Simultaneous Analysis of the p16 Gene and Protein in Canine Lymphoma Cells and Their Correlation with pRb Phosphorylation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Treatment of Cell Lines with 5-aza-2-Deoxycytidine
2.3. Real-Time Polymerase Chain Reaction (PCR)
2.4. Western Blot Analysis
2.5. Statistical Methods
3. Results
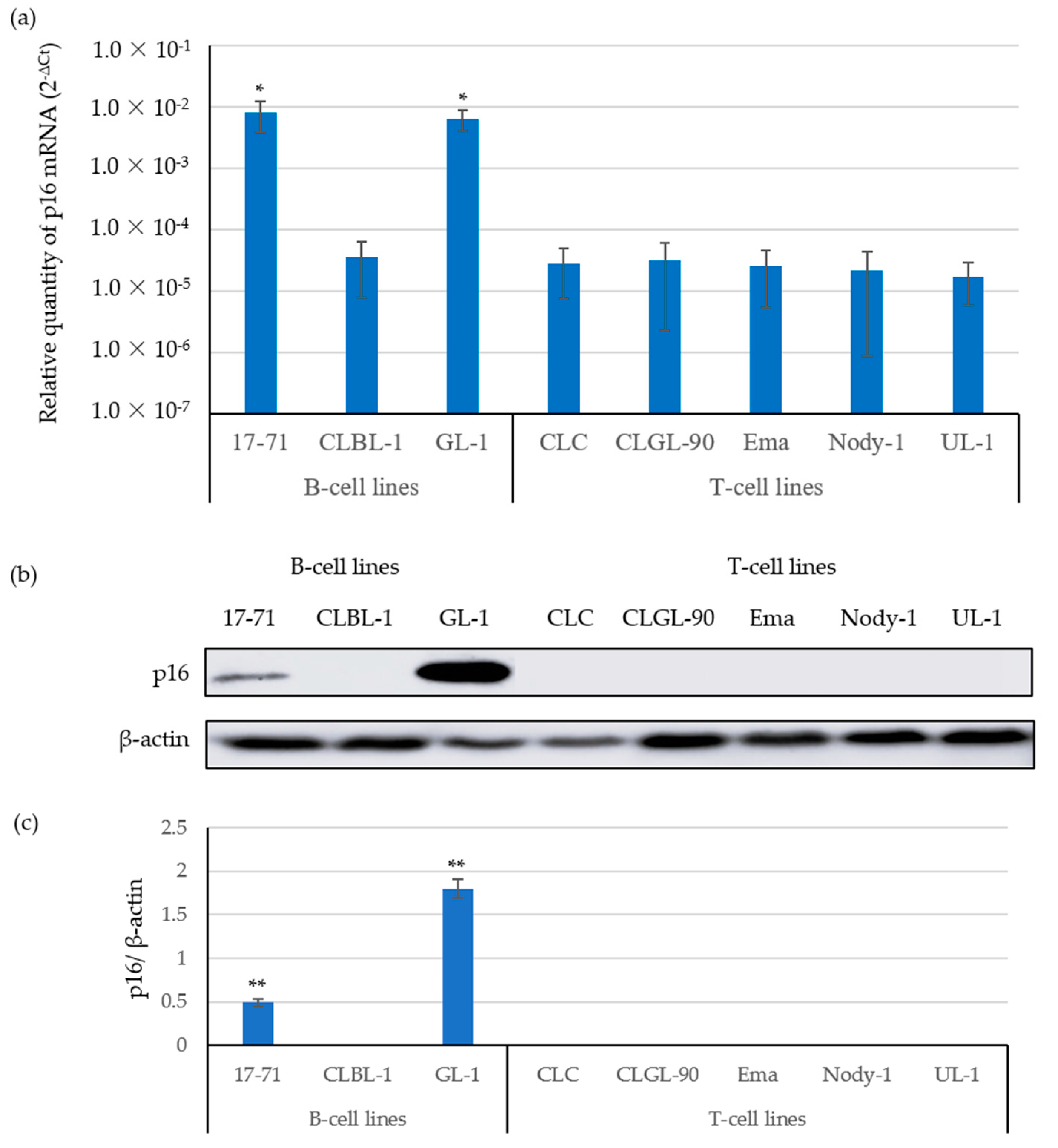
3.1. The Expression of p16 mRNA Was Correlated with p16 Protein Expressions
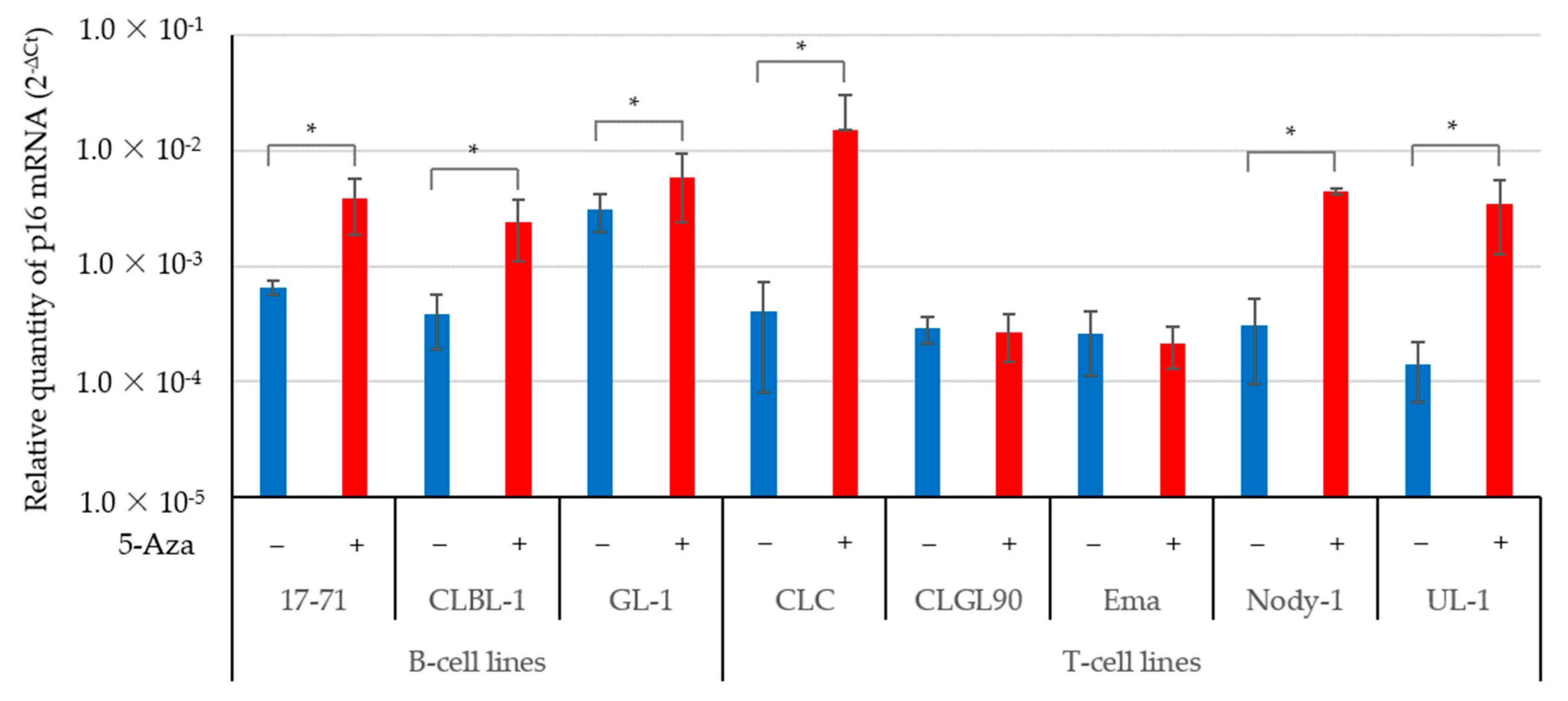
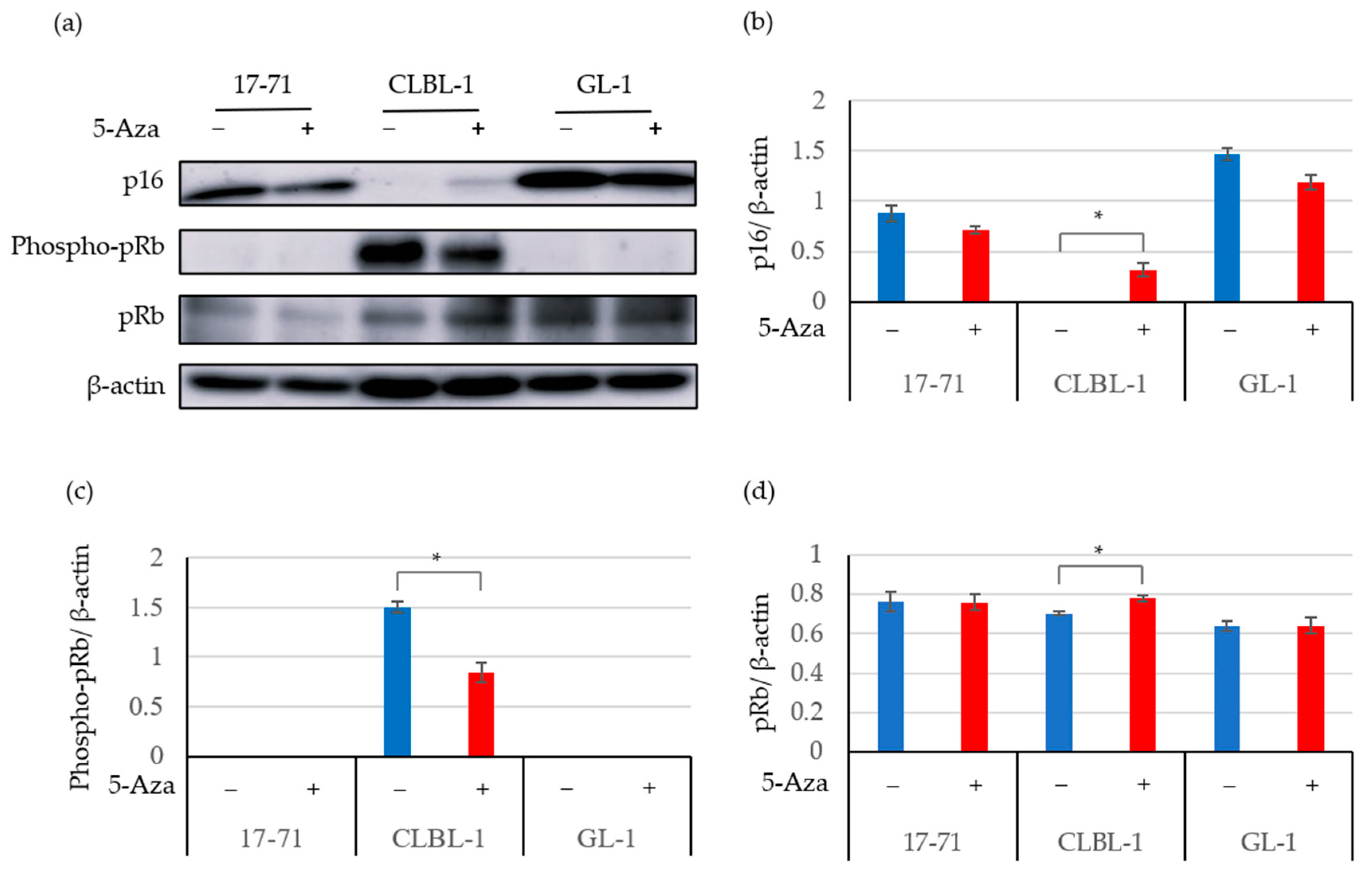
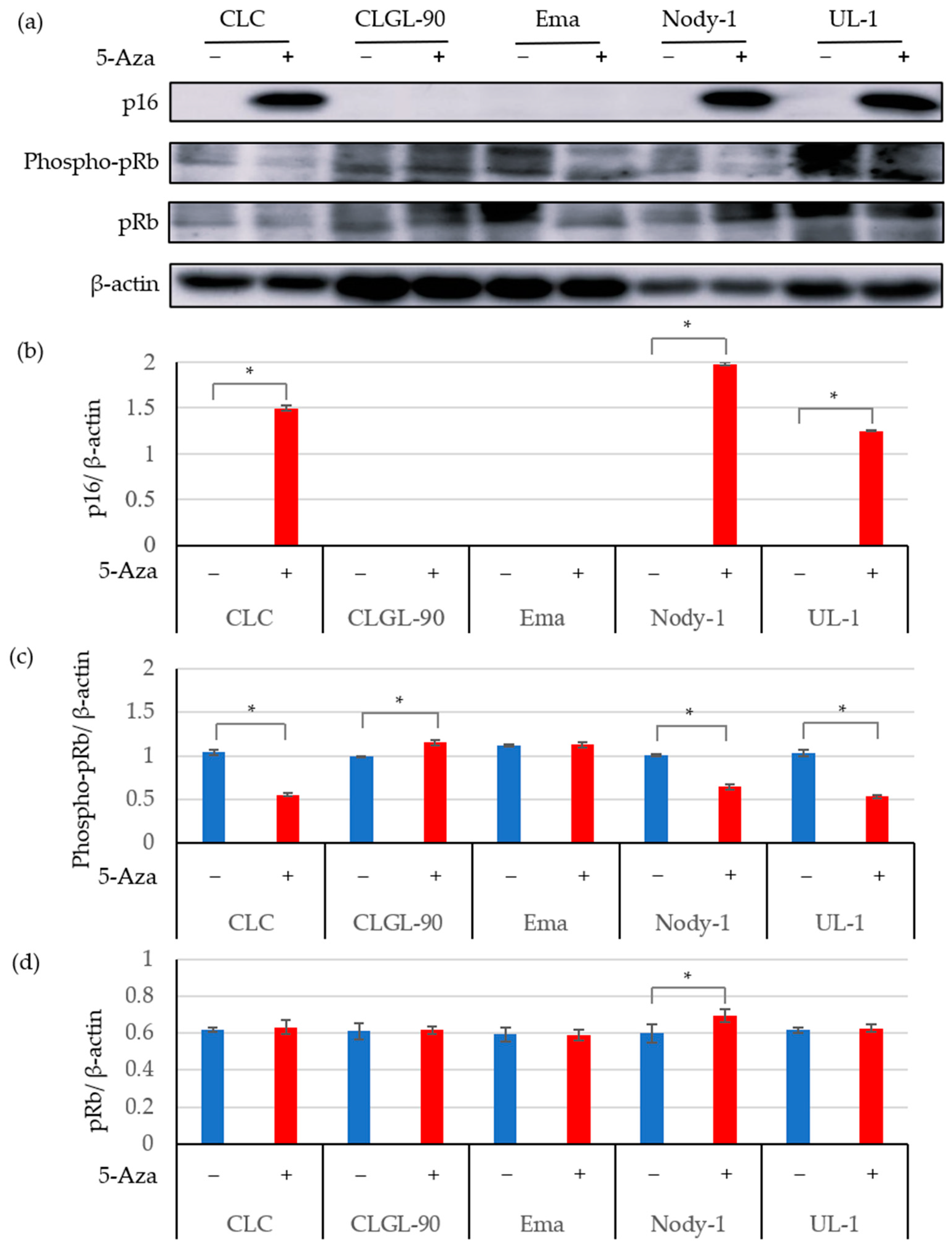
3.2. The 5-Aza Treatment Induced the Expressions of the p16 Gene and Protein; and Decreased pRb Phosphorylation in Some Canine Lymphoma and Leukemia Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [PubMed]
- Ettinger, S.J.; Feldman, E.C. Textbook of Veterinary Internal Medicine, Diseases of the Dog and the Cat, 7th ed.; Elsevier: St. Louis, MO, USA, 2010; pp. 2148–2157. [Google Scholar]
- Ponce, F.; Marchal, T.; Magnol, J.P.; Turinelli, F.; Ledieu, D.; Bonnefont, C.; Pastor, M.; Delignette, M.L.; Fournell-Floury, C. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet. Pathol. 2010, 47, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Vezzali, E.; Parodi, A.L.; Marcato, P.S.; Bettini, G. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet. Comp. Oncol. 2009, 8, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Modiano, J.F.; Breen, M.; Burnett, M.C.; Parker, H.G.; Inusah, S.; Thomas, R.; Avery, P.R.; Linblad-Toh, K.; Ostrander, E.A.; Cutter, G.C.; et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005, 65, 5654–5661. [Google Scholar] [CrossRef]
