The Swine Erysipelas Vaccine SER-ME Effectively Protects Pigs against Challenge with the Erysipelothrix rhusiopathiae M203/I257 SpaA-Type Variant
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Conditions and Colony-Forming Unit (CFU) Assay
2.2. Sequencing of SpaA Genes
2.3. Vaccine Used for Immunization
2.4. Vaccination and Challenge Exposure in Pigs
2.5. Collection of Blood and Organs Samples from Experiment Pigs
2.6. Measuring Antibody Titers in Pigs (ELISA and GA Titers)
2.7. Statistical Analyses
3. Results
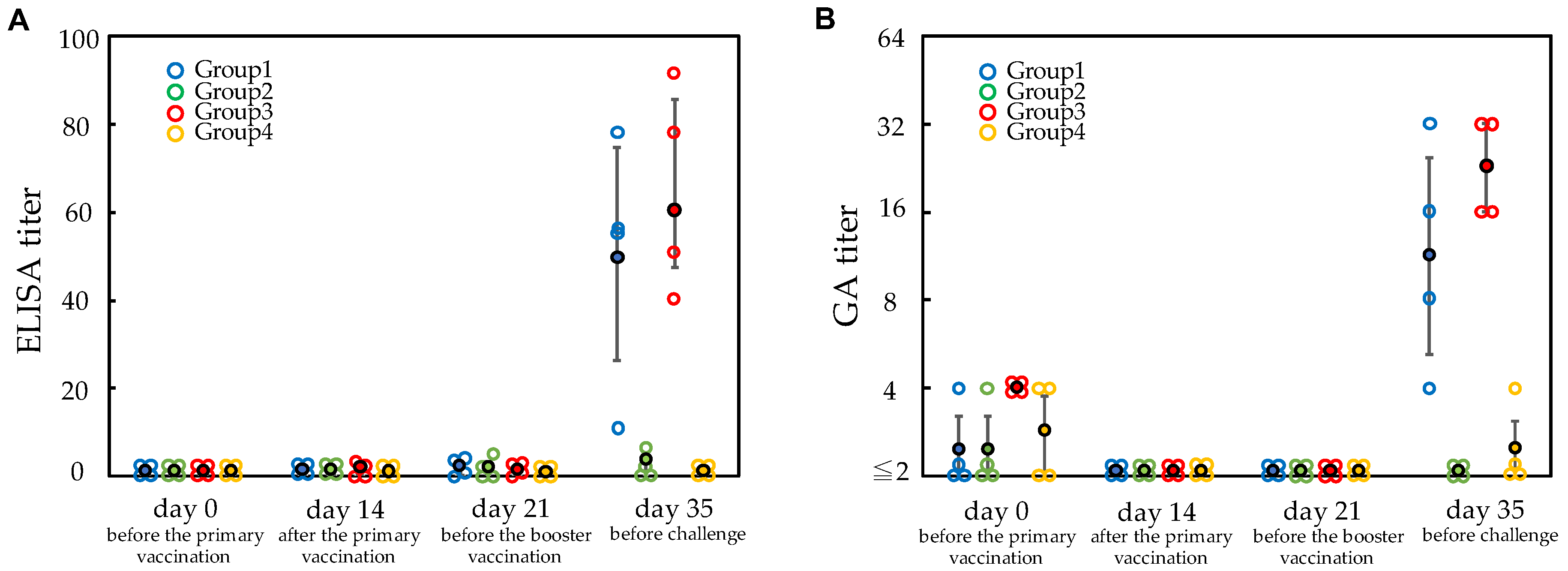
3.1. Vaccination and Serum Titers for E. rhusiopathiae
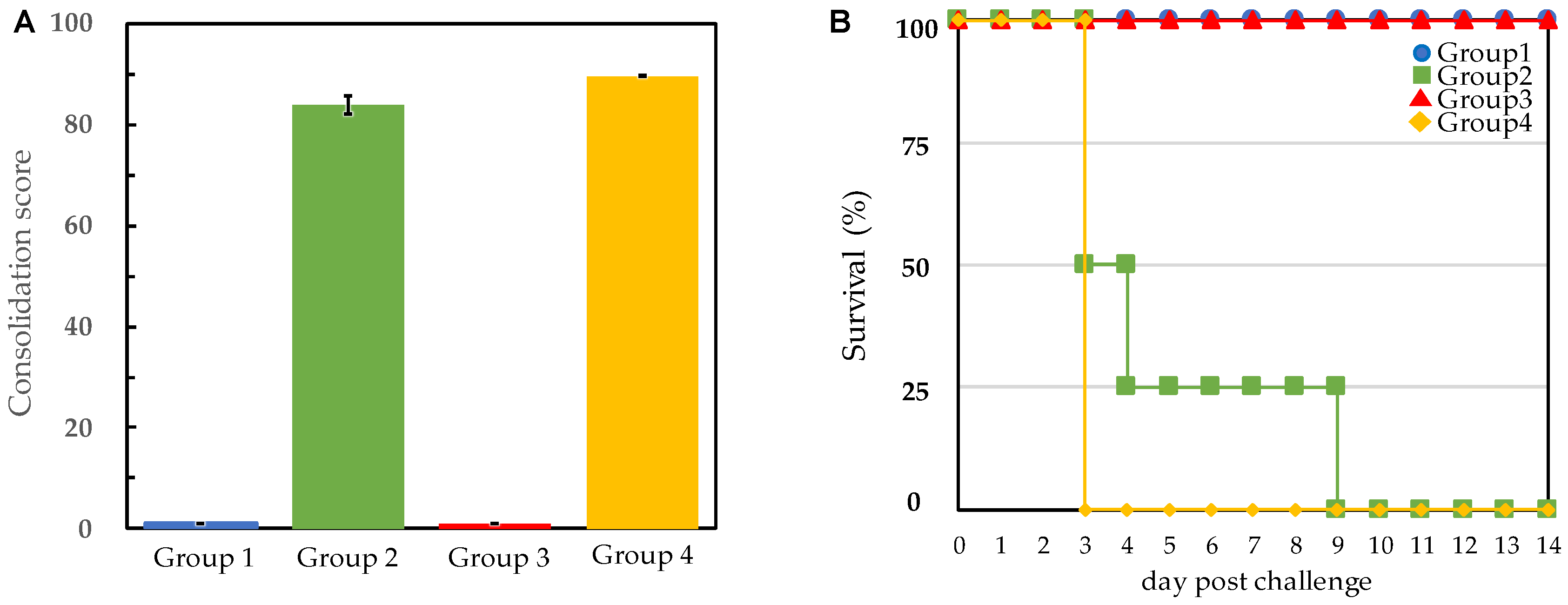
3.2. Protection of Pigs against E. rhusiopathiae Challenge
3.3. Isolation of E. rhusiopathiae from Challenged Pigs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opriessnig, T.; Coutinho, A.T. Erysipelas. In Diseases of Swine; Zimmerman, J.J., Karriker, A.L., Ramirez, A., Schwartz, J.K., Stevenson, W.G., Zhang, J., Eds.; Wiley-Blackwell: New Jersey, NJ, USA, 2019; pp. 835–843. ISBN 9781119350927. [Google Scholar]
- To, H.; Someno, S.; Nagai, S.; Koyama, T.; Nagano, T. Immunization with Truncated Recombinant Protein SpaC of Erysipelothrix rhusiopathiae Strain 715 Serovar 18 Confers Protective Immunity against Challenge with Various Serovars. Clin. Vaccine Immunol. 2010, 17, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Forde, T.; Shimoji, Y. Erysipelothrix Spp.: Past, Present, and Future Directions in Vaccine Research. Front. Vet. Sci. 2020, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Imada, Y.; Goji, N.; Ishikawa, H.; Kishima, M.; Sekizaki, T. Truncated Surface Protective Antigen (SpaA) of Erysipelothrix rhusiopathiae Serotype 1a Elicits Protection against Challenge with Serotypes 1a and 2b in Pigs. Infect. Immun. 1999, 67, 4376–4382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wu, C.; Kang, C.; Cai, C.; Wang, Y.; Li, J.; Zhang, Q.; Sun, X.; Jin, M. Evaluation of the Protective Efficacy of Four Newly Identified Surface Proteins of Erysipelothrix rhusiopathiae. Vaccine 2018, 36, 8079–8083. [Google Scholar] [CrossRef] [PubMed]
