Canine Gastric Cancer: Current Treatment Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology and Risk Factors
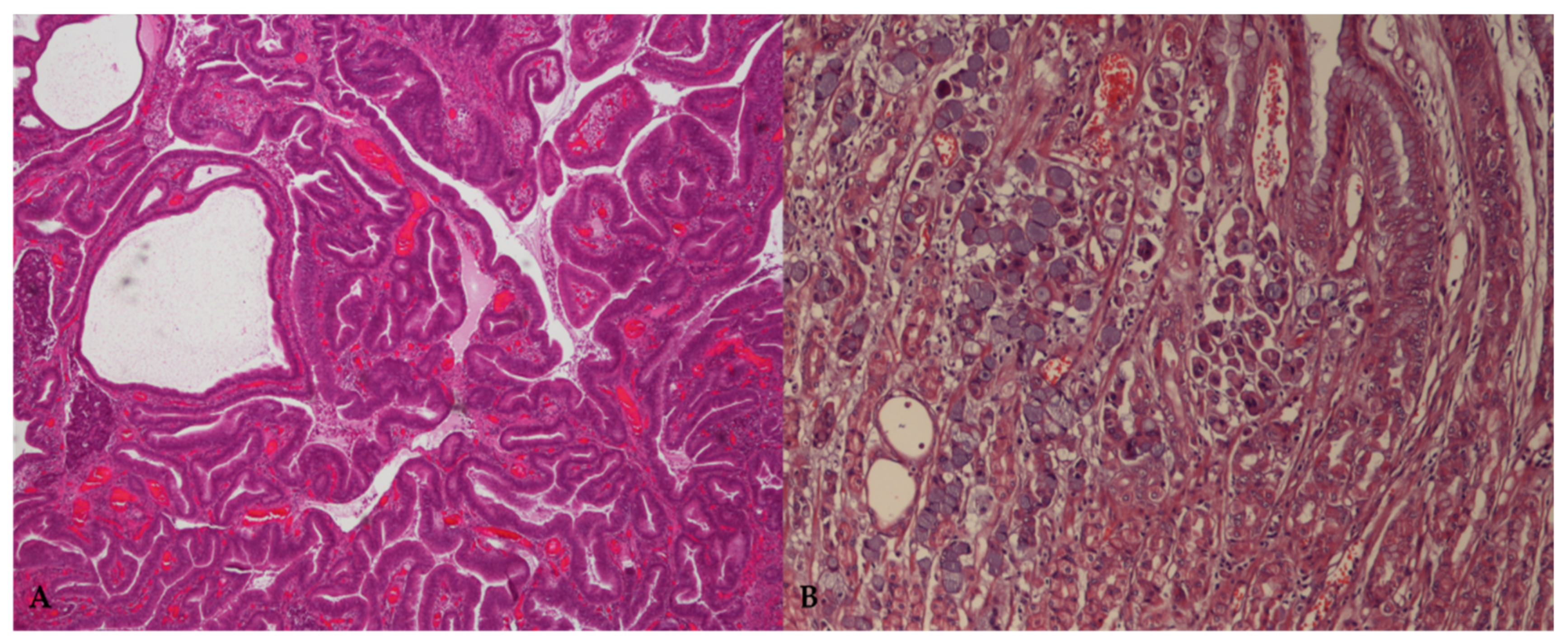
3. Diagnosis and Prognosis
4. Treatment Strategies
4.1. Surgical Resection Approach
4.2. Pharmacological Approach
4.2.1. Chemotherapy Schemes
Carboplatin and Cisplatin
Doxorubicin
| Adjuvant Therapy | Dose and Frequency | Surgical Removal | Survival Time | Histology | Reference |
|---|---|---|---|---|---|
| Carboplatin | 250 mg/m2 carboplatin for the first injection and 200 mg/m2 for the 3 subsequent treatments, 4 times for 13 weeks | Yes | 30 months | Adenocarcinoma | [26] |
| 280 mg/m2 IV every 3 weeks × 5/6 intended doses | Yes | 272 days | [11] | ||
| 300 mg/m2 IV every 3 weeks × 4/4 doses | Yes | 93 days | |||
| 300 mg/m2 IV every 3 weeks × 4/4 doses | Yes | 383 days ** | |||
| Carboplatin/5-FU | Carboplatin: 275 mg/m2 IV, 1 dose single agent Carboplatin/5-FU: 200 mg/m2/150 mg/m2 IV every 3 weeks × 5/5 doses | Yes | 553 days ** | ||
| 5-FU: 150 mg/m2 IV as a slow push Carboplatin: 200 mg/m2 IV over 10 min, 1 h after 5-FU | - | 79 days | Metastatic GC | [37] | |
| - | 26 days | ||||
| Carboplatin Toceranib * | Carboplatin: 285 mg/m2 IV every 3 weeks × 6/6 doses Toceranib: 2.5 mg/kg MWF × 3 months | Yes | 354 days | Adenocarcinoma | [11] |
| Carboplatin Mitoxantrone * | Carboplatin: 240 mg/m2 IV × 1 dose Mitoxantrone: 5 mg/m2 IV every 3 weeks × 6/6 doses | Yes | 190 days | ||
| Gemcitabine/Carboplatin Toceranib * | Week 1—gemcitabine/carboplatin: 57 mg/m2/285 mg/m2 IV Week 2—gemcitabine: 57 mg/m2 IV × 4/4 cycles Toceranib: 3.3 mg/kg PO MWF | Yes | 564 days | ||
| Carboplatin Cyclophosphamide Doxorubicin | Carboplatin: 300 mg/m2 every 3 weeks × 4/4 doses Cyclophosphamide: 15 mg/m2 daily × 2 months Doxorubicin: dose not reported, every 3 weeks × 3 doses | Yes | 274 days | ||
| Toceranib Cyclophosphamide | Toceranib: 1.7 mg/kg PO MWF Cyclophosphamide: 12.5 mg/m2 PO every other day | Yes | 1902 days | ||
| Doxorubicin | 30 mg/m2 IV on day 43 following surgery and repeated on days 69, 90, and 111 | Yes | 114 days | [27] | |
| 30 mg/m2 IV 5 doses | Yes | 81 days | [12] | ||
| 30 mg/m2 IV every 3 weeks × 4/4 doses | Yes | 177 days | Adenocarcinoma | [11] | |
| 6 treatments | Yes | 1 year | Carcinoma | [36] | |
| Doxorubicin Cyclophosphamide | Week 1—doxorubicin: 27 mg/m2 IV Weeks 2 and 3—cyclophosphamide: 222 mg/m2 PO divided over 2 days × 2/5 intended cycles | Yes | 101 days | Adenocarcinoma | [11] |
| Doxorubicin: 25 mg/m2 IV Cyclophosphamide: 50 mg/m2 PO for 4 days | Yes | 9 weeks | [4] | ||
| Doxorubicin Carboplatin | 1 treatment | No | 21 days | Carcinoma | [36] |
| Gemcitabine | 222 mg/m2 IV × 1 dose | Yes | 71 days | Adenocarcinoma | [11] |
