Effects of Saccharomyces boulardii Supplementation on Nutritional Status, Fecal Parameters, Microbiota, and Mycobiota in Breeding Adult Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Nutritional Parameters
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.B.; Suchodolski, J.S. Importance of gut microbiota for the health and disease of dogs and cats. Anim. Front. 2016, 6, 37–42. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Zadjali, F. Use of germ-free animal models in microbiota-related research. J. Microbiol. Biotechnol. 2015, 25, 1583–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The gastrointestinal microbiome: A review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Pilla, R.; Suchodolski, J.S. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [Green Version]
- Broom, D.M.; Kirkton, R.D. Welfare, stress, behaviour and pathophysiology. Vet. Pathophysiol. 2004, 337–369. [Google Scholar]
- Beerda, B.; Schilder, M.B.; Van Hooff, J.A.; De Vries, H.W.; Mol, J.A. Behavioural and hormonal indicators of enduring environmental stress in dogs. Anim. Welf.-Potters Bar 2000, 9, 49–62. [Google Scholar]
- Wells, D.L.; Graham, L.; Hepper, P.G. The influence of auditory stimulation on the behaviour of dogs housed in a rescue shelter. Anim. Welf. 2002, 11, 385–393. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Mycotoxins in Food; Food & Agriculture Organization: Rome, Italy, 2001; Available online: https://apps.who.int/iris/handle/10665/42467 (accessed on 19 July 2020).
- Redfern, A.; Suchodolski, J.; Jergens, A. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Rec. 2017, 181, 370. [Google Scholar] [CrossRef]
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Ther. Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Szajewska, H.; Kołodziej, M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2015, 42, 793–801. [Google Scholar] [CrossRef]
- Kabbani, T.A.; Pallav, K.; Dowd, S.E.; Villafuerte-Galvez, J.; Vanga, R.R.; Castillo, N.E.; Hansen, J.; Dennis, M.; Leffler, D.A.; Kelly, C.P. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes 2017, 8, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Tomičić, M.Z.; Čolović, R.R.; Čabarkapa, S.I.; Vukmirović, M.Đ.; Đuragić, M.O.; Tomičić, M.R. Beneficial properties of probiotic yeast Saccharomyces boulardii. Food Feed. Res. 2016, 43, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Pais, G.M.; Liu, J.; Zepcan, S.; Avedissian, S.N.; Rhodes, N.J.; Downes, K.J.; Moorthy, J.S.; Scheetz, M.H. Vancomycin-induced kidney injury: Animal models of toxicodynamics, mechanisms of injury, human translation, and potential strategies for prevention. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 438–454. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Laflamme D.R.P.C. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Greco, D.S. Quick Resource Guide. Diagnosis and Dietary Management of Gastrointestinal Diseases. 2016. Available online: https://www.purinaveterinarydiets.com/media/1202/gi_quick_reference_guide.pdf (accessed on 19 July 2020).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Mota-Gutierrez, J.; Ferrocino, I.; Rantsiou, K.; Cocolin, L. Metataxonomic comparison between internal transcribed spacer and 26S ribosomal large subunit (LSU) rDNA gene. Int. J. Food Microbiol. 2019, 290, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019, 9, 253–258. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Fracassi, F.; Bresciani, F.; Galuppi, R.; Diana, A.; Linta, N.; Bettini, G.; Morini, M.; Pietra, M. Effect of Saccharomyces boulardii in dogs with chronic enteropathies: Double-blinded, placebo-controlled study. Vet. Rec. 2018, 182, 258. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011. [Google Scholar] [CrossRef] [Green Version]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, B.; Laflamme, D. Effect of diet on markers of intestinal health in dogs. Res. Vet. Sci. 2002, 72, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, N.; Hill, S.; Parnell, N.K.; Mansell, J.; Suchodolski, J.S.; Steiner, J.M. Fecal and urinary N-methylhistamine concentrations in dogs with chronic gastrointestinal disease. Vet. J. 2014, 201, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Grellet, A.; Heilmann, R.M.; Lecoindre, P.; Feugier, A.; Day, M.J.; Peeters, D.; Freiche, V.; Hernandez, J.; Grandjean, D.; Suchodolski, J.S.; et al. Fecal calprotectin concentrations in adult dogs with chronic diarrhea. Am. J. Vet. Res. 2013, 74, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Grellet, A.; Mila, H.; Heilmann, R.M.; Feugier, A.; Gruetzner, N.; Suchodolski, J.S.; Steiner, J.M.; Chastant-Maillard, S. Effect of age, gestation and lactation on faecal IgA and calprotectin concentrations in dogs. J. Nutr. Sci. 2014, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Walsham, N.E.; Sherwood, R.A. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2016, 9, 21. [Google Scholar]
