Microbiome Profile of Dogs with Stage IV Multicentric Lymphoma: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use and Clinical Data Collection
2.2. Sampling and Sequencing Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Lymphoma Type Classification
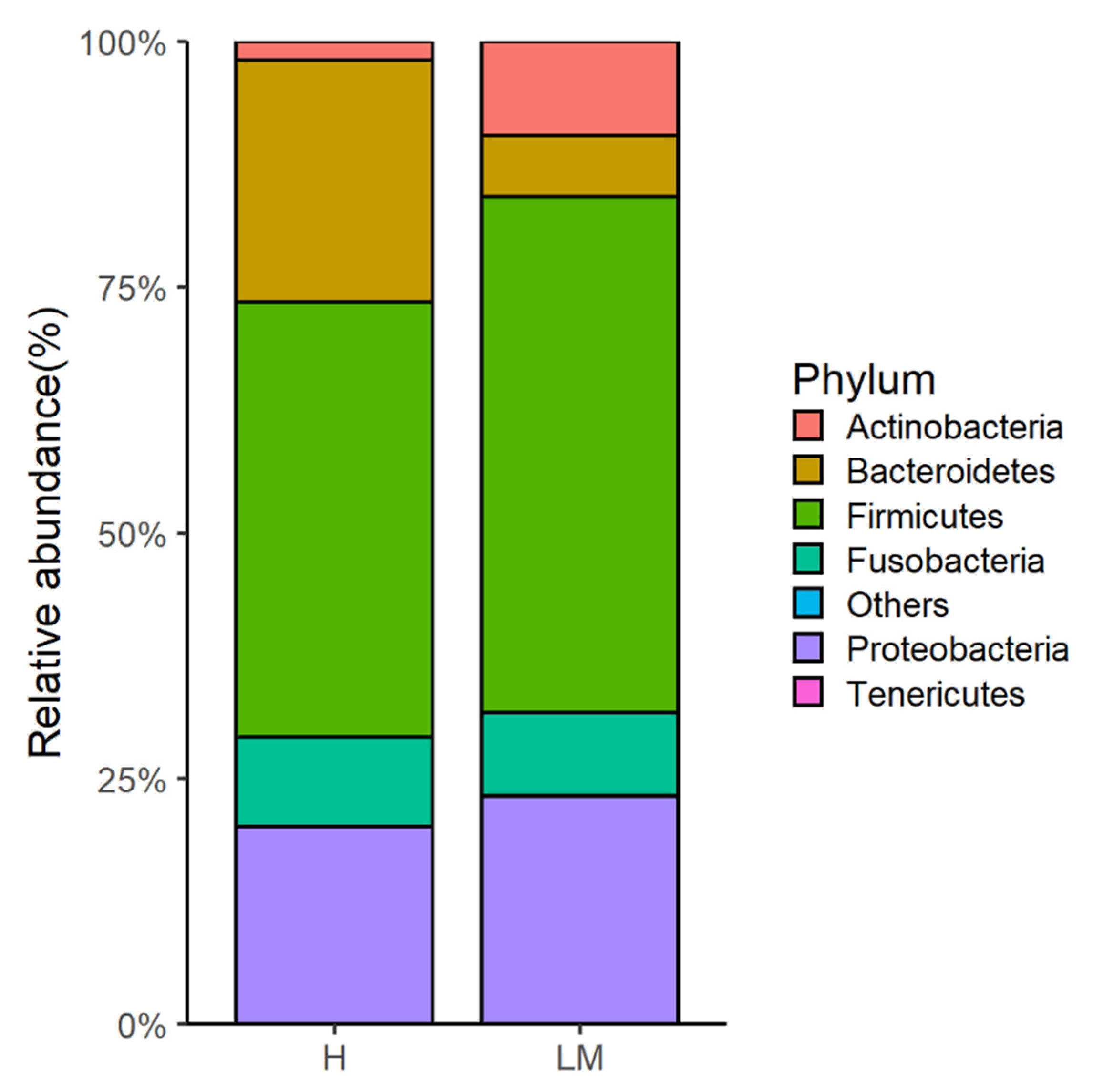
3.3. Gut Microbiome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, V.; Clark, J.; Doré, J. Fecal microbiota analysis: An overview of sample collection methods and sequencing strategies. Future Microbiol. 2015, 10, 1485–1504. [Google Scholar] [CrossRef] [PubMed]
- Indiani, C.M.d.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: A systematic review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Mazmanian, S.K. Disruption of the gut microbiome as a risk factor for microbial infections. Curr. Opin. Microbiol. 2013, 16, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Daillère, R.; Roberti, M.P.; Routy, B.; Kroemer, G. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 2017, 15, 465–478. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Larouche-Lebel, É.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Gut dysbiosis and its associations with gut microbiota-derived metabolites in dogs with myxomatous mitral valve disease. Msystems 2021, 6, e00111-21. [Google Scholar] [CrossRef]
- Craig, J.M. Atopic dermatitis and the intestinal microbiota in humans and dogs. Vet. Med. Sci. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Park, J.S.; Guevarra, R.B.; Kim, B.-R.; Lee, J.H.; Lee, S.H.; Cho, J.H.; Kim, H.; Cho, J.H.; Song, M.; Lee, J.-H. Intestinal microbial dysbiosis in Beagles naturally infected with canine parvovirus. J. Microbiol. Biotechnol. 2019, 29, 1391–1400. [Google Scholar] [CrossRef]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Valli, V.; Myint, M.S.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.; Johnson, Y.; Jones, C. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Elshafie, N.O.; Nascimento, N.C.d.; Lichti, N.I.; Kasinski, A.L.; Childress, M.O.; Santos, A.P.d. MicroRNA Biomarkers in Canine Diffuse Large B-Cell Lymphoma. Vet. Pathol. 2021, 58, 34–41. [Google Scholar] [CrossRef]
- Loftus, M.; Hassouneh, S.A.-D.; Yooseph, S. Bacterial community structure alterations within the colorectal cancer gut microbiome. BMC Microbiol. 2021, 21, 98. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Owen, L.; World Health Organization. TNM Classification of Tumours in Domestic Animals/Edited by LN Owen; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- You, I.; Kim, M.J. Comparison of gut microbiota of 96 healthy dogs by individual traits: Breed, age, and body condition score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Seelig, D.M.; Avery, A.C.; Ehrhart, E.; Linden, M.A. The comparative diagnostic features of canine and human lymphoma. Vet. Sci. 2016, 3, 11. [Google Scholar] [CrossRef]
- Villiers, E.; Ristić, J. BSAVA Manual of Canine and Feline Clinical Pathology; British Small Animal Veterinary Association: London, UK, 2016. [Google Scholar]
