Effects of Intrauterine Infusion of Micronised Purified Flavonoid Fraction (MPFF) in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum
Abstract
Simple Summary
Abstract
1. Introduction
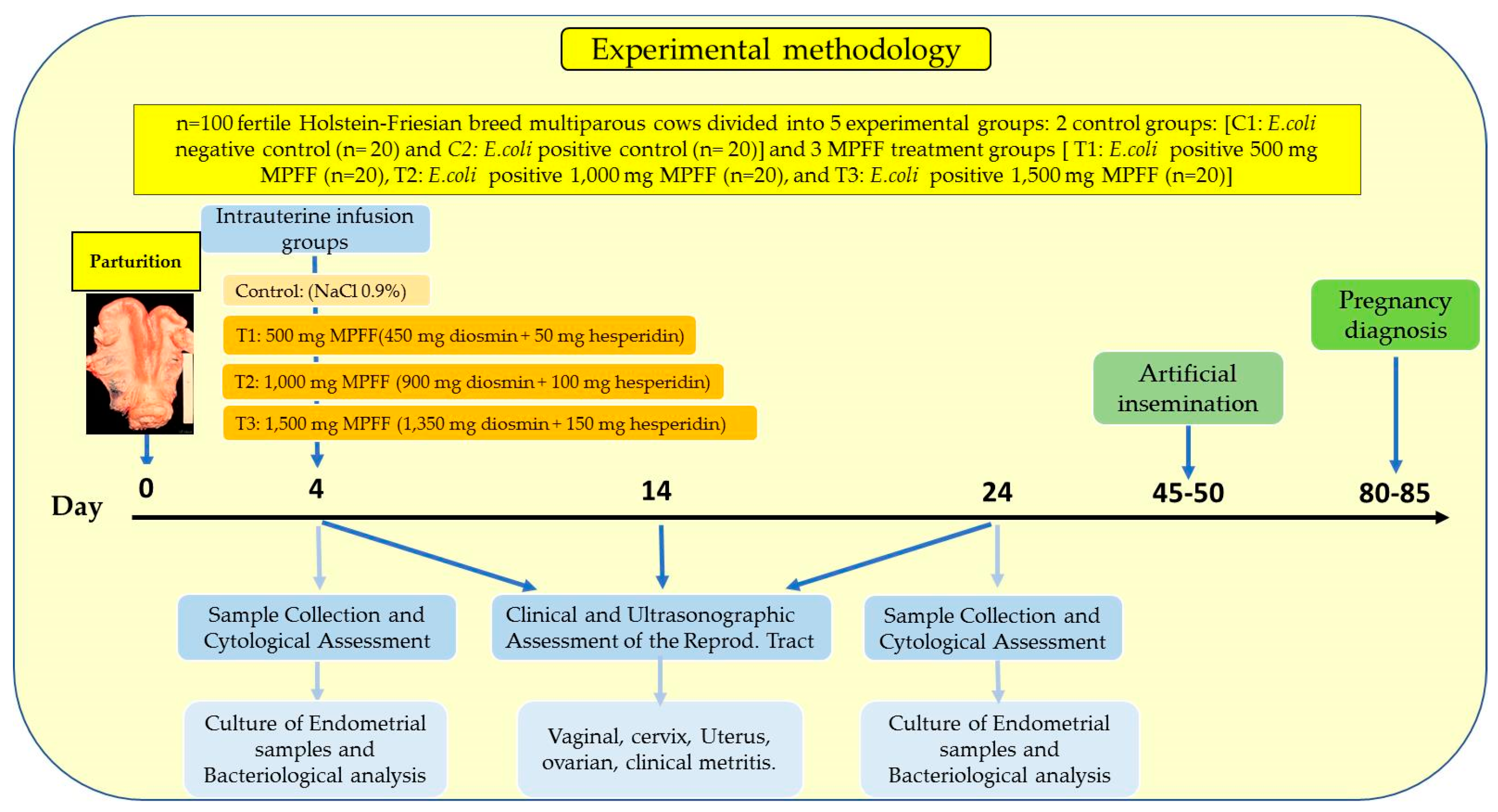
2. Materials and Methods
2.1. Reagents and Media
2.2. Environmental Conditions, Experimental Animals and Facilities
2.3. Experimental Design
2.4. Experimental MPFF Intrauterine Infusion Treatments
2.5. Clinical and Ultrasonographic Reproductive Tract Evaluation
2.6. Sample Collection and Cytological Assessment
2.7. Culture of Endometrial Samples and Bacteriological Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of MPFF Intrauterine Infusion on the Success Rate (% Cure) in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum Period
3.2. Effect of MPFF Intrauterine Treatments on Reproductive Tract Anatomical Dynamics in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum Period
3.3. Effect of MPFF Intrauterine Application on Uterine Bacteriological Profiles in Metritis-Diagnosed Dairy Cows during the Early Postpartum Period
3.4. Effects of MPFF Intrauterine Treatments on Uterine Cytological Characteristics in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum Period
3.5. Effects of MPFF Intrauterine Application during the Early Postpartum Period on the Reproductive Performance Parameters in Metritis-Diagnosed Dairy Cows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, I.M.; Dobson, H. Postpartum Uterine Health in Cattle. Anim. Reprod. Sci. 2004, 82, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Elmetwally, M.A. Uterine Involution and Ovarian Activity in Postpartum Holstein Dairy Cows. A Review. J. Vet. Healthc. 2018, 1, 29–40. [Google Scholar] [CrossRef]
- Arthur, G.H.; Noakes, D.E.; Pearson, H.; Arthur, G.H. Veterinary Reproduction and Obstetrics: The Riogenology, 6th ed.; Baillière Tindall: London, UK, 1989; ISBN 9780702012884. [Google Scholar]
- Hafez, S.E. Reproduction in Farm Animals, 7th ed.; Lea Febiger: Philadelphia, PA, USA, 2000. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Osawa, T.; Dubuc, J. Reproductive Tract Defense and Disease in Postpartum Dairy Cows. Theriogenology 2011, 76, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Reproductive Tract Inflammatory Disease in Postpartum Dairy Cows. Animal 2014, 8 (Suppl. 1), 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining Postpartum Uterine Disease in Cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Lewis, G.S. Uterine Health and Disorders. J. Dairy Sci. 1997, 80, 984–994. [Google Scholar] [CrossRef]
- Dadarwal, D.; Palmer, C.; Griebel, P. Mucosal Immunity of the Postpartum Bovine Genital Tract. Theriogenology 2017, 104, 62–71. [Google Scholar] [CrossRef]
- Górriz-Martín, L.; Ulbrich, S.E.; Schmicke, M.; Hirsbrunner, G.; Keller, C.; Yücesoy, N.; Pfarrer, C.; Bollwein, H.; Heppelmann, M. The Myometrial Contractility during Late Pregnancy in Dairy Cows, in Vitro. Anim. Reprod. Sci. 2017, 181, 130–140. [Google Scholar] [CrossRef]
- Breen, K.M.; Billings, H.J.; Debus, N.; Karsch, F.J. Endotoxin Inhibits the Surge Secretion of Gonadotropin-Releasing Hormone via a Prostaglandin-Independent Pathway. Endocrinology 2004, 145, 221–227. [Google Scholar] [CrossRef][Green Version]