- Borrathybay, E.; Gong, F.J.; Zhang, L.; Nazierbieke, W. Role of Surface Protective Antigen A in the Pathogenesis of Erysipelothrix rhusiopathiae Strain C43065. J. Microbiol. Biotechnol. 2015, 25, 206–216. [Google Scholar] [CrossRef]
- Rosenow, C.; Ryan, P.; Weiser, J.N.; Johnson, S.; Fontan, P.; Ortqvist, A.; Masure, H.R. Contribution of Novel Choline-Binding Proteins to Adherence, Colonization and Immunogenicity of Streptococcus Pneumoniae. Mol. Microbiol. 1997, 25, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.-I.; Yamamoto, K.; Murakami, S.; Shirahata, T.; Uemura, K.; Sawada, T.; Wakamoto, H.; Morita, Y. Properties of Repeat Domain Found in a Novel Protective Antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb. Pathog. 1998, 25, 101–109. [Google Scholar] [CrossRef]
- Zhu, W.; Cai, C.; Wang, Y.; Li, J.; Wu, C.; Kang, C.; Sun, X.; Jin, M. Characterization of Roles of SpaA in Erysipelothrix rhusiopathiae Adhesion to Porcine Endothelial Cells. Microb. Pathog. 2017, 113, 176–180. [Google Scholar] [CrossRef]
- To, H.; Nagai, S. Genetic and Antigenic Diversity of the Surface Protective Antigen Proteins of Erysipelothrix rhusiopathiae. Clin. Vaccine Immunol. 2007, 14, 813–820. [Google Scholar] [CrossRef]
- Ingebritson, A.L.; Roth, J.A.; Hauer, P.J. Erysipelothrix rhusiopathiae: Association of Spa-Type with Serotype and Role in Protective Immunity. Vaccine 2010, 28, 2490–2496. [Google Scholar] [CrossRef]
- Shiraiwa, K.; Ogawa, Y.; Nishikawa, S.; Eguchi, M.; Shimoji, Y. Multiplex PCR Assay for the Simultaneous Detection and Differentiation of Clonal Lineages of Erysipelothrix rhusiopathiae Serovar 1a Strains Currently Circulating in Japan. J. Vet. Med. Sci. 2017, 79, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- To, H.; Sato, H.; Tazumi, A.; Tsutsumi, N.; Nagai, S.; Iwata, A.; Nagano, T. Characterization of Erysipelothrix rhusiopathiae Strains Isolated from Recent Swine Erysipelas Outbreaks in Japan. J. Vet. Med. Sci. 2012, 74, 949–953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uchiyama, M.; Shimazaki, Y.; Isshiki, Y.; Kojima, A.; Hirano, F.; Yamamoto, K.; Kijima, M.; Nagai, H. Pathogenic Characterization of Erysipelothrix rhusiopathiae Met-203 Type SpaA Strains from Chronic and Subacute Swine Erysipelas in Japan. J. Vet. Med. Sci. 2017, 79, 18–21. [Google Scholar] [CrossRef]