- Kluin, P.; Schuuring, E. Molecular cytogenetics of lymphoma: Where do we stand in 2010? Histopathology 2011, 58, 128–144. [Google Scholar] [CrossRef]
- Boxer, L.M.; Dang, C.V. Translocations involving c-myc and c-myc function. Oncogene 2001, 20, 5595–5610. [Google Scholar] [CrossRef]
- Ott, G.; Rosenwald, A.; Campo, E. Understanding MYC-driven aggressive B-cell lymphomas: Pathogenesis and classification. Blood 2013, 122, 3884–3891. [Google Scholar] [CrossRef]
- Kos, I.A.; Thurner, L.; Bittenbring, J.T.; Christofyllakis, K.; Kaddu-Mulindwa, D. Advances in lymphoma molecular diagnostics. Diagnostics 2021, 11, 2174. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nomura, K.; Matsumoto, S.; Ueda, K.; Nakao, M.; Nishida, K.; Sakabe, H.; Yokota, S.; Horiike, S.; Nakamine, H.; et al. Detection of t(14;18) in follicular lymphoma by dual-color fluorescence in situ hybridization on paraffin-embedded tissue sections. Cancer Genet. Cytogenet. 2004, 150, 22–26. [Google Scholar] [CrossRef]
- Einerson, R.R.; Kurtin, P.J.; Dayharsh, D.A.; Kimlinger, T.K.; Remstein, E.D. FISH is superior to PCR in detecting t(14;18) (q32;q21)–IgH/bcl-2 in follicular lymphoma using paraffin-embedded tissue samples. Am. J. Clin. Pathol. 2005, 124, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Martinez, L.; Davies-Hill, T.; Fend, F.; Calzada-Wack, J.; Sorbara, L.; Campo, E.; Jaffe, E.S.; Raffeld, M. Sequestration of p27Kip1 protein by cyclin D1 in typical and blastic variants of mantle cell lymphoma (MCL): Implications for pathogenesis. Blood 2003, 101, 