- Földi, J.; Kulcsár, M.; Pécsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.C.M.; Cox, P.; Huszenicza, G. Bacterial Complications of Postpartum Uterine Involution in Cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.E.; Galvão, K.N.; Rodriguez-Lecompte, J.C.; Costa, M.C. The Cattle Microbiota and the Immune System: An Evolving Field. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Königsson, K.; Gustafsson, H.; Gunnarsson, A.; Kindahl, H. Clinical and Bacteriological Aspects on the Use of Oxytetracycline and Flunixin in Primiparous Cows with Induced Retained Placenta and Post-Partal Endometritis. Reprod. Domest. Anim. 2001, 36, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, M.J.W.; Joop, K.; Sturk, A.; Bols, P.E.J.; Lohuis, J.A.C.M. Relationship between Intra-Uterine Bacterial Contamination, Endotoxin Levels and the Development of Endometritis in Postpartum Cows with Dystocia or Retained Placenta. Theriogenology 2000, 54, 1019–1032. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between Virulence Factors of Escherichia Coli, Fusobacterium Necrophorum, and Arcanobacterium Pyogenes and Uterine Diseases of Dairy Cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Herath, S.; England, G.C.W.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Effect of Escherichia Coli Infection of the Bovine Uterus from the Whole Animal to the Cell. Animal 2008, 2, 1153–1157. [Google Scholar] [CrossRef]
- Ghasemi, F.; Gonzalez-Cano, P.; Griebel, P.J.; Palmer, C. Proinflammatory Cytokine Gene Expression in Endometrial Cytobrush Samples Harvested from Cows with and without Subclinical Endometritis. Theriogenology 2012, 78, 1538–1547. [Google Scholar] [CrossRef]
- Pantaleo, M.; Rizzo, A.; D’Onghia, G.; D’Onghia, G.; Roncetti, M.; Piccinno, M.; Mutinati, M.; Terlizzi, M.; Sciorsci, R. Immunological Aspects of Metritis in Dairy Cows: A Review. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 196–205. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms Linking Bacterial Infections of the Bovine Endometrium to Disease and Infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Galvão, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium Review: The Uterine Microbiome Associated with the Development of Uterine Disease in Dairy Cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef]
- Kluciński, W.; Targowski, S.P.; Miernik-Degórska, E.; Winnicka, A. The Phagocytic Activity of Polymorphonuclear Leucocytes Isolated from Normal Uterus and That with Experimentally Induced Inflammation in Cows. Zentralbl. Veterinarmed. A 1990, 37, 506–512. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.; Buddle, B.M.; Heuer, C.; Hussein, H.; Zheng, T.; LeBlanc, S.J.; McDougall, S. Associations between Intrauterine Bacterial Infection, Reproductive Tract Inflammation, and Reproductive Performance in Pasture-Based Dairy Cows. Theriogenology 2015, 83, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Dini, P.; Farhoodi, M.; Hostens, M.; Van Eetvelde, M.; Pascottini, O.B.; Fazeli, M.H.; Opsomer, G. Effect of Uterine Lavage on Neutrophil Counts in Postpartum Dairy Cows. Anim. Reprod. Sci. 2015, 158, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; Dini, P.; Hostens, M.; Ducatelle, R.; Opsomer, G. A Novel Cytologic Sampling Technique to Diagnose Subclinical Endometritis and Comparison of Staining Methods for Endometrial Cytology Samples in Dairy Cows. Theriogenology 2015, 84, 1438–1446. [Google Scholar] [CrossRef]
- Lee, S.C.; Jeong, J.K.; Choi, I.S.; Kang, H.G.; Jung, Y.H.; Park, S.B.; Kim, I.H. Cytological Endometritis in Dairy Cows: Diagnostic Threshold, Risk Factors, and Impact on Reproductive Performance. J. Vet. Sci. 2018, 19, 301–308. [Google Scholar] [CrossRef]
- Barlund, C.S.; Carruthers, T.D.; Waldner, C.L.; Palmer, C.W. A Comparison of Diagnostic Techniques for Postpartum Endometritis in Dairy Cattle. Theriogenology 2008, 69, 714–723. [Google Scholar] [CrossRef]
- Runciman, D.J.; Anderson, G.A.; Malmo, J.; Davis, G.M. Effect of Intrauterine Treatment with Cephapirin on the Reproductive Performance of Seasonally Calving Dairy Cows at Risk of Endometritis Following Periparturient Disease. Aust. Vet. J. 2008, 86, 250–258. [Google Scholar] [CrossRef]