- Garnica, T.K.; Lesbon, J.C.; Ávila, A.C.; Rochetti, A.L.; Matiz, O.R.; Ribeiro, R.; Zoppa, A.; Nishiya, A.T.; Costa, M.T.; de Nardi, A.B. Liquid biopsy based on small extracellular vesicles predicts chemotherapy response of canine multicentric lymphomas. Sci. Rep. 2020, 10, 20371. [Google Scholar] [CrossRef] [PubMed]
- Teske, E. Canine malignant lymphoma: A review and comparison with human non-Hodgkin’s lymphoma. Vet. Q. 1994, 16, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Gavazza, A.; Rossi, G.; Lubas, G.; Cerquetella, M.; Minamoto, Y.; Suchodolski, J. Faecal microbiota in dogs with multicentric lymphoma. Vet. Comp. Oncol. 2018, 16, E169–E175. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.C.; Wouda, R.M.; Higginbotham, M.L. Preliminary evaluation of probiotic effects on gastrointestinal signs in dogs with multicentric lymphoma undergoing multi-agent chemotherapy: A randomised, placebo-controlled study. Vet. Rec. Open 2021, 8, e2. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.I. Prospective evaluation of the fecal microbiome in dogs with large-cell lymphoma receiving chop chemotherapy. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2021. [Google Scholar]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Su, Z.; Li, Y.; Li, Y.; Liu, K.; Chu, F.; Liu, T.; Chen, R.; Ding, X. Dysbiosis of gut microbiota is associated with gastric carcinogenesis in rats. Biomed. Pharmacother. 2020, 126, 110036. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef] [Green Version]
- den Hengst, C.D.; Buttner, M.J. Redox control in actinobacteria. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 1201–1216. [Google Scholar] [CrossRef]
- Gladysheva, I.V.; Cherkasov, S.V.; Khlopko, Y.A.; Plotnikov, A.O. Genome Characterization and Probiotic Potential of Corynebacterium amycolatum Human Vaginal Isolates. Microorganisms 2022, 10, 249. [Google Scholar] [CrossRef]
- Hawk, M.A.; McCallister, C.; Schafer, Z.T. Antioxidant activity during tumor progression: A necessity for the survival of cancer cells? Cancers 2016, 8, 92. [Google Scholar] [CrossRef]
- Bottari, N.B.; Munhoz, T.D.; Torbitz, V.D.; Tonin, A.A.; Anai, L.A.; Semolin, L.M.; Jark, P.C.; Bollick, Y.S.; Moresco, R.N.; França, R.T. Oxidative stress in dogs with multicentric lymphoma: Effect of chemotherapy on oxidative and antioxidant biomarkers. Redox Rep. 2015, 20, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Nomura, K.; Ishikawa, D.; Okahara, K.; Ito, S.; Haga, K.; Takahashi, M.; Arakawa, A.; Shibuya, T.; Osada, T.; Kuwahara-Arai, K. Bacteroidetes species are correlated with disease activity in ulcerative colitis. J. Clin. Med. 2021, 10, 1749. [Google Scholar] [CrossRef]
- Tsvetikova, S.A.; Koshel, E.I. Microbiota and cancer: Host cellular mechanisms activated by gut microbial metabolites. Int. J. Med. Microbiol. 2020, 310, 151425. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Subramanian, U.; Venkidasamy, B.; Thirupathi, P.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Chung, I.-M.; Rengasamy, K.R. Emerging role of nutritional short-chain fatty acids (SCFAs) against cancer via modulation of hematopoiesis. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef]