| 675 mg/m2 IV every 2 weeks × 4/4 doses | Yes | 97 days | |||
| Toceranib | 1.7 mg/kg PO MWF | Yes | 280 days | ||
| 2.7 mg/kg PO × 2 doses | Yes | 403 days | |||
| 3 mg/kg PO MWF | Yes | 135 days | |||
| 1.5 mg/kg PO × 2 doses | Yes | 49 days | |||
| 3.4 mg/kg PO MWF | Yes | 132 days ** | |||
| Mitoxantrone | 5.5 mg/m2 diluted 1:1 in 0.9% NaCl, then again in 1 mL/4.5 kg, intracavitary | Yes | 311 days | ||
| 5- FU/Cyclophosphamide | 5-FU: 150 mg/m2 IV Cyclophosphamide: 50 mg/m2 PO for 4 days every 2 weeks for two cycles | No | 9 weeks | Adenocarcinoma | [4] |
| FAC protocol Cis-platinum | FAC protocol: doxorubicin, 25 mg/m2 IV, and cyclophosphamide, 75 mg/m2 PO for 4 days on week 1; 5-FU, 150 mg/m2 IV on weeks 2 and 3 for 8 cycles Cis-platinum: 60 mg/m2 IV over 6 h, every 3 weeks for two cycles | No | 7.5 months | ||
| Prednisolone | 0.5–1.0 mg/kg/day | Yes | 104 days ** | [38] | |
| Piroxicam | 0.3 mg/kg/day | No | 374 days | ||
| Piroxicam Cyclophosphamide | Piroxicam: 0.3 mg/kg/day Cyclophosphamide: 15.0 mg/m2/day | Yes | 1366 days | ||
| Piroxicam Prednisolone | Piroxicam: 0.3 mg/kg/day Prednisolone: 0.5–1.0 mg/kg/day | Yes | 1250 days ** |
Mitoxantrone
Gemcitabine
5-Fluororacil
Masitinib and Toceranib
Cyclophosphamide
Prednisolone
Piroxicam
Combination Therapies
4.2.2. Ethical Implications of Chemotherapy
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugen, S.; Thomas, R.E.; German, A.J.; Burgener, I.A.; Mandigers, P.J.J. Gastric carcinoma in canines and humans, a review. Vet. Comp. Oncol. 2017, 15, 692–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patnaik, A.K.; Hurvitz, A.I.; Johnson, G.F. Canine gastrointestinal neoplasms. Vet. Pathol. 1977, 14, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonda, D.; Gualtieri, M.; Scanziani, E. Gastric carcinoma in the dog: A clinicopathological—Study of 11 cases. J. Small Anim. Pract. 1989, 30, 353–360. [Google Scholar] [CrossRef]
- Swann, H.M.; Holt, D.E. Canine Gastric Adenocarcinoma and Leiomyosarcoma: A Retrospective Study of 21 Cases (1986–1999) and Literature Review. J. Am. Anim. Hosp. Assoc. 2002, 38, 157–164. [Google Scholar] [CrossRef]
- Seim-Wikse, T.; Jörundsson, E.; Nødtvedt, A.; Grotmol, T.; Bjornvad, C.R.; Kristensen, A.T.; Skancke, E. Breed predisposition to canine gastric carcinoma—A study based on the Norwegian canine cancer register. Acta Vet. Scand. 2013, 55, 25. [Google Scholar] [CrossRef] [Green Version]
- Simpson, K.W. Diseases of the stomach. In BSAVA Manual of Canine and Feline Gastroenterology, 2nd ed.; Edward, J., Hall, J.W.S., Williams, D.A., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2005; pp. 151–175. [Google Scholar]
- Bray, J.; Neiger, R. Tumours of the stomach. In BSAVA Manual of Canine and Feline Oncology, 3rd ed.; Dobson, J.M., Lascelles, B.D.X., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2011; pp. 208–211. [Google Scholar]