- Ohlsson, B.; Roth, B.; Larsson, E.; Höglund, P. Calprotectin in serum and zonulin in serum and feces are elevated after introduction of a diet with lower carbohydrate content and higher fiber, fat and protein contents. Biomed. Rep. 2017, 6, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Otoni, C.C.; Heilmann, R.M.; García-Sancho, M.; Sainz, A.; Ackermann, M.R.; Suchodolski, J.S.; Steiner, J.M.; Jergens, A.E. Serologic and fecal markers to predict response to induction therapy in dogs with idiopathic inflammatory bowel disease. J. Vet. Intern. Med. 2018, 32, 999–1008. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Guard, B.C.; Weber, K.; Suchodolski, J.S.; Steiner, J.M. Development and analytical validation of an enzyme-linked immunosorbent assay for the quantification of canine calprotectin in serum and feces from dogs. J. Vet. Intern. Med. 2011, 25, 693. [Google Scholar]
- Benyacoub, J.; Czarnecki-Maulden, G.L.; Cavadini, C.; Sauthier, T.; Anderson, R.E.; Schiffrin, E.J.; von der Weid, T. Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J. Nutr. 2003, 133, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Sjaastad, O.V.; Sand, O.; Hove, K. Physiology of Domestic Animals; Scandinavian Veterinary Press AS: Oslo, Norway, 2010. [Google Scholar]
- Staufenbiel, S.M.; Penninx, B.W.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 2013, 38, 1220–1235. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Saettone, V.; Biasato, I.; Radice, E.; Schiavone, A.; Bergero, D.; Meineri, G. State-of-the-Art of the Nutritional Alternatives to the Use of Antibiotics in Humans and Monogastric Animals. Animals 2020, 10, 2199. [Google Scholar] [CrossRef]
- Bayazit, V. Evaluation of cortisol and stress in captive animals. Aust. J. Basic Appl. Sci. 2009, 3, 1022–1031. [Google Scholar]
- Cornale, P.; Macchi, E.; Miretti, S.; Renna, M.; Lussiana, C.; Perona, G.; Mimosi, A. Effects of stocking density and environmental enrichment on behavior and fecal corticosteroid levels of pigs under commercial farm conditions. J. Vet. Behav. 2015, 10, 569–576. [Google Scholar] [CrossRef]
- Uetake, K.; Yang, C.H.; Endo, A.; Tanaka, T. Effects of sheltering on behavior and fecal corticosterone level of elderly dogs. Front. Vet. Sci. 2016, 3, 103. [Google Scholar] [CrossRef] [Green Version]
- Dalla Villa, P.; Barnard, S.; Di Fede, E.; Podaliri, M.; Candeloro, L.; Di Nardo, A.; Siracusa, C.; Serpell, J.A. Behavioural and physiological responses of shelter dogs to long-term confinement. Vet. Ital. 2013, 49, 231–241. [Google Scholar]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, J.M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, S.; Kennedy, D.; Watson, R.; Valsecchi, P.; Arnott, G. Revisiting a previously validated temperament test in shelter dogs, including an examination of the use of fake model dogs to assess conspecific sociability. Animals 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef]