- Saito, M.; Gao, J.; Basso, K.; Kitagawa, Y.; Smith, P.M.; Bhagat, G.; Pernis, A.; Pasqualucci, L.; Dalla-Favera, R. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell 2007, 12, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Vasanwala, F.H.; Kusam, S.; Toney, L.M.; Dent, A.L. Repression of AP-1 function: A mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J. Immunol. 2002, 169, 1922–1929. [Google Scholar] [CrossRef] [Green Version]
- Cozen, W.; Yu, G.; Gail, M.H.; Ridaura, V.K.; Nathwani, B.N.; Hwang, A.E.; Hamilton, A.S.; Mack, T.M.; Gordon, J.I.; Goedert, J.J. Fecal microbiota diversity in survivors of adolescent/young adult Hodgkin lymphoma: A study of twins. Br. J. Cancer 2013, 108, 1163–1167. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Borges-Canha, M.; Portela-Cidade, J.P.; Dinis-Ribeiro, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Role of colonic microbiota in colorectal carcinogenesis: A systematic review. Rev. Esp. Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Liu, L.; Chang, E.B.; Wang, J.-Y.; Raufman, J.-P. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol. Cancer 2015, 14, 180. [Google Scholar] [CrossRef] [Green Version]
- Piva, S.; Pietra, M.; Serraino, A.; Merialdi, G.; Magarotto, J.; Giacometti, F. First description of Streptococcus lutetiensis from a diseased cat. Lett. Appl. Microbiol. 2019, 69, 96–99. [Google Scholar] [CrossRef]
- Jin, D.; Chen, C.; Li, L.; Lu, S.; Li, Z.; Zhou, Z.; Jing, H.; Xu, Y.; Du, P.; Wang, H. Dynamics of fecal microbial communities in children with diarrhea of unknown etiology and genomic analysis of associated Streptococcus lutetiensis. BMC Microbiol. 2013, 13, 141. [Google Scholar] [CrossRef] [Green Version]
- Chirouze, C.; Patry, I.; Duval, X.; Baty, V.; Tattevin, P.; Aparicio, T.; Pagenault, M.; Carbonnel, F.; Couetdic, G.; Hoen, B. Streptococcus bovis/Streptococcus equinus complex fecal carriage, colorectal carcinoma, and infective endocarditis: A new appraisal of a complex connection. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1171–1176. [Google Scholar] [CrossRef]
- Frischmann, A.; Knoll, A.; Hilbert, F.; Zasada, A.A.; Kämpfer, P.; Busse, H.-J. Corynebacterium epidermidicanis sp. nov., isolated from skin of a dog. Int. J. Syst. Evol. Microbiol. 2012, 62, 2194–2200. [Google Scholar] [CrossRef]
- Dalal, A.; Urban, C.; Segal-Maurer, S. Endocarditis due to Corynebacterium amycolatum. J. Med. Microbiol. 2008, 57, 1299–1302. [Google Scholar] [CrossRef] [Green Version]
- Chauvelot, P.; Ferry, T.; Tafani, V.; Diot, A.; Tasse, J.; Conrad, A.; Chidiac, C.; Braun, E.; Lustig, S.; Laurent, F. Bone and joint infection involving Corynebacterium spp.: From clinical features to pathophysiological pathways. Front. Med. 2021, 7, 539501. [Google Scholar] [CrossRef]
- Bernard, K. Coryneform Gram-positive rods. In Manual of Clinical Microbiology, 12th ed.; ASM Publishing: Washington, DC, USA, 2019; pp. 488–524. [Google Scholar]