- Morimoto, M.; Kato, A.; Kojima, H.; Akaike, Y.; Nogami, K.; Sasakawa, C.; Nagai, S.; To, H. Serovars and SpaA Types of Erysipelothrix rhusiopathiae Isolated from Pigs in Japan from 2012 to 2019. Curr. Microbiol. 2021, 78, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Kato, A.; Akaike, Y.; Nogami, K.; Ono, H.; Furusawa, T.; Kojima, H.; Sasakawa, C. Comparative Study of the Phenotype and Virulence of Recent Serovar 1a, 1b, and 2a Isolates of Erysipelothrix rhusiopathiae in Japan. Vet. Microbiol. 2022, 270, 109458. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Yamamoto, K.; Ochiai, M.; Yamamoto, T.; Hirano, F.; Imamura, S.; Nagai, H.; Ohishi, K.; Horiuchi, N.; Kijima, M. Prevalence of Met-203 Type SpaA Variant in Erysipelothrix rhusiopathiae Isolates and the Efficacy of Swine Erysipelas Vaccines in Japan. Biologicals 2014, 42, 109–113. [Google Scholar] [CrossRef]
- Ogawa, Y.; Ooka, T.; Shi, F.; Ogura, Y.; Nakayama, K.; Hayashi, T.; Shimoji, Y. The Genome of Erysipelothrix rhusiopathiae, the Causative Agent of Swine Erysipelas, Reveals New Insights into the Evolution of Firmicutes and the Organism’s Intracellular Adaptations. J. Bacteriol. 2011, 193, 2959–2971. [Google Scholar] [CrossRef]
- Ishida, S.; Tien, L.H.T.; Osawa, R.; Tohya, M.; Nomoto, R.; Kawamura, Y.; Takahashi, T.; Kikuchi, N.; Kikuchi, K.; Sekizaki, T. Development of an Appropriate PCR System for the Reclassification of Streptococcus Suis. J. Microbiol. Methods 2014, 107, 66–70. [Google Scholar] [CrossRef]
- Nagai, S.; To, H.; Kanda, A. Differentiation of Erysipelothrix rhusiopathiae Strains by Nucleotide Sequence Analysis of a Hypervariable Region in the SpaA Gene: Discrimination of a Live Vaccine Strain from Field Isolates. J. Vet. Diagnostic Investig. 2008, 20, 336–342. [Google Scholar] [CrossRef]
