390/vetsci9070344
Chicago/Turabian StyleGrant, Malin, Caroline Bröjer, Siamak Zohari, Maria Nöremark, Henrik Uhlhorn, and Désirée S. Jansson. 2022. "Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020–2021 Season" Veterinary Sciences 9, no. 7: 344. https://doi.org/10.3390/vetsci9070344
APA StyleGrant, M., Bröjer, C., Zohari, S., Nöremark, M., Uhlhorn, H., & Jansson, D. S. (2022). Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020–2021 Season. Veterinary Sciences, 9(7), 344. https://doi.org/10.3390/vetsci9070344