- Sengupta, M.; Naina, P.; Balaji, V.; Anandan, S. Corynebacterium amycolatum: An unexpected pathogen in the ear. J. Clin. Diagn. Res. JCDR 2015, 9, DD01. [Google Scholar] [CrossRef]

| Disease Extent | Signs | Stage |
|---|---|---|
| Involve a limited area of the body | One group of lymph nodes A single body organ (extranodal lymphoma) | Stage I |
| Two or more groups of lymph nodes in the front half or back half of the body Extranodal lymphoma and the presence of one or more groups of lymph nodes on the same side of the diaphragm | Stage II | |
| Advanced disease | Generalized lymphadenopathy | Stage III |
| Generalized lymphadenopathy with involvement of the liver, and/or spleen | Stage IV | |
| Stage I to IV with involvement of blood or bone marrow | Stave V | |
| Substages | No systemic signs | A substage |
| Unexplained weight loss Fever Night sweats Hypercalcemia | B Substage |
| Index | H | LM | p Value |
|---|---|---|---|
| Observed species | 78.5 ± 7.4 | 94.9 ± 12.7 | 0.389 |
| Chao1 | 79.1 ± 7.4 | 96.6 ± 13.2 | 0.415 |
| Shannon | 3.7 ± 0.1 | 4.2 ± 0.3 | 0.239 |
| Simpson | 0.8 ± 0 | 0.9 ± 0 | 0.122 |
| Bacteria | H | LM | p Value |
|---|---|---|---|
| Phylum | |||
| Actinobacteria | 1.9 ± 0.7 | 9.6 ± 3.7 | 0.024 |
| Bacteroidetes | 24.6 ± 6.4 | 6.2 ± 4.1 | 0.037 |
| Genus | |||
| Corynebacterium | 1.2 ± 0.5 | 8.3 ± 3.1 | 0.018 |
| Kineothrix | 1.4 ± 0.3 | 0.7 ± 0.6 | 0.036 |
| Caproiciproducens | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.032 |
| Peptostreptococcus | 0.0 ± 0.0 | 0.3 ± 0.2 | 0.010 |
| Proteus | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.042 |
| Roseburia | 0.4 ± 0.3 | 0.0 ± 0.0 | 0.033 |
| Species | |||
| Corynebacterium amycolatum | 0.5 ± 0.4 | 4.3 ± 1.5 | 0.016 |
| Blautia schinkii | 3.5 ± 1.4 | 0.0 ± 0.0 | 0.016 |
| [Clostridium] spiroforme | 1.5 ± 0.9 | 0.1 ± 0.1 | 0.042 |
| Kineothrix alysoides | 1.4 ± 0.3 | 0.7 ± 0.6 | 0.036 |
| Caproiciproducens galactitolivorans | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.023 |
| Peptostreptococcus canis | 0.0 ± 0.0 | 0.3 ± 0.2 | 0.046 |
| Proteus mirabilis | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.042 |
| Roseburia intestinalis | 0.4 ± 0.3 | 0.0 ± 0.0 | 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahiddine, F.Y.; You, I.; Park, H.; Kim, M.J. Microbiome Profile of Dogs with Stage IV Multicentric Lymphoma: A Pilot Study. Vet. Sci. 2022, 9, 409. https://doi.org/10.3390/vetsci9080409
Mahiddine FY, You I, Park H, Kim MJ. Microbiome Profile of Dogs with Stage IV Multicentric Lymphoma: A Pilot Study. Veterinary Sciences. 2022; 9(8):409. https://doi.org/10.3390/vetsci9080409
Chicago/Turabian StyleMahiddine, Feriel Yasmine, Inhwan You, Heekee Park, and Min Jung Kim. 2022. "Microbiome Profile of Dogs with Stage IV Multicentric Lymphoma: A Pilot Study" Veterinary Sciences 9, no. 8: 409. https://doi.org/10.3390/vetsci9080409
APA StyleMahiddine, F. Y., You, I., Park, H., & Kim, M. J. (2022). Microbiome Profile of Dogs with Stage IV Multicentric Lymphoma: A Pilot Study. Veterinary Sciences, 9(8), 409. https://doi.org/10.3390/vetsci9080409