3181–3187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, M. Epigenetic alterations and advancement of treatment in peripheral T-cell lymphoma. Clin. Epigenetics 2020, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Elvers, I.; Turner-Maier, J.; Swofford, R.; Koltookian, M.; Johnson, J.; Stewart, C.; Zhang, C.Z.; Schumacher, S.E.; Beroukhim, R.; Rosenberg, M.; et al. Exome sequencing of lymphomas from three dog breeds reveals somatic mutation patterns reflecting genetic background. Genome Res. 2015, 25, 1634–1645. [Google Scholar] [CrossRef]
- Fosmire, S.P.; Thomas, R.; Jubala, C.M.; Wojcieszyn, J.W.; Valli, V.E.O.; Getzy, D.M.; Smith, T.L.; Gardner, L.A.; Ritt, M.G.; Bell, J.S.; et al. Inactivation of the p16 cyclin-dependent kinase inhibitor in high-grade canine non-Hodgkin’s T-cell lymphoma. Vet. Pathol. 2007, 44, 467–478. [Google Scholar] [CrossRef]
- Zhao, R.; Choi, B.Y.; Lee, M.H.; Bode, A.M.; Dong, Z. Implications of genetic and epigenetic alterations of CDKN2A (p16INK4a) in cancer. eBioMedicine 2016, 8, 30–39. [Google Scholar] [CrossRef]
- Robaina, M.C.S.; Faccion, R.S.; Arruda, V.O.; de Rezende, L.M.M.; Vasconcelos, G.M.; Apa, A.G.; Bacchi, C.E.; Klumb, C.E. Quantitative analysis of CDKN2A methylation, mRNA, and p16INK4a protein expression in children and adolescents with Burkitt lymphoma: Biological and clinical implications. Leuk. Res. 2015, 39, 248–256. [Google Scholar] [CrossRef]
- Fan, Z.; Pei, R.; Sha, K.; Chen, L.; Wang, T.; Lu, Y. Comprehensive characterization of driver genes in diffuse large B cell lymphoma. Oncol. Lett. 2020, 20, 382–390. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell 2017, 171, 481–494. [Google Scholar] [CrossRef]