- Schatz, S.; Palme, R. Measurement of faecal cortisol metabolites in cats and dogs: A non-invasive method for evaluating adrenocortical function. Vet. Res. Commun. 2001, 25, 271–287. [Google Scholar] [CrossRef]
- Righi, C.; Menchetti, L.; Orlandi, R.; Moscati, L.; Mancini, S.; Diverio, S. Welfare assessment in shelter dogs by using physiological and immunological parameters. Animals 2019, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Gazzano, A.; Mariti, C.; Alvares, S.; Cozzi, A.; Tognetti, R.; Sighieri, C. The prevention of undesirable behaviors in dogs: Effectiveness of veterinary behaviorists’ advice given to puppy owners. J. Vet. Behav. 2008, 3, 125–133. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Serpell, J.A.; Poole, T.B. Correlates of pen size and housing conditions on the behaviour of kennelled dogs. Appl. Anim. Behav. Sci. 1992, 34, 365–383. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Companion animals symposium: Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 2011, 89, 1520–1530. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S.; Xenoulis, P.G.; Paddock, C.G.; Steiner, J.M.; Jergens, A.E. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet. Microbiol. 2010, 142, 394–400. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Daristotle, L.; Quesnell, R.; Reinhart, G.A.; Frantz, N.Z. Altered fecal microbiota, IgA, and fermentative end-products in adult dogs fed prebiotics and a nonviable Lactobacillus acidophilus. J. Anim. Sci. 2021, 99, skab347. [Google Scholar] [CrossRef]
- You, I.; Kim, M.J. Comparison of gut microbiota of 96 healthy dogs by individual traits: Breed, age, and body condition score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- García-Belenguer, S.; Grasa, L.; Valero, O.; Palacio, J.; Luño, I.; Rosado, B. Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment. Animals 2021, 11, 3121. [Google Scholar] [CrossRef]
- Pereira, A.M.; Clemente, A. Dogs’ microbiome from tip to toe. Top. Companion Anim. Med. 2021, 45, 100584. [Google Scholar] [CrossRef]
- Doron, I.; Leonardi, I.; Li, X.V.; Fiers, W.D.; Semon, A.; Bialt-DeCelie, M.; Migaud, M.; Gao, I.H.; Lin, W.-Y.; Kusakabe, T. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 2021, 184, 1017–1031. [Google Scholar] [CrossRef]