- To, H.; Tsutsumi, N.; Akihiro, T.; Kamada, T.; Nagano, T.; Nagai, S.; Iwata, A.; Nunoya, T. Protection of Immunized Mice and Swine to Challenge Exposure with Erysipelothrix rhusiopathiae Strains Obtained from Recent Swine Erysipelas Outbreaks in Japan. Acta Vet. Brno 2013, 82, 119–124. [Google Scholar] [CrossRef]
- Sawada, T.; Muramatsu, M.; Seko, K. Response of Growth Agglutinating Antibody and Protection of Pigs Inoculated with Swine Erysipelas Live Vaccine. Nihon Juigaku Zasshi 1979, 41, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chang, B.J.; Riley, T.V. Erysipelothrix rhusiopathiae. Vet. Microbiol. 2010, 140, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Sawada, T.; Muramatsu, M.; Tamura, Y.; Fujisawa, T.; Benno, Y.; Mitsuoka, T. Serotype, Antimicrobial Susceptibility, and Pathogenicity of Erysipelothrix rhusiopathiae Isolates from Tonsils of Apparently Healthy Slaughter Pigs. J. Clin. Microbiol. 1987, 25, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Shimoji, Y.; Osaki, M.; Ogawa, Y.; Shiraiwa, K.; Nishikawa, S.; Eguchi, M.; Yamamoto, T.; Tsutsui, T. Wild Boars: A Potential Source of Erysipelothrix rhusiopathiae Infection in Japan. Microbiol. Immunol. 2019, 63, 465–468. [Google Scholar] [CrossRef] [PubMed]
| Strains and SpaA-Type Variant (Serovar) | Difference of Nucleotide (Amino Acid) in spaA Gene | |||
|---|---|---|---|---|
| 555 (185) | 584 (195) | 609 (203) | 769 (257) | |
| Koganei 65-0.15 (1a) | CCC (P) | GAT (D) | ATT (I) | ATT (I) |
| Tama-96 (2a) | CCA (P) | GAT (D) | ATT (I) | CTT (K) |
| Fujisawa (1a) | CCC (P) | GAT (D) | ATT (I) | CTT (K) |
| 2012 Miyazaki, M203/I257 SpaA-type (1a) | CCC (P) | GAT (D) | ATG (M) | ATT (I) |
| Group No. | Pig No. | Immunization | Challenge Strain | Presence of Clinical Signs after Challenge | Bacterial Isolation from Blood *3 | |||
|---|---|---|---|---|---|---|---|---|
| Pyrexia (°C) *1 | Erythema *2 | Depression | Mortality | |||||
| 1 | 1 | Vaccinated (SER-ME) | Fujisawa (reference) | None | − | − | Survive | Below detection limit |
| 2 | None | − | − | Survive | Below detection limit | |||
| 3 | None | − | − | Survive | Below detection limit | |||