- Fujiwara-Igarashi, A.; Goto-Koshino, Y.; Mochizuki, H.; Maeda, S.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Simultaneous inactivation of the p16, p15 and p14 genes encoding cyclin-dependent kinase inhibitors in canine T-lymphoid tumor cells. J. Vet. Med. Sci. 2013, 75, 733–742. [Google Scholar] [CrossRef]
- Fujiwara-Igarashi, A.; Goto-Koshino, Y.; Mochizuki, H.; Sato, M.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Inhibition of p16 tumor suppressor gene expression via promoter hypermethylation in canine lymphoid tumor cells. Res. Vet. Sci. 2014, 97, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara-Igarashi, A.; Goto-Koshino, Y.; Sato, M.; Maeda, S.; Igarashi, H.; Takahashi, M.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Prognostic significance of the expression levels of the p16, p15, and p14 genes in dogs with high-grade lymphoma. Vet. J. 2014, 199, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Modiano, J.F.; Breen, M.; Valli, V.E.O.; Wojcieszyn, J.W.; Cutter, G.C. Predictive value of p16 or Rb inactivation in a model of naturally occurring canine non-Hodgkin’s lymphoma. Leukemia 2007, 21, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.G.; Mok, M.Y.; York, D.; Rebhun, R.; Woolard, K.D.; Hillman, C.; Dickinson, P.; Skorupski, K. Evaluation of p16 expression in canine appendicular osteosarcoma. BMC Vet. Res. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Rosales, C.; Jeglum, K.A.; Obrocka, M.; Steplewski, Z. Cytolytic activity of murine anti-dog lymphoma monoclonal antibodies with canine effector cells and complement. Cell. Immunol. 1988, 115, 420–428. [Google Scholar] [CrossRef]