- Galvão, K.N.; Greco, L.F.; Vilela, J.M.; Sá Filho, M.F.; Santos, J.E.P. Effect of Intrauterine Infusion of Ceftiofur on Uterine Health and Fertility in Dairy Cows. J. Dairy Sci. 2009, 92, 1532–1542. [Google Scholar] [CrossRef]
- Brick, T.A.; Schuenemann, G.M.; Bas, S.; Daniels, J.B.; Pinto, C.R.; Rings, D.M.; Rajala-Schultz, P.J. Effect of Intrauterine Dextrose or Antibiotic Therapy on Reproductive Performance of Lactating Dairy Cows Diagnosed with Clinical Endometritis. J. Dairy Sci. 2012, 95, 1894–1905. [Google Scholar] [CrossRef]
- Mari, G.; Iacono, E.; Toni, F.; Predieri, P.G.; Merlo, B. Evaluation of the Effectiveness of Intrauterine Treatment with Formosulphathiazole of Clinical Endometritis in Postpartum Dairy Cows. Theriogenology 2012, 78, 189–200. [Google Scholar] [CrossRef]
- Denis-Robichaud, J.; Dubuc, J. Randomized Clinical Trial of Intrauterine Cephapirin Infusion in Dairy Cows for the Treatment of Purulent Vaginal Discharge and Cytological Endometritis. J. Dairy Sci. 2015, 98, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Tison, N.; Bouchard, E.; DesCôteaux, L.; Lefebvre, R.C. Effectiveness of Intrauterine Treatment with Cephapirin in Dairy Cows with Purulent Vaginal Discharge. Theriogenology 2017, 89, 305–317. [Google Scholar] [CrossRef]
- Brodzki, P.; Lisiecka, U.; Brodzki, A.; Krakowski, L.; Szczubiał, M.; Dąbrowski, R.; Junkuszew, A.; Bochniarz, M. Selected Leukocyte Subpopulations in Peripheral Blood and Uterine Washings in Cows before and after Intrauterine Administration of Cefapirin and Methisoprinol. Anim. Sci. J. 2020, 91, e13306. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between Negative Energy Balance, Metabolic Diseases, Uterine Health and Immune Response in Transition Dairy Cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.; Mira, L.; Corvo, M. Molecular Mechanisms of Anti-Inflammatory Activity Mediated by Flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Huang, Y.J.; Jiang, Y.W.; Hu, S.H. Persistence of Oxytetracycline Residues in Milk after the Intrauterine Treatment of Lactating Cows for Endometritis. Vet. Rec. 2007, 161, 585–587. [Google Scholar] [CrossRef]
- Gorden, P.J.; Ydstie, J.A.; Kleinhenz, M.D.; Wulf, L.W.; Gehring, R.; Lee, C.J.; Wang, C.; Coetzee, J.F. A Study to Examine the Relationship between Metritis Severity and Depletion of Oxytetracycline in Plasma and Milk after Intrauterine Infusion. J. Dairy Sci. 2016, 99, 8314–8322. [Google Scholar] [CrossRef]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential Therapeutic Anti-Inflammatory and Immunomodulatory Effects of Dihydroflavones, Flavones, and Flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Noakes, D.E. Comparison of Three Treatments for Bovine Endometritis. Vet. Rec. 1998, 142, 575–579. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.W.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical Evaluation of Postpartum Vaginal Mucus Reflects Uterine Bacterial Infection and the Immune Response in Cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of Endometritis and Its Effects on Reproductive Performance of Dairy Cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Sannmann, I.; Arlt, S.; Heuwieser, W. A Critical Evaluation of Diagnostic Methods Used to Identify Dairy Cows with Acute Post-Partum Metritis in the Current Literature. J. Dairy Res. 2012, 79, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.G.; Lowder, M.Q.; Wenzel, J.G.W. Retrospective Analysis of the Management of 78 Cases of Postpartum Metritis in the Cow. Theriogenology 1994, 42, 455–463. [Google Scholar] [CrossRef]
- Kegley, E.B.; Ball, J.J.; Beck, P.A. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: Impact of Mineral and Vitamin Status on Beef Cattle Immune Function and Health. J. Anim. Sci. 2016, 94, 5401–5413. [Google Scholar] [CrossRef]