| 4 | None | − | − | Survive | Below detection limit | |||
| 2 | 5 | Unvaccinated control | 41.5 | +++ | + | Dead (Day9) | 2.2 × 102 | |
| 6 | 41.4 | +++ | + | Dead (Day3) | 4.9 × 105 | |||
| 7 | 41.7 | +++ | + | Dead (Day3) | 2.3 × 103 | |||
| 8 | 41.8 | +++ | + | Dead (Day4) | 5.0 × 103 | |||
| 3 | 9 | Vaccinated (SER-ME) | 2012 Miyazaki (variant) | None | − | − | Survive | Below detection limit |
| 10 | None | − | − | Survive | Below detection limit | |||
| 11 | None | − | − | Survive | Below detection limit | |||
| 12 | None | − | − | Survive | Below detection limit | |||
| 4 | 13 | Unvaccinated control | 41.7 | +++ | + | Dead (Day3) | 8.2 × 105 | |
| 14 | 41.7 | +++ | + | Dead (Day3) | 1.8 × 107 | |||
| 15 | 41.7 | +++ | + | Dead (Day3) | 1.2 × 106 | |||
| 16 | 41.9 | +++ | + | Dead (Day3) | 9.1 × 105 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, M.; Kato, A.; Nogami, K.; Akaike, Y.; Furusawa, T.; Kojima, H.; Sasakawa, C. The Swine Erysipelas Vaccine SER-ME Effectively Protects Pigs against Challenge with the Erysipelothrix rhusiopathiae M203/I257 SpaA-Type Variant. Vet. Sci. 2022, 9, 382. https://doi.org/10.3390/vetsci9080382
Morimoto M, Kato A, Nogami K, Akaike Y, Furusawa T, Kojima H, Sasakawa C. The Swine Erysipelas Vaccine SER-ME Effectively Protects Pigs against Challenge with the Erysipelothrix rhusiopathiae M203/I257 SpaA-Type Variant. Veterinary Sciences. 2022; 9(8):382. https://doi.org/10.3390/vetsci9080382
Chicago/Turabian StyleMorimoto, Misako, Atsushi Kato, Kotoe Nogami, Yuta Akaike, Takaaki Furusawa, Hiroe Kojima, and Chihiro Sasakawa. 2022. "The Swine Erysipelas Vaccine SER-ME Effectively Protects Pigs against Challenge with the Erysipelothrix rhusiopathiae M203/I257 SpaA-Type Variant" Veterinary Sciences 9, no. 8: 382. https://doi.org/10.3390/vetsci9080382
APA StyleMorimoto, M., Kato, A., Nogami, K., Akaike, Y., Furusawa, T., Kojima, H., & Sasakawa, C. (2022). The Swine Erysipelas Vaccine SER-ME Effectively Protects Pigs against Challenge with the Erysipelothrix rhusiopathiae M203/I257 SpaA-Type Variant. Veterinary Sciences, 9(8), 382. https://doi.org/10.3390/vetsci9080382