- Canzonieri, V.; Giordano, A. Gastric Cancer In The Precision Medicine Era; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Biller, B.; Berg, J.; Garrett, L.; Ruslander, D.; Wearing, R.; Abbott, B.; Patel, M.; Smith, D.; Bryan, C. 2016 AAHA Oncology Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2016, 52, 181–204. [Google Scholar] [CrossRef] [Green Version]
- Lana, S.E.; Dobson, J.M. Principles of chemotherapy. In BSAVA Manual of Canine and Feline Oncology, 3rd ed.; Dobson, J.M., Lascelles, B.D.X., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2011; pp. 60–79. [Google Scholar]
- Abrams, B.; Wavreille, V.A.; Husbands, B.D.; Matz, B.M.; Massari, F.; Liptak, J.M.; Cray, M.T.; de Mello Souza, C.H.; Wustefeld-Janssens, B.G.; Oblak, M.L.; et al. Perioperative complications and outcome after surgery for treatment of gastric carcinoma in dogs: A Veterinary Society of Surgical Oncology retrospective study of 40 cases (2004–2018). Vet. Surg. 2019, 48, 923–932. [Google Scholar] [CrossRef]
- Babo, V.V.; Eberle, N.; Mischke, R.; Meyer-Lindenberg, A.; Hewicker-Trautwein, M.; Nolte, I.; Betz, D. Canine non-hematopoietic gastric neoplasia. Tierärztliche Prax. Kleintiere 2012, 40, 243–249. [Google Scholar]
- Scanziani, E.; Giusti, A.M.; Gualtieri, M.; Fonda, D. Gastric a Carcinoma in the Belgian shepherd dog. J. Small Anim. Pract. 1991, 32, 465–469. [Google Scholar] [CrossRef]
- Massimo, G.; Maria, G.M. Gastric Neoplasia. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 415–440. [Google Scholar] [CrossRef]
- Sullivan, M.; Lee, R.; Fisher, E.; Nash, A.; McCandlish, I. A study of 31 cases of gastric carcinoma in dogs. Vet. Rec. 1987, 120, 79–83. [Google Scholar] [CrossRef]
- Penninck, D.G.; Moore, A.S.; Gliatt, J. Ultrasonography of Canine Gastric Epithelial Neoplasia. Vet. Radiol. Ultrasound 1998, 39, 342–348. [Google Scholar] [CrossRef]
- Bilek, A.; Hirt, R. Breed-associated increased occurrence of gastric carcinoma in chow-chows. Wien. Tierarztl. Mon. 2007, 94, 71–79. [Google Scholar]
- Carrasco, V.; Canfran, S.; Rodriguez-Franco, F.; Benito, A.; Sainz, A.; Rodriguez-Bertos, A. Canine gastric carcinoma: Immunohistochemical expression of cell cycle proteins (p53, p21, and p16) and heat shock proteins (Hsp27 and Hsp70). Vet. Pathol. 2011, 48, 322–329. [Google Scholar] [CrossRef]
- Withrow, S.J. Cancer of the Gastrointestinal Tract—SECTION E Gastric Cancer. In Small Animal Clinical Oncology; MacEwen’s, W., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 381–431. [Google Scholar]
- Avital, I.; Nissan, A.; Golan, T.; Lawrence, Y.R.; Stojadinovic, A. Cancer of the Stomach. In Cancer Principles & Pratice of Oncology; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Wilcock, B. Histopathology. In Canine and Feline Gastroenterology; Washabau, R.J., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 333–385. [Google Scholar]
- Head, K.; Cullen, J.; Dubielzig, R.; Else, R.; Misdorp, W.; Patnaik, A.; Tateyama, S.; Gaag, I. Histological Classification of Tumors of the Alimentary System of Domestic Animals; Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology: Washington, DC, USA, 2003. [Google Scholar]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt to a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Eisele, J.; McClaran, J.K.; Runge, J.J.; Holt, D.E.; Culp, W.T.; Liu, S.; Long, F.; Bergman, P.J. Evaluation of risk factors for morbidity and mortality after pylorectomy and gastroduodenostomy in dogs. Vet. Surg. 2010, 39, 261–267. [Google Scholar] [CrossRef]
- Sellon, R.K.; Bissonnette, K.; Bunch, S.E. Long—Term Survival After Total Gastrectomy for Gastric. J. Vet. Lnternal Med. 1996, 10, 333–335. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, J.H.; Jee, C.H.; Lee, J.H.; Moon, J.H.; Kim, N.H.; Sur, J.H.; Cho, K.W.; Kang, B.T.; Ha, J.; et al. A case of gastric adenocarcinoma in a Shih Tzu dog: Successful treatment of early gastric cancer. J. Vet. Med. Sci. 2014, 76, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.; Anderson, G.M. Metastasis of gastric adenocarcinoma to the abdominal wall following placement of a gastrostomy tube in a dog. Can. Vet. J. 2005, 46, 641–643. [Google Scholar]
- Reiss, K.A.; Calvert, A.H.; O’Dwyer, P.J. Platinum Analogs. In Cancer Principles & Practice of Oncology, 11th ed.; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Gustafson, D.L.; Page, R.L. Cancer Chemotherapy. In Withrow & MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 157–179. [Google Scholar]
- Papich, M.G. Saunders Handbook of Veterinary Drugs: Small and Large Animal, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Wittenburg, L.A.; Gustafson, D.L. Chemotherapy of Neoplastic Diseases. In Veterinary Pharmacology and Therapeutics, 10th ed.; Riviere, J.E., Papich, M.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1191–1225. [Google Scholar]
- Fox, L.E. Carboplatin. J. Am. Anim. Hosp. Assoc. 2000, 36, 13–14. [Google Scholar] [CrossRef]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Vijgh, W.J.F. Clinical Pharmacokinetics of Carboplatin. Clin. Pharm. 1991, 21, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Whitwhouse, J.M.A. Cis-platinum: A new anticancer agent. Br. Med. J. 1979, 1, 1689–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koterbay, A.M.; Muthupalani, S.; Fox, J.G.; McNiel, E.A. Risk and characteristics of gastric carcinoma in the chow chow dog. Can. Vet. J. 2020, 61, 396–400. [Google Scholar]