- Thurmond, M.C.; Jameson, C.M.; Picanso, J.P. Effect of Intrauterine Antimicrobial Treatment in Reducing Calving-to-Conception Interval in Cows with Endometritis. J. Am. Vet. Med. Assoc. 1993, 203, 1576–1578. [Google Scholar] [PubMed]
- Smith, B.I.; Donovan, G.A.; Risco, C.; Littell, R.; Young, C.; Stanker, L.H.; Elliott, J. Comparison of Various Antibiotic Treatments for Cows Diagnosed with Toxic Puerperal Metritis. J. Dairy Sci. 1998, 81, 1555–1562. [Google Scholar] [CrossRef]
- Liu, W.B.; Chuang, S.T.; Shyu, C.L.; Chang, C.C.; Jack, A.; Peh, H.C.; Chan, J. Strategy for the Treatment of Puerperal Metritis and Improvement of Reproductive Efficiency in Cows with Retained Placenta. Acta Vet. Hung. 2011, 59, 247–256. [Google Scholar] [CrossRef]
- Haimerl, P.; Heuwieser, W. Invited Review: Antibiotic Treatment of Metritis in Dairy Cows: A Systematic Approach. J. Dairy Sci. 2014, 97, 6649–6661. [Google Scholar] [CrossRef]
- Armengol, R.; Fraile, L. Comparison of Two Treatment Strategies for Cows with Metritis in High-Risk Lactating Dairy Cows. Theriogenology 2015, 83, 1344–1351. [Google Scholar] [CrossRef]
- Haimerl, P.; Arlt, S.; Borchardt, S.; Heuwieser, W. Antibiotic Treatment of Metritis in Dairy Cows—A Meta-Analysis. J. Dairy Sci. 2017, 100, 3783–3795. [Google Scholar] [CrossRef]
- Emre, B.; Korkmaz, Ö.; Temamoğulları, F.; Zonturlu, A.K.; Koyuncu, İ.; Ozkaraca, M.; Cengiz, M. Effect of Intrauterine Infusion of Momordica Charantia, L. on Oxidative Stress and Pregnancy Rate in Infertile Cows. J. Vet. Res. 2017, 61, 489–496. [Google Scholar] [CrossRef]
- Hajibemani, A.; Mirzaei, A.; Ghasrodashti, A.R.; Memarzadeh, M.R. The Effect of Zataria Multiflora Extract on the Clinical Endometritis and Reproductive Indices in Lactating Holstein Dairy Cows. Vet. Res. Forum 2016, 7, 309–315. [Google Scholar] [PubMed]
- Verma, S.; Choudhary, A.; Maini, S.; Ravikanth, K. Evaluation of Efficacy of Herbal Intrauterine Infusion Uterofix Liquid in Treatment of Various Reproductive Disorders in Cows: A Field Study. Pharmacogn. Res. 2016, 8, 173–175. [Google Scholar] [CrossRef]
- Pinedo, P.J.; Velez, J.S.; Bothe, H.; Merchan, D.; Piñeiro, J.M.; Risco, C.A. Effect of Intrauterine Infusion of an Organic-Certified Product on Uterine Health, Survival, and Fertility of Dairy Cows with Toxic Puerperal Metritis. J. Dairy Sci. 2015, 98, 3120–3132. [Google Scholar] [CrossRef]
- Hassan, A.A.; Thabet, N.M.; Kh, M.; Rafei, A. Hyaluronan as a Mediator for the Hepatoprotective Effect of Diosmin/Hesperidin Complex. Pak. J. Pharm. Sci. 2018, 31, 1191–1201. [Google Scholar]
- Serra, R.; Grande, R.; Butrico, L.; Buffone, G.; Caliò, F.G.; Squillace, A.; Rizzo, B.A.; Massara, M.; Spinelli, F.; Ferrarese, A.G.; et al. Effects of a New Nutraceutical Substance on Clinical and Molecular Parameters in Patients with Chronic Venous Ulceration. Int. Wound J. 2016, 13, 88–96. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, T.; Yin, N.; Ma, X.; Zhang, Z.; Zhu, Z.; Shaukat, A.; Deng, G. Luteoloside Protects the Uterus from Staphylococcus Aureus-Induced Inflammation, Apoptosis, and Injury. Inflammation 2018, 41, 1702–1716. [Google Scholar] [CrossRef]
- Santos, S.; Haslinger, C.; Klaic, K.; Faleschini, M.T.; Mennet, M.; Potterat, O.; Von Mandach, U.; Hamburger, M.; Simões-Wüst, A.P. A Bufadienolide-Enriched Fraction of Bryophyllum Pinnatum Inhibits Human Myometrial Contractility In Vitro. Planta Med. 2019, 85, 385–393. [Google Scholar] [CrossRef]
- Nicolaides, A.N. From Symptoms to Leg Edema: Efficacy of Daflon 500 Mg. Angiology 2003, 54 (Suppl. 1), S33–S44. [Google Scholar] [CrossRef]
- Takase, S.; Pascarella, L.; Lerond, L.; Bergan, J.J.; Schmid-Schönbein, G.W. Venous Hypertension, Inflammation and Valve Remodeling. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 484–493. [Google Scholar] [CrossRef]