- Li, X.V.; Leonardi, I.; Iliev, I.D. Gut mycobiota in immunity and inflammatory disease. Immunity 2019, 50, 1365–1379. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Zhou, H.; Yu, B.; He, J.; Wu, A.; Huang, Z.; Zheng, P.; Mao, X.; Yu, J. The Nutritional Significance of Intestinal Fungi: Alteration of Dietary Carbohydrate Composition Triggers Colonic Fungal Community Shifts in a Pig Model. Appl. Environ. Microbiol. 2021, 87, e00038-21. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Intestinal microbiota of dogs and cats: A bigger world than we thought. Vet. Clin. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Morris, E.K.; Allenspach, K.; Jergens, A.E.; Harmoinen, J.A.; Westermarck, E.; Steiner, J.M. Prevalence and identification of fungal DNA in the small intestine of healthy dogs and dogs with chronic enteropathies. Vet. Microbiol. 2008, 132, 379–388. [Google Scholar] [CrossRef]
- Shi, T.; Yan, X.; Sun, H.; Fu, Y.; Hao, L.; Zhou, Y.; Liu, Y.; Han, W.; Bao, G.; Suo, X. An Investigation of the Relationship between Cyniclomyces guttulatus and Rabbit Diarrhoea. Pathogens 2021, 10, 880. [Google Scholar] [CrossRef]
- Holmes, M.J.; Shah, P.; Wek, R.C.; Sullivan, W.J., Jr. Simultaneous ribosome profiling of human host cells infected with Toxoplasma gondii. Msphere 2019, 4, e00292-19. [Google Scholar] [CrossRef] [Green Version]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. WJG 2014, 20, 16489. [Google Scholar] [CrossRef] [PubMed]
- van der Aa Kühle, A.; Jespersen, L. The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S rDNA, the ITS1-5.8 S rDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Syst. Appl. Microbiol. 2003, 26, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.L.; Dowd, S.E.; Stephenson, C.; Steiner, J.M.; Suchodolski, J.S. Characterization of the fungal microbiome (mycobiome) in fecal samples from dogs. Vet. Med. Int. 2013, 2013, 658373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spatz, M.; Richard, M.L. Overview of the potential role of Malassezia in gut health and disease. Front. Cell. Infect. Microbiol. 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, E.A.; Schreijen, E.M.; Patil, A.R.; Hussein, H.S.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C., Jr. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 2003, 81, 2008–2018. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]


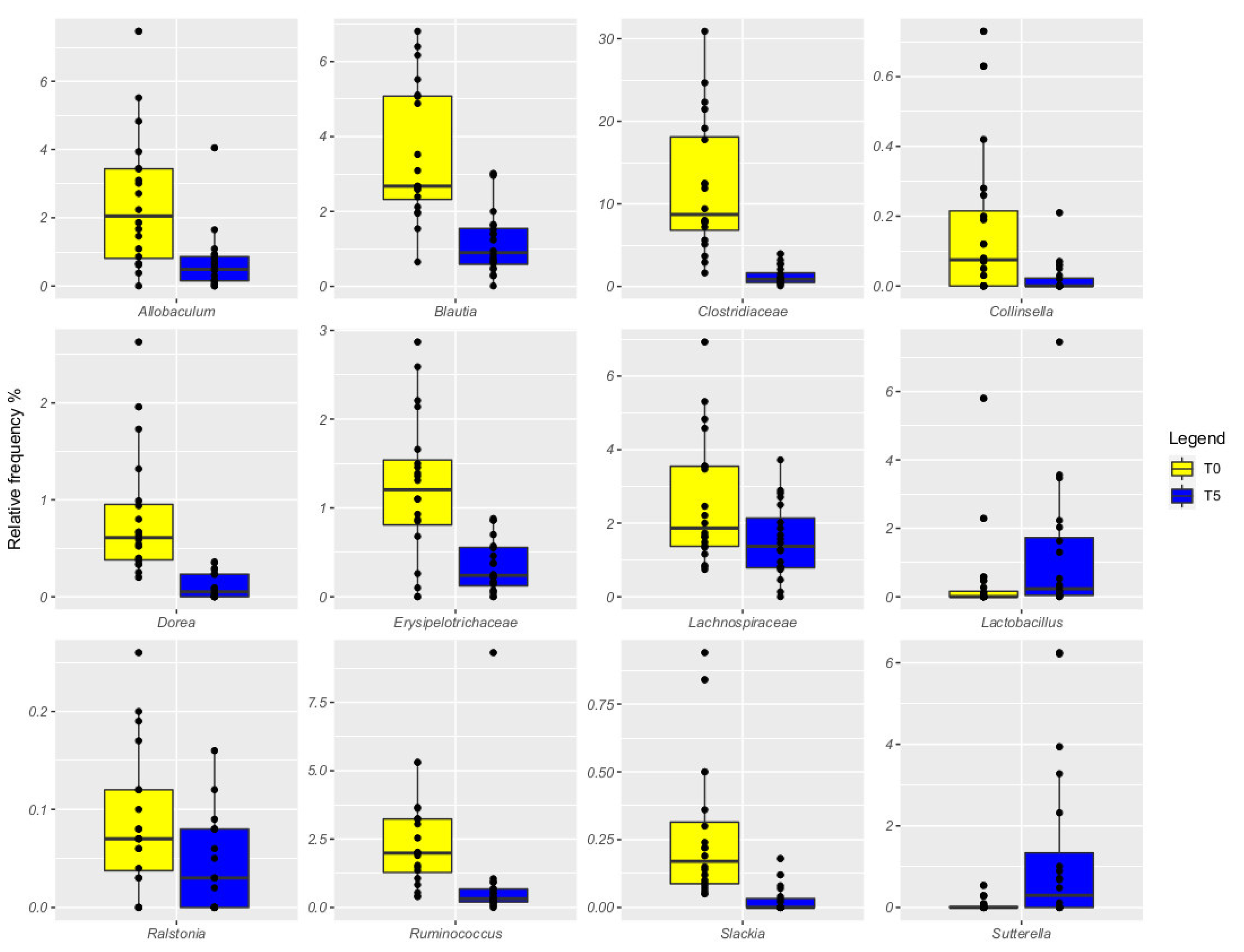
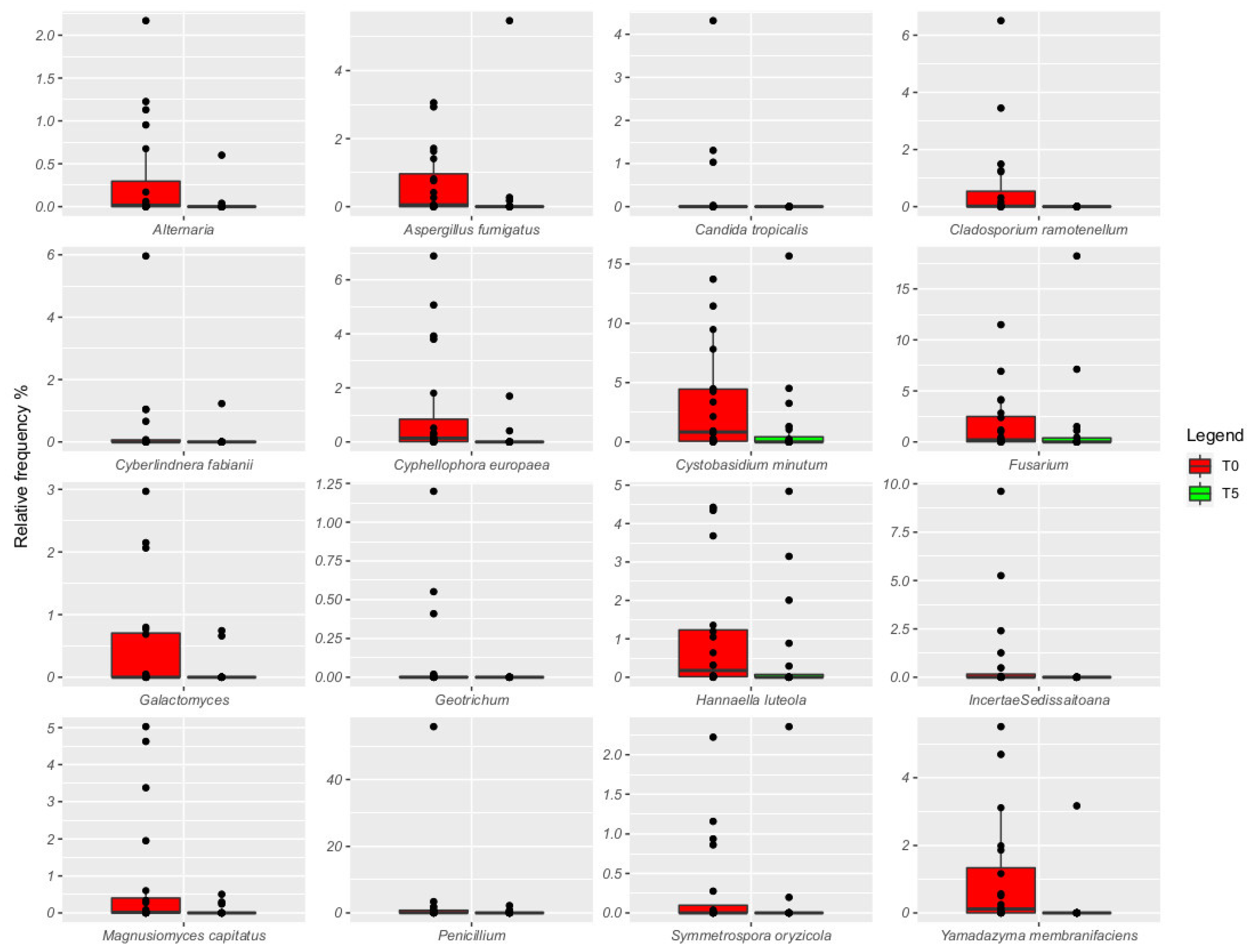

| Group (G) | Time (T) | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | TRT | T0 | T1 | T2 | T3 | T4 | T5 | G | T | G | T | G × T | |