- Rütgen, B.C.; Hammer, S.E.; Gerner, W.; Christian, M.; de Arespacochaga, A.G.; Willmann, M.; Kleiter, M.; Schwendenwein, I.; Saalmüller, A. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk. Res. 2010, 34, 932–938. [Google Scholar] [CrossRef]
- Nakaichi, M.; Taura, Y.; Kanki, M.; Mamba, K.; Momoi, Y.; Tsujimoto, H.; Nakama, S. Establishment and characterization of a new canine B-cell leukemia cell line. J. Vet. Med. Sci. 1996, 58, 469–471. [Google Scholar] [CrossRef]
- Umeki, S.; Ema, Y.; Suzuki, R.; Kubo, M.; Hayashi, T.; Okamura, Y.; Yamazaki, J.; Tsujimoto, H.; Tani, K.; Hiraoka, H.; et al. Establishment of five canine lymphoma cell lines and tumor formation in a xenotransplantation model. J. Vet. Med. Sci. 2013, 75, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In vitro canine distemper virus infection of canine lymphoid cells: A prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef]
- Yamazaki, J.; Baba, K.; Goto-Koshino, Y.; Setoguchi-Mukai, A.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Quantitative assessment of minimal residual disease (MRD) in canine lymphoma by using real-time polymerase chain reaction. Vet. Immunol. Immunopathol. 2008, 126, 321–331. [Google Scholar] [CrossRef]
- Bonnefont-Rebeix, C.; Fournel-Fleury, C.; Ponce, F.; Belluco, S.; Watrelot, D.; Bouteille, S.E.; Rapiteau, S.; Razanajaona-Doll, D.; Pin, J.J.; Leroux, C.; et al. Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology. Immunobiology 2016, 221, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Peters, I.R.; Peeters, D.; Helps, C.R.; Day, M.J. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 2007, 117, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Pinyol, M.; Cobo, F.; Bea, S.; Jares, P.; Nayach, I.; Fernandez, P.L.; Montserrat, E.; Cardesa, A.; Campo, E. p16INK4a gene inactivation by deletions, mutations, and hypermethylation is associated with transformed and aggressive variants of non-Hodgkin’s lymphomas. Blood 1998, 91, 2977–2984. [Google Scholar] [CrossRef]
- Baran, M.; Canoz, O.; Altuntas, H.; Sivgin, S.; Cetin, M.; Yay, A.; Ketenci, S. Immunohistochemical investigation of P16, P53 and Ki-67’s prognostic values in diffuse large B-Cell lymphomas. Bratisl. Med. J. 2017, 118, 602–608. [Google Scholar] [CrossRef]
- Jardin, F.; Jais, J.P.; Molina, T.J.; Parmentier, F.; Picquenot, J.M.; Ruminy, P.; Tilly, H.; Bastard, C.; Salles, G.A.; Feugier, P.; et al. Diffuse large B-cell lymphomas with CDKN2A deletion have a distinct gene expression signature and a poor prognosis under R-CHOP treatment: A GELA study. Blood 2010, 116, 1092–1104. [Google Scholar] [CrossRef]
| Target Gene | Primer Name | Sequence (5′-3′) | Product Length (bp) | GeneBank Accession No. |
|---|---|---|---|---|
| p16 | 16F | GGTCGGAGCCCGATTCA | 95 | AB675384 |
| 16R | ACGGGGTCGGCACAGTT | |||
| RPL32 | RPL32F | TGGTTACAGGAGCAACAAGAA | 100 | XM848016 |
| RPL32R | GCACATCAGCAGCACTTCA |
| Target Protein | Clone | Manufacture | Diluted with | Dilution |
|---|---|---|---|---|
| p16 | F-8 | Santa Cruz Biotechnology | 5% nonfat milk/TBST | 1:500 |
| phospho-pRb | Phospho-T826 | Abcam | 5% nonfat milk/TBST | 1:1000 |