- Perrin, M.; Ramelet, A.A. Pharmacological Treatment of Primary Chronic Venous Disease: Rationale, Results and Unanswered Questions. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 117–125. [Google Scholar] [CrossRef]
- Pietrzycka, A.; Kózka, M.; Urbanek, T.; Stepniewski, M.; Kucharzewski, M. Effect of Micronized Purified Flavonoid Fraction Therapy on Endothelin-1 and TNF-α Levels in Relation to Antioxidant Enzyme Balance in the Peripheral Blood of Women with Varicose Veins. Curr. Vasc. Pharmacol. 2015, 13, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Bebe, F.N.; Panemangalore, M. Biosafety of Flavonoids in Rats: Effects on Copper and Zinc Homeostasis and Interaction with Low-Level Pesticide Exposure. Biol. Trace Elem. Res. 2009, 129, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vidlář, A.; Zatloukalová, M.; Stuchlík, M.; Vacek, J.; Šimánek, V.; Ulrichová, J. Biosafety and Antioxidant Effects of a Beverage Containing Silymarin and Arginine. A Pilot, Human Intervention Cross-over Trial. Food Chem. Toxicol. 2013, 56, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Onsare, J.G.; Arora, D.S. Antibiofilm Potential of Flavonoids Extracted from Moringa Oleifera Seed Coat against Staphylococcus Aureus, Pseudomonas Aeruginosa and Candida Albicans. J. Appl. Microbiol. 2015, 118, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Sood, H. In Vitro Antimicrobial Potential of Extracts and Phytoconstituents from Gymnema Sylvestre R.Br. Leaves and Their Biosafety Evaluation. AMB Express 2017, 7, 115. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic Potential of Natural Compounds in Inflammation and Chronic Venous Insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Rycroft, A.N.; Dogan, B.; Craven, M.; Bromfield, J.J.; Chandler, A.; Roberts, M.H.; Price, S.B.; Gilbert, R.O.; Simpson, K.W. Specific Strains of Escherichia Coli Are Pathogenic for the Endometrium of Cattle and Cause Pelvic Inflammatory Disease in Cattle and Mice. PLoS ONE 2010, 5, e9192. [Google Scholar] [CrossRef]
- Royal, M.; Mann, G.E.; Flint, A.P.F. Strategies for Reversing the Trend towards Subfertility in Dairy Cattle. Vet. J. 2000, 160, 53–60. [Google Scholar] [CrossRef]
- MacMillan, K.L. Recent Advances in the Synchronization of Estrus and Ovulation in Dairy Cows. J. Reprod. Dev. 2010, 56, S42–S47. [Google Scholar] [CrossRef]
- Machado, V.S.; Oikonomou, G.; Ganda, E.K.; Stephens, L.; Milhomem, M.; Freitas, G.L.; Zinicola, M.; Pearson, J.; Wieland, M.; Guard, C.; et al. The Effect of Intrauterine Infusion of Dextrose on Clinical Endometritis Cure Rate and Reproductive Performance of Dairy Cows. J. Dairy Sci. 2015, 98, 3849–3858. [Google Scholar] [CrossRef]
- Maquivar, M.G.; Barragan, A.A.; Velez, J.S.; Bothe, H.; Schuenemann, G.M. Effect of Intrauterine Dextrose on Reproductive Performance of Lactating Dairy Cows Diagnosed with Purulent Vaginal Discharge under Certified Organic Management. J. Dairy Sci. 2015, 98, 3876–3886. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Acconcia, F.; Ambra, R.; Rinna, A.; Totta, P.; Marino, M. Nutritional Flavonoids Modulate Estrogen Receptor Alpha Signaling. IUBMB Life 2004, 56, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Moriyoshi, M.; Kawata, K. Effect of Postpartum Intrauterine Treatment with 2% Polyvinyl-Pyrrolidone-Iodine Solution on Reproductive Efficiency in Cows. Theriogenology 1988, 30, 1033–1043. [Google Scholar] [CrossRef]
- Heuwieser, W.; Tenhagen, B.A.; Tischer, M.; Lühr, J.; Blum, H. Effect of Three Programmes for the Treatment of Endometritis on the Reproductive Performance of a Dairy Herd. Vet. Rec. 2000, 146, 338–341. [Google Scholar] [CrossRef]
- Knutti, B.; Küpfer, U.; Busato, A. Reproductive Efficiency of Cows with Endometritis after Treatment with Intrauterine Infusions or Prostaglandin Injections, or No Treatment. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2000, 47, 609–615. [Google Scholar] [CrossRef]