| Laboratory analytes (unit) | |||||||||||||
| pH | 6.51 | 6.50 | 6.50 | 6.54 | 6.50 | 6.52 | 6.46 | 6.50 | 0.06 | 0.05 | 0.982 | 0.152 | 0.161 |
| Calprotectin (µg/g) | 5.95 | 5.57 | 5.99 ab | 6.04 a | 5.94 b | 5.63 cd | 5.64 c | 5.32 d | 0.85 | 0.60 | 0.753 | <0.001 | 0.108 |
| Lactoferrin (µg/g) | 1.53 | 1.32 | 1.45 | 1.45 | 1.31 | 1.38 | 1.49 | 1.44 | 0.22 | 0.16 | 0.489 | 0.260 | 0.330 |
| Zonulin (ng/mL) | 52.51 | 50.36 | 49.58 | 52.35 | 49.84 | 53.79 | 50.18 | 52.96 | 0.77 | 1.16 | 0.046 | 0.250 | 0.710 |
| Cortisol (pg/mg) | 0.61 | 0.55 | 0.60 | 0.65 | 0.57 | 0.60 | 0.53 | 0.54 | 0.02 | 0.02 | 0.090 | 0.100 | <0.001 |
| Immunoglobulin A (mg/g) | 47.71 | 48.17 | 48.87 | 48.68 | 48.33 | 47.40 | 47.66 | 46.75 | 1.70 | 1.23 | 0.849 | 0.100 | 0.116 |
| Short chain fatty acids (μmol/g) | 143.56 | 146.96 | 148.11 | 145.94 | 139.55 | 146.24 | 145.04 | 146.77 | 21.39 | 15.54 | 0.912 | 0.112 | 0.180 |
| Indole/skatole (μmol/g) | 1.76 | 1.60 | 1.67 | 1.73 | 1.63 | 1.66 | 1.69 | 1.68 | 0.04 | 0.06 | <0.001 | 0.937 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meineri, G.; Martello, E.; Atuahene, D.; Miretti, S.; Stefanon, B.; Sandri, M.; Biasato, I.; Corvaglia, M.R.; Ferrocino, I.; Cocolin, L.S. Effects of Saccharomyces boulardii Supplementation on Nutritional Status, Fecal Parameters, Microbiota, and Mycobiota in Breeding Adult Dogs. Vet. Sci. 2022, 9, 389. https://doi.org/10.3390/vetsci9080389
Meineri G, Martello E, Atuahene D, Miretti S, Stefanon B, Sandri M, Biasato I, Corvaglia MR, Ferrocino I, Cocolin LS. Effects of Saccharomyces boulardii Supplementation on Nutritional Status, Fecal Parameters, Microbiota, and Mycobiota in Breeding Adult Dogs. Veterinary Sciences. 2022; 9(8):389. https://doi.org/10.3390/vetsci9080389
Chicago/Turabian StyleMeineri, Giorgia, Elisa Martello, David Atuahene, Silvia Miretti, Bruno Stefanon, Misa Sandri, Ilaria Biasato, Maria Rita Corvaglia, Ilario Ferrocino, and Luca Simone Cocolin. 2022. "Effects of Saccharomyces boulardii Supplementation on Nutritional Status, Fecal Parameters, Microbiota, and Mycobiota in Breeding Adult Dogs" Veterinary Sciences 9, no. 8: 389. https://doi.org/10.3390/vetsci9080389
APA StyleMeineri, G., Martello, E., Atuahene, D., Miretti, S., Stefanon, B., Sandri, M., Biasato, I., Corvaglia, M. R., Ferrocino, I., & Cocolin, L. S. (2022). Effects of Saccharomyces boulardii Supplementation on Nutritional Status, Fecal Parameters, Microbiota, and Mycobiota in Breeding Adult Dogs. Veterinary Sciences, 9(8), 389. https://doi.org/10.3390/vetsci9080389