| pRb | G3-248 | BD Pharmingen | 5% nonfat milk/TBST | 1:1000 |
| β-actin | AC-15 | Sigma–Aldrich | 0.5% nonfat milk/TBST | 1:5000 |
| Canine Lymphoma Cell Lines | p16 | Phospho-pRb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Expression | Protein Expression | Methylation | Protein Expression | Hyper-Phosphorylation | |||||
| 5-Aza | 5-Aza | ||||||||
| (−) | (+) | (−) | (+) | (−) | (+) | ||||
| B-cell lines | 17-71 | ++ | +++ | ++ | ++ | − | − | − | − |
| CLBL-1 * | ++ | +++ | − | + | + | ++++ | ++ | + | |
| GL-1 | +++ | ++++ | +++ | +++ | − | − | - | − | |
| T-cell lines | CLC * | + | ++++ | − | +++ | + | ++ | + | + |
| CLGL-90 | + | + | − | − | − | ++ | +++ | + | |
| Ema | + | + | − | − | − | ++ | ++ | + | |
| Nody-1 * | + | +++ | − | +++ | + | ++ | + | + | |
| UL-1 * | + | +++ | − | +++ | + | ++ | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maylina, L.; Kambayashi, S.; Baba, K.; Okuda, M. Simultaneous Analysis of the p16 Gene and Protein in Canine Lymphoma Cells and Their Correlation with pRb Phosphorylation. Vet. Sci. 2022, 9, 393. https://doi.org/10.3390/vetsci9080393
Maylina L, Kambayashi S, Baba K, Okuda M. Simultaneous Analysis of the p16 Gene and Protein in Canine Lymphoma Cells and Their Correlation with pRb Phosphorylation. Veterinary Sciences. 2022; 9(8):393. https://doi.org/10.3390/vetsci9080393
Chicago/Turabian StyleMaylina, Leni, Satoshi Kambayashi, Kenji Baba, and Masaru Okuda. 2022. "Simultaneous Analysis of the p16 Gene and Protein in Canine Lymphoma Cells and Their Correlation with pRb Phosphorylation" Veterinary Sciences 9, no. 8: 393. https://doi.org/10.3390/vetsci9080393