- Gao, F.; Wei, D.; Bian, T.; Xie, P.; Zou, J.; Mu, H.; Zhang, B.; Zhou, X. Genistein Attenuated Allergic Airway Inflammation by Modulating the Transcription Factors T-Bet, GATA-3 and STAT-6 in a Murine Model of Asthma. Pharmacology 2012, 89, 229–236. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular Link Mechanisms between Inflammation and Cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef]
- Burnett, B.P.; Bitto, A.; Altavilla, D.; Squadrito, F.; Levy, R.M.; Pillai, L. Flavocoxid Inhibits Phospholipase A2, Peroxidase Moieties of the Cyclooxygenases (COX), and 5-Lipoxygenase, Modifies COX-2 Gene Expression, and Acts as an Antioxidant. Mediat. Inflamm. 2011, 2011, 385780. [Google Scholar] [CrossRef]
- Pang, L.; Zou, S.; Shi, Y.; Mao, Q.; Chen, Y. Apigenin Attenuates PM2.5-Induced Airway Hyperresponsiveness and Inflammation by down-Regulating NF-ΚB in Murine Model of Asthma. Int. J. Clin. Exp. Pathol. 2019, 12, 3700–3709. [Google Scholar] [PubMed]
- Koujan, A.; Eissa, H.M.; Hussein, M.A.; Ayoub, M.M.; Afiefy, M.M. Therapeutic Efficacy of Povidone-Iodlne (Betadine) and Dichloroxylenol (Septocid) in Holstein Cows Affected with Endometritis and/or Cervicitis. Acta Vet. Hung. 1996, 44, 111–119. [Google Scholar] [PubMed]
| Experimental Groups | (−) Control (n = 20; Healthy Non-Treated) | (+) Control (n = 20; Metritis Non-Treated) | Low Dose (n = 20; Metritis MPFF: 500 mg) | Medium Dose (n = 20; Metritis MPFF: 1000 mg) | High Dose (n = 20; Metritis MPFF: 1500 mg) |
|---|---|---|---|---|---|
| Success Rate (%Cure; Day 20) (Day 24 Postpartum) Post-treatment | 0.0 A | 5.0 A | 15.0 B | 45.0 C | 60.0 D |
| (0/20) | (1/20) | (3/20) | (9/20) | (12/20) |
| Uterine Anatomical Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lumen Length (mm) | Lumen Width (mm) | Thickness (mm) | |||||||
| Day 0 (Day 4 Post-part) (Pre-Treat.) | Day 10 (Day 14 Post-Part.) (Post-Treat) | Day 20 (Day 24 Post-Part.) (Post-Treat) | Day 0 (Day 4 Post-Part.) (Pre-Treat) | Day 10 (Day 14 Post-Part.) (Post- Treat.) | Day 20 (Day 24 Post-Part) (Post-Treat) | Day 0 (Day 4 Post-Part) (Pre-Treat) | Day 10 (Day 14 Post-Part) (Post-Treat) | Day 20 (Day 24 Post-Part) (Post-Treat) | |
| (−) Control | 79.92 ± 0.30 Aa | 70.01 ± 0.21 Ba | 67.69 ± 0.15 Ba | 54.22 ± 3.91 Aa | 46.05 ± 3.16 ABa | 40.88 ± 2.92 Ba | 11.93 ± 0.90 Aa | 10.74 ± 0.46 Ba | 7.03 ± 0.31 Ca |
| (+) Control | 83.11 ± 0.33 Aa | 74.21 ± 0.23 ABa | 69.75 ± 0.19 Ba | 59.19 ± 4.56 Aa | 54.62 ± 3.84 ABa | 49.20 ± 2.87 Bb | 13.11 ± 0.95 Ab | 13.22 ± 0.42 Ab | 12.37 ± 0.69 Ab |
| Low Dose (500 mg) | 84.24 ± 0.40 Aa | 74.90 ± 0.32 ABa | 71.22 ± 0.28 Ba | 58.35 ± 4.61 Aa | 52.12 ± 2.98 ABa | 48.89 ± 3.73 Bb | 13.35 ± 1.33 Ab | 13.40 ± 0.67 Ab | 12.69 ± 0.32 Ab |
| Medium Dose (1000 mg) | 85.64 ± 0.39 Aa | 71.93 ± 0.28 Ba | 69.04 ± 0.19 Ba | 56.90 ± 4.39 Aa | 49.53 ± 3.81 ABa | 45.11 ± 3.19 Ba | 13.65 ± 1.41 Ab | 13.01 ± 0.52 Ab | 11.78 ± 0.46 Bb |
| High Dose (1500 mg) | 85.07 ± 0.45 Aa | 71.52 ± 0.22 Ba | 68.5 ± 0.24 Ba | 55.57 ± 5.03 Aa | 48.35 ± 4.88 ABa | 42.83 ± 4.77 Ba | 13.82 ± 1.25 Ab | 11.79 ± 0.61 Bc | 10.69 ± 0.30 Cc |
| Experimental Groups | Cervical Anatomical Parameters | |||||
|---|---|---|---|---|---|---|
| Diameter (mm) | Thickness (mm) | |||||
| Day 0 (Day 4 Postpartum) (Pre-Treatment) | Day 10 (Day 14 Postpartum) (Post-Treatment) | Day 20 (Day 24 Postpartum) (Post-Treatment) | Day 0 (Day 4 Postpartum) (Pre-Treatment) | Day 10 (Day 14 Postpartum) (Post-Treatment) | Day 20 (Day 24 Postpartum) (Post-Treatment) | |
| (−) Control (n = 20; Healthy Non-Treated) | 44.25 ± 4.03 Aa | 42.56 ± 3.12 Aba | 35.89 ± 1.56 Ba | 14.10 ± 2.87 Aa | 9.99 ± 0.90 Aba | 6.78 ± 1.05 Ba |