- Menard, K.; Flesner, B.K.; Glahn, A.; Boudreaux, B.; Bryan, J.N. Concurrent 5-fluorouracil and carboplatin for the treatment of canine carcinomas. Vet. Comp. Oncol. 2018, 16, 590–595. [Google Scholar] [CrossRef]
- Ohmi, A.; Ohno, K.; Chambers, J.K.; Uchida, K.; Nakagawa, T.; Tomiyasu, H.; Tsujimoto, H. Clinical and histopathological features and prognosis of gastrointestinal adenocarcinomas in Jack Russell Terriers. J. Vet. Med. Sci. 2021, 83, 167–173. [Google Scholar] [CrossRef]
- Lee, J.J.; Chu, E. Antimetabolites. In Cancer Principles & Practice of Oncology, 11th ed.; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- London, C.A. Molecular/Targeted Therapy of Cancer—SECTION B Signal Transduction and Cance. In Withrow & MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 215–244. [Google Scholar]
- Marech, I.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Introna, M.; Zito, A.F.; Gadaleta, C.D.; Ranieri, G. Masitinib (AB1010), from canine tumor model to human clinical development: Where we are? Crit. Rev. Oncol. Hematol. 2014, 91, 98–111. [Google Scholar] [CrossRef]
- Hahn, K.A.; Oglivie, G.; Rusk, T.; Devauchelle, P.; Leblanc, A.; Legendre, A.; Powers, B.; Leventhal, P.S.; Kinet, J.-P.; Palmerini, F.; et al. Masitinib is Safe and Effective for the Treatment of Canine Mast Cell Tumors. J. Vet. Intern. Med. 2008, 22, 1301–1309. [Google Scholar] [CrossRef]
- Hahn, K.A.; Legendre, A.M.; Shaw, N.G.; Phillips, B.; Ogilvie, G.K.; Prescott, D.M.; Atwater, S.W.; Carreras, J.K.; Lana, S.E.; Ladue, T.; et al. Evaluation of 12- and 24-month survival rates after treatment with masitinib in dogs with nonresectable mast cell tumors. AJVR 2010, 71, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, H.; Tanaka, T.; Mie, K.; Nishida, H.; Miura, N.; Akiyoshi, H. Assessment of postoperative adjuvant treatment using toceranib phosphate against adenocarcinoma in dogs. J. Vet. Intern. Med. 2020, 34, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Tew, K.D. Alkylating Agents. In Cancer Principles & Practice of Oncology; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Pickup, M.E. Clinical Pharmacokinetics of Prednisone and Prednisolone. Clin. Pharm. 1979, 4, 111–128. [Google Scholar] [CrossRef]
- Frey, B.M.; Frey, F.J. Clinical Pharmacokinetics of Prednisone and Prednisolone. Clin. Pharm. 1990, 19, 126–146. [Google Scholar] [CrossRef]
- Krensky, A.M.; Azzi, J.R.; Hafler, D.A. Immunosuppressants and Tolerogens. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 637–653. [Google Scholar]
- Schimmer, B.P.; Funder, J.W. Adrenocorticotropic Hormone, Adrenal Steroids, and the Adrenal Cortex. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 845–861. [Google Scholar]
- Olkkola, K.T.; Brunetto, A.V.; Mattila, M.J. Pharmacokinetics of Oxicam Nonsteroidal Anti-Inflammatory Agents. Clin. Pharm. 1994, 26, 107–120. [Google Scholar] [CrossRef]
- Grosser, T.; Smyth, E.M.; FitzGerald, G.A. Pharmacotherapy of Inflammation, Fever, Pain, and Gout. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 685–709. [Google Scholar]
- Mutsaers, A.J. Molecular/Targeted Therapy of Cancer—SECTION C Antiangiogenic and Metronomic Therapy. In Withrow & MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 229–237. [Google Scholar]
- Knapp, D.W.; McMillan, S.K. Tumors of the Urinary System. In Withrow & MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 572–582. [Google Scholar]
- Knapp, D.W.; Richardson, R.C.; Bottoms, G.D.; Teclaw, R.; Chan, T.C.K. Phase I trial of piroxicam in 62 dogs bearing naturally occurring tumors. Cancer Chemother Pharm. 1992, 29, 214–218. [Google Scholar] [CrossRef]
- Dobson, J.M. Introduction: Cancer in cats and dogs. In BSAVA Manual of Canine and Feline Oncology, 3rd ed.; Dobson, J.M., Lascelles, B.D.X., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2011; pp. 1–5. [Google Scholar]
- Rollin, B.E. When to treat animals with cancer. In BSAVA Manual of Canine and Feline Oncology, 3rd ed.; Dobson, J.M., Lascelles, B.D.X., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2011; pp. 40–43. [Google Scholar]
- Rollin, B.E. Euthanasia, moral stress, and chronic illness in veterinary medicine. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 651–659. [Google Scholar] [CrossRef]
- Lascelles, B.D.X. Supportive Care for the Cancer Patient. In Withrow & MacEwen’s Small Animal Clinical Oncology, 5th ed.; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 245–259. [Google Scholar]
- Trumpatori, B.J.; Lascelles, B.D.X. Relief of chronic cancer pain. In BSAVA Manual of Canine and Feline Oncology, 3rd ed.; Dobson, J.M., Lascelles, B.D.X., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2011; pp. 111–129. [Google Scholar]
- Williams, J.; Phillips, C.; Byrd, H.M. Factors Which Influence Owners When Deciding to Use Chemotherapy in Terminally Ill Pets. Animals 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.; Adega, F.; Chaves, R. The Importance of Cancer Cell Lines as in vitro Models in Cancer Methylome Analysis and Anticancer Drugs Testing. In Oncogenomics and Cancer Proteomics—Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer; InTech: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
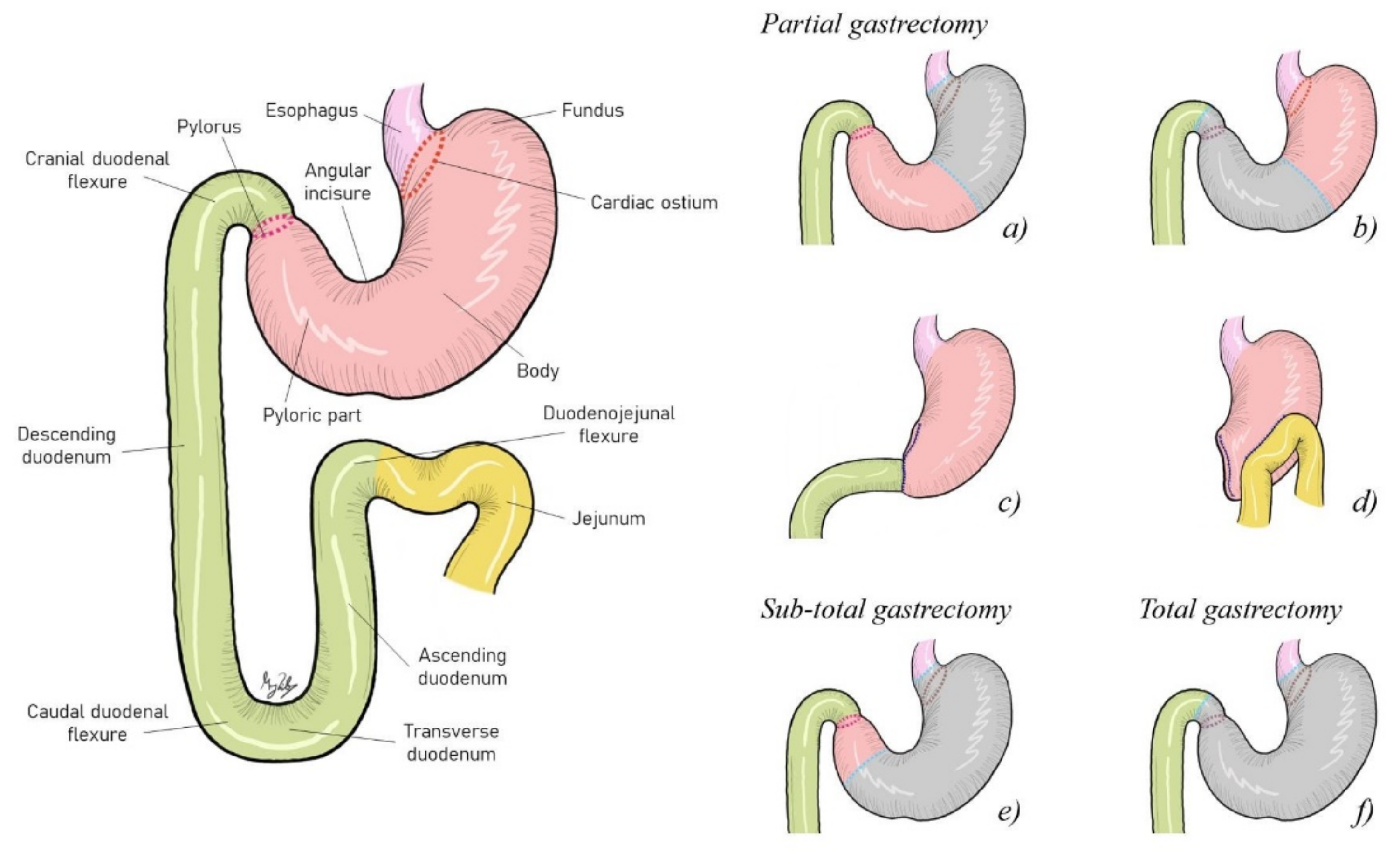

| Surgery | Complications | Survival Time | References | |
|---|---|---|---|---|
| Partial gastrectomy | Major intra- and postoperative complications | Spillage of gastric contents and septic peritonitis | 1 day | [11] |
| Inadvertent stab incision into small bowel and septic peritonitis | 190 days | |||
| Major postoperative complications | Cardiopulmonary arrest | 2 days | ||
| Septic peritonitis | 2 days | |||
| Gastric stasis, pancreatitis, and cardiopulmonary arrest | 15 days | |||
| Intraoperative complications | Minor hemorrhage | 2 days | ||
| 3 days | ||||
| 177 days | ||||
| Minor postoperative complications | Hypertension | 274 days | ||
| Hyporexia | 132 days | |||
| Postoperative complications | Pericardial effusion | 7 days | [12] | |
| Partial gastrectomy and splenectomy | - | Disseminated intravascular coagulation and ventricular arrhythmias | 2 days | |
| Partial distal gastrectomy and gastroduodenal anastomosis | Postoperative complications | Discomfort, vomiting, and diarrhea for 10–15 days | 3 years | [14] |
| 4 years | ||||
| 30 days–20 months * | ||||
| 3 days | ||||
| 5 months | ||||
| Billroth I | Major postoperative complication | Pancreatitis | 16 days | [11] |
| Minor intraoperative complication | Hypertension | 71 days | ||
| Postoperative complications | Ascending cholangiohepatitis and pancreatitis | 49 days | ||
| Minor intraoperative complications | Major hemorrhage and 2nd-degree atrioventricular block | 258 days | ||
| Major postoperative complications | Severe pancreatitis and intermittent hypoglycemia | 183 days | ||
| Pulled out the gastrostomy tube | ||||
| - | Persistent vomiting | 3 days | [4] | |
| - | Vomiting and anorexia | 6 weeks | ||
| - | Vomiting and anorexia | 6 weeks | ||
| - | Vomiting and anorexia | 10 months | ||
| Billroth II | - | Vomiting and anorexia | 4 weeks | |
| - | Vomiting and anorexia | 5 weeks | ||
| Subtotal gastrectomy | Major intra- and postoperative complications | Septic peritonitis | 13 days | [11] |
| Minor intra- and postoperative complications | Hemorrhage and vomiting | 93 days | ||
| Total gastrectomy | - | Discomfort during and after eating | 240 days | [25] |
| Pylorectomy and gastroduodenostomy | - | 578 days ** | [24] | |
| 33 days *** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, D.; Cabral, I.; Vale, N.; Amorim, I. Canine Gastric Cancer: Current Treatment Approaches. Vet. Sci. 2022, 9, 383. https://doi.org/10.3390/vetsci9080383
Araújo D, Cabral I, Vale N, Amorim I. Canine Gastric Cancer: Current Treatment Approaches. Veterinary Sciences. 2022; 9(8):383. https://doi.org/10.3390/vetsci9080383
Chicago/Turabian StyleAraújo, Diana, Inês Cabral, Nuno Vale, and Irina Amorim. 2022. "Canine Gastric Cancer: Current Treatment Approaches" Veterinary Sciences 9, no. 8: 383. https://doi.org/10.3390/vetsci9080383
APA StyleAraújo, D., Cabral, I., Vale, N., & Amorim, I. (2022). Canine Gastric Cancer: Current Treatment Approaches. Veterinary Sciences, 9(8), 383. https://doi.org/10.3390/vetsci9080383