| (+) Control (n = 20; Metritis Non-Treated) | 45.12 ± 3.55 Aa | 44.79 ± 3.32 Aa | 41.23 ± 1.81 Ab | 15.77 ± 3.03 Aa | 14.11 ± 0.82 Ab | 11.66 ± 1.58 Ab |
| Low Dose (n = 20; Metritis 500 mg) | 45.56 ± 4.67 Aa | 44.21 ± 3.78 Aa | 40.72 ± 1.45 Ab | 15.49 ± 2.99 Aa | 13.79 ± 1.97 Ab | 12.21 ± 1.90 Ab |
| Medium Dose (n = 20; Metritis 1000 mg) | 45.78 ± 3.37 Aa | 44.10 ± 2.67 Aba | 39.02 ± 1.63 Ba | 15.19 ± 1.83 Aa | 12.12 ± 1.29 ABab | 9.50 ± 1.10 Bab |
| High Dose (n = 20; Metritis 500 mg) | 45.46 ± 3.72 Aa | 43.87 ± 4.00 ABa | 37.90 ± 1.98 Ba | 15.88 ± 2.27 Aa | 11.89 ± 1.98 ABa | 8.85 ± 0.93 Bab |
| Bacterial Species/Type | Associated to Endometritis Cases/Considered Uterine Pathogen | Prevalence (% Total) | Prevalence (% Metritis +) | |
|---|---|---|---|---|
| GRAM− | ||||
| Acinetobacter baumannii* | Yes | Yes | 2/120 (1.6%) | 2/60 (3.3%) |
| Coliforms(≠spp.) * | Yes | Yes | 44/120 (36.6%) | 44/60(73.3%) |
| Citrobacter freundii* | Yes | Yes | 6/120 (5.0%) | 6/60 (10.0%) |
| Escherichia coli* | Yes | Yes | 60/120 (50%) | 60/60 (100%) |
| Klebsiella oxytoca | No | No | 4/120 (3.3%) | 4/60 (6.6%) |
| Pseudomonas aeuroginosa | No | No | 6/120 (5.0%) | 6/60 (10.0%) |
| Proteus mirabilis | No | No | 6/120 (5.0%) | 6/60 (10.0%) |
| Shigella flexneri | No | No | 2/120 (1.6%) | 2/60 (3.3%) |
| GRAM+ | ||||
| Bacillus(≠spp.) * | Yes | Yes | 11/60 (18.3%) | 11/30 (36.6%) |
| Clostridium(≠spp.) | No | No | 2/60 (3.3%) | 2/30 (6.6%) |
| Enterococcus faecalis* | Yes | Yes | 5/60 (8.3%) | 5/30 (16.6%) |
| Lactobacillus(≠spp.) | No | No | 19/60 (31.6%) | 19/30 (63.3%) |
| Micrococcus(≠spp.) | No | No | 3/60 (5.0%) | 3/30 (10.0%) |
| Staphylococcus(≠spp.) | No | No | 5/60 (8.3%) | 5/30 (16.6%) |
| Staphylococcus aureus (coag.+)* | Yes | Yes | 9/60 (15.0%) | 9/30 (30.0%) |
| Staphylococcus epidermidis (coag.−) | No | No | 27/60 (45.0%) | 27/30 (90.0%) |
| Staphylococcus hominis | No | No | 1/60 (1.6%) | 1/30 (3.3%) |
| Streptococcus(≠spp.) | No | No | 8/60 (13.3%) | 8/30 (26.6%) |
| Streptococcus agalactiae* | No | No | 4/60 (6.6%) | 4/30 (13.3%) |
| Streptococcus γ-haemolytic* | Yes | Yes | 6/60 (10.0%) | 6/30 (20.0%) |
| Streptococcus viridans* | Yes | Yes | 1/60 (1.6%) | 1/30 (3.3%) |
| Total | 998 | 908 | 90 | 90 |
| Bacterial Spp./Types | Antimicrobial Sensitivity Patterns | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | |
| GRAM− | ||||||||||||||||||||
| Acinetob. baumannii | ++ | ++ | ++ | ++ | - | ++ | + | - | ++ | |||||||||||
| Coliforms (≠spp.) | ++ | ++ | ++ | ++ | ++ | + | - | ++ | ||||||||||||
| Citrob. freundii | ++ | ++ | ++ | ++ | + | + | + | + | - | + | ||||||||||
| E. coli | ++ | ++ | ++ | ++ | + | + | - | + | ||||||||||||
| GRAM+ | ||||||||||||||||||||
| Bacillus(≠spp.) | ++ | - | + | ++ | - | + | - | - | + | ++ | ++ | |||||||||
| Enter. faecalis | ++ | ++ | ++ | + | + | + | ++ | |||||||||||||
| Staph. aureus(c+) | ++ | - | ++ | ++ | - | ++ | + | - | - | ++ | ++ | ++ | - | |||||||
| Strep.γ-hemolytic | ++ | + | ++ | ++ | - | + | - | + | ++ | |||||||||||
| Strep. viridans | ++ | + | ++ | + | + | - | - | - | + | - | ||||||||||
| Total HS (++) | 8 | 6 | 5 | 7 | 7 | 4 | 3 | 6 | 1 | 1 | 4 | 1 | 1 | 2 | 3 | 0 | 2 | 1 | 3 | 0 |
| Total R (−) | 0 | 2 | 0 | 0 | 0 | 5 | 0 | 2 | 7 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Experimental Groups (Number of Pathogenic Bacterial Isolates) | |||||
|---|---|---|---|---|---|
| Timepoints | (−) Control (n = 20; Healthy Non-Treated) | (+) Control (n = 20; Metritis Non-Treated) | Low Dose (n = 20; Metritis MPFF: 500 mg) | Medium Dose (n = 20; Metritis MPFF: 1000 mg) | High Dose (n = 20; Metritis MPFF: 1500 m) |
| Day 0 (Day 4 Postpartum) (Pre-Treatment) | 0/20 (0.00) Aa | 48/20 (2.40) Ba | 52/20 (2.60) Ba | 50/20 (2.50) Ba | 55/20 (2.75) Ba |
| Day 10 (Day 14 Postpartum) (Post-treatment) | 0/20 (0.00) Aa | 50/20 (2.50) Ba | 46/20 (2.30) Ba | 40/20 (2.00) BCb | 35/20 (1.75) Cb |
| Day 20 (Day 24 Postpartum) (Post-treatment) | 0/20 (0.00) Aa | 41/20 (2.05) Ba | 43/20 (2.15) Ba | 34/20 (1.70) BCab | 30/20 (1.50) Cab |
| Total reduction (%) from Day 0 to Day 20 | 7 | 9 | 16 | 25 | |
| Bacterial Species/Type | Experimental Groups (Antimicrobial Sensitivity) | |||
|---|---|---|---|---|
| (+) Control (n = 20; Metritis Non-Treated) | Low Dose (n = 20; Metritis MPFF: 500 mg) | Medium Dose (n = 20; Metritis MPFF: 1000 mg) | High Dose (n = 20; Metritis MPFF:1500 mg) | |
| Acinetobacter baumannii* | − | + | ++ | +++ |
| Bacillus(≠spp.) * | − | − | − | + |
| Citrobacter freundii* | − | + | ++ | +++ |
| Coliforms(≠spp.) * | − | − | − | + |
| Enterococcus faecalis* | − | + | + | ++ |
| Escherichia coli* | − | − | − | + |
| Staphylococcus aureus (c+)* | − | − | − | + |
| Streptococcus γ-haemolytic * | − | + | ++ | +++ |
| Streptococcus viridans* | − | + | + | ++ |
| Experimental Groups (% of Polymorphonuclears; % PMNs) | |||||
|---|---|---|---|---|---|
| Timepoints | (−) Control (n = 20; Healthy Non-Treated) | (+) Control (n = 20; Metritis Non-Treated) | Low Dose (n = 20; Metritis MPFF: 500 mg) | Medium Dose (n = 20; Metritis MPFF: 1000 mg) | High Dose (n = 20; Metritis MPFF: 1500 mg) |
| Day 0 (Day 4 Postpartum) (Pre-Treatment) | 8.2 ± 4.1 Aa | 18.8 ± 9.2 Ba | 19.4 ± 11.6 Ba | 22.1 ± 10.1 Ba | 21.5 ± 13.6 Ba |
| Day 10 (Day 14 Postpartum) (Post-treatment) | 35.8 ± 9.7 Ab | 54.1 ± 14.2 Bb | 50.9 ± 18.7 Bb | 44.2 ± 20.1 ABb | 40.9 ± 16.8 Ab |
| Day 20 (Day 24 Postpartum) (Post-treatment) | 16.1 ± 6.0 Aa | 50.0 ± 19.6 Bb | 41.2 ± 14.4 Bb | 30.1 ± 16.5 Ca | 24.6 ± 18.1 ACa |
| Total reduction (%) from Day 10 to Day 20 | 19.7 A | 5.5 B | 9.7 B | 14.1 AB | 16.3 AB |
| Reproductive Performance Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| (−) Control (n = 20; Healthy Non-Treated) | (+) Control (n = 20; Metritis Non-Treated) | Low Dose (n = 20; Metritis MPFF: 500 mg) | Medium Dose (n = 20; Metritis MPFF: 1000 mg) | High Dose (n = 20; Metritis MPFF: 1500 mg) | |
| Calving–Conception Interval (CCI; Days) | 106 ± 29 A | 136 ± 41 B | 131 ± 45 B | 120 ± 40 C | 117 ± 37 C |
| Calving to first service Interval (CFSI; Days) | 85 ± 21 A | 112 ± 34 B | 107 ± 39 B | 95 ± 44 C | 93 ± 40 AC |
| Conception Rate (%) | 75.0 A | 25.0 B | 35.0 C | 50.0 D | 60.0 E |
| (15/20) | (5/20) | (7/20) | (10/20) | (12/20) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Reinoso, M.A.; Uquilla, J.B.; Barona, F.A.; Guano, M.E.; Chicaiza, G.N.; García-Herreros, M. Effects of Intrauterine Infusion of Micronised Purified Flavonoid Fraction (MPFF) in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum. Vet. Sci. 2022, 9, 362. https://doi.org/10.3390/vetsci9070362
Gutiérrez-Reinoso MA, Uquilla JB, Barona FA, Guano ME, Chicaiza GN, García-Herreros M. Effects of Intrauterine Infusion of Micronised Purified Flavonoid Fraction (MPFF) in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum. Veterinary Sciences. 2022; 9(7):362. https://doi.org/10.3390/vetsci9070362
Chicago/Turabian StyleGutiérrez-Reinoso, Miguel A., José B. Uquilla, Francisco A. Barona, Manuel E. Guano, Gloria N. Chicaiza, and Manuel García-Herreros. 2022. "Effects of Intrauterine Infusion of Micronised Purified Flavonoid Fraction (MPFF) in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum" Veterinary Sciences 9, no. 7: 362. https://doi.org/10.3390/vetsci9070362
APA StyleGutiérrez-Reinoso, M. A., Uquilla, J. B., Barona, F. A., Guano, M. E., Chicaiza, G. N., & García-Herreros, M. (2022). Effects of Intrauterine Infusion of Micronised Purified Flavonoid Fraction (MPFF) in Metritis-Diagnosed Dairy Cows Naturally Infected by E. coli during the Early Postpartum. Veterinary Sciences, 9(7), 362. https://doi.org/10.3390/vetsci9070362








