The Microbiological Quality of Concentrates for Horses—A Retrospective Study on Influencing Factors and Associations with Clinical Symptoms Reported by Owners or Referring Vets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Examination Methods
2.2. Evaluation of Examination Results
2.3. Statistical Analyses
3. Results
3.1. Proportional Frequency of Deficiencies
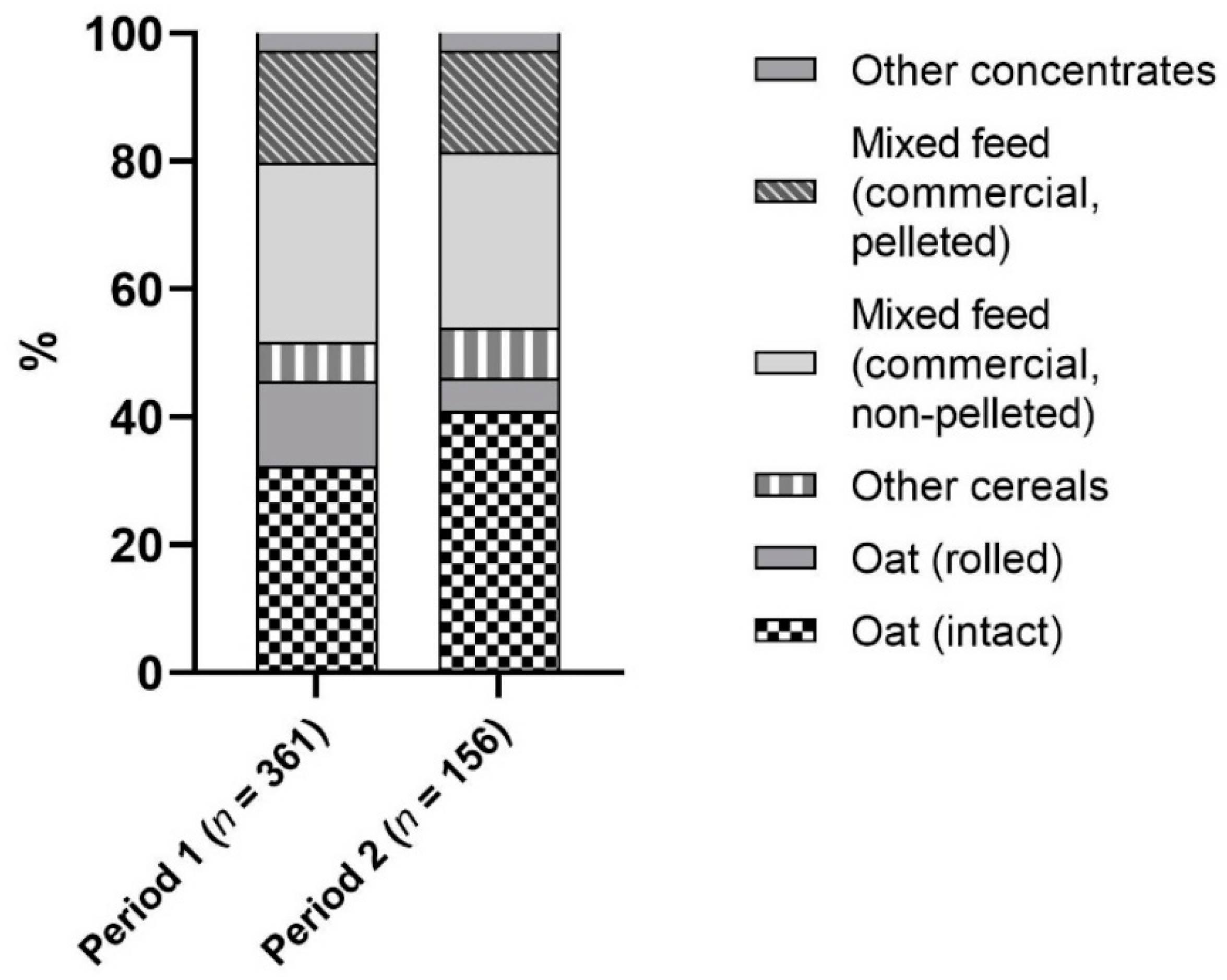
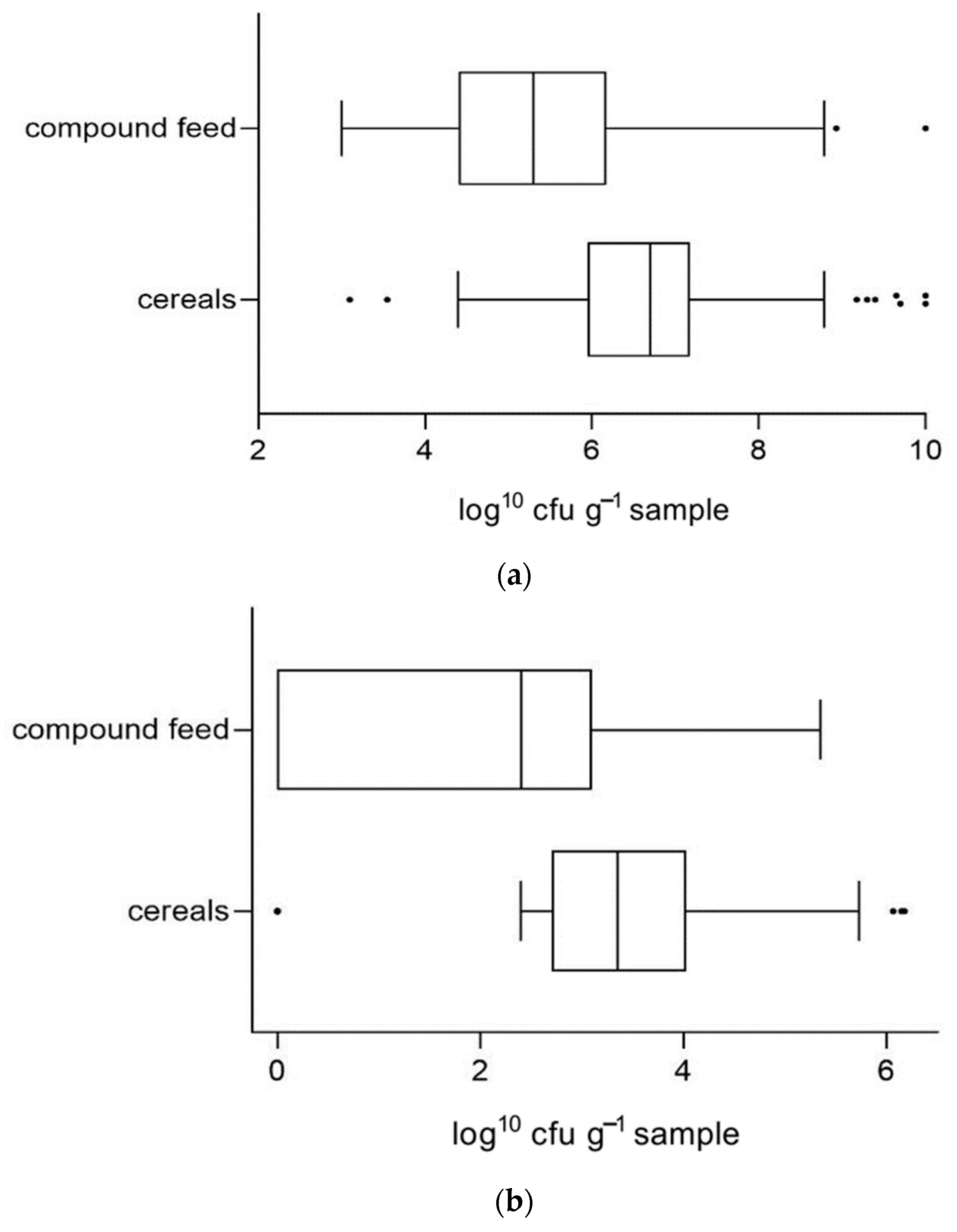
3.2. Comparison of Microbiological Quality in Two Investigation Periods
3.3. Relations between Low Dry Matter Content and Microbiological Load of Feeds
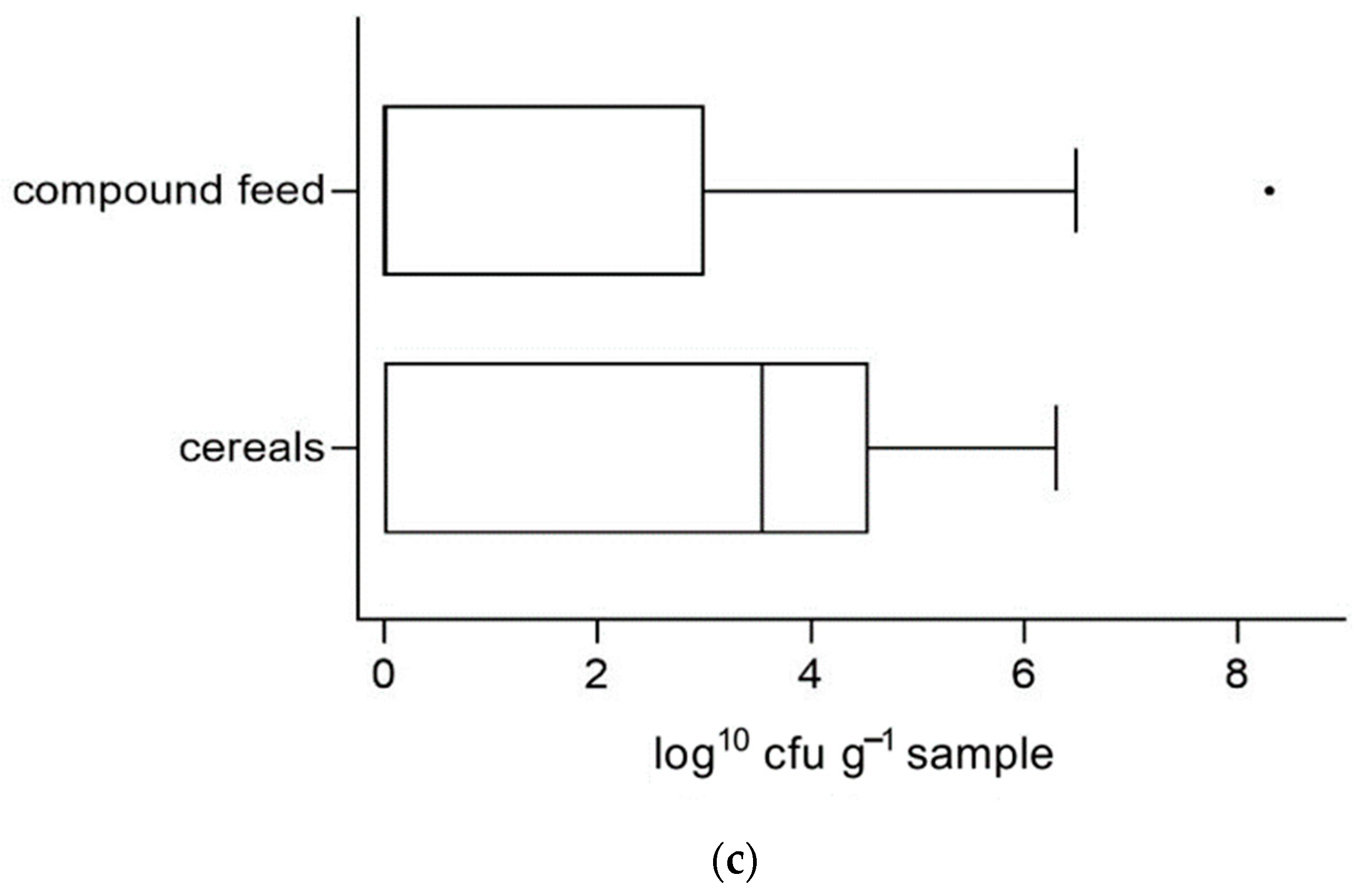
3.4. Associations of Microbiological Quality and Occurrence of Disease Symptoms Reported by Sender
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindberg, J. Feedstuffs for horses. In Equine Applied and Clinical Nutrition; Saunders: Philadelphia, PA, USA, 2013; pp. 319–331. [Google Scholar] [CrossRef]
- Meyer, H.; Coenen, M. Pferdefütterung; Georg Thieme Verlag: New York, NY, USA, 2014. [Google Scholar]
- Hill, J. Impacts of nutritional technology on feeds offered to horses: A review of effects of processing on voluntary intake, digesta characteristics and feed utilisation. Anim. Feed. Sci. Technol. 2007, 138, 92–117. [Google Scholar] [CrossRef]
- Van Saun, R.J. Feed Additives, Nutritional Supplements and Nutraceuticals for Horses. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2006. [Google Scholar]
- Douglas, C.D. Quality Improvement in Oat. J. Crop Prod. 2002, 5, 165–189. [Google Scholar] [CrossRef]
- Meyer, H.; Heckotter, E.; Merkt, M.; Bernoth, E.; Kienzle, E.; Kamphues, J. Current problems in veterinary advice on feeding. 6. Adverse effects of feeds in horses. Dtsch. Tierarztl. Wochenschr. 1986, 93, 486–490. [Google Scholar] [PubMed]
- Wolf, P.; Kloetzer, P.; Paulus, C.; Kamphues, J. A survey on the hygienic standard of feeds for horses and its implication for environmental conditions and animal health. In Proceedings of the Sustainable Animal Husbandry: Prevention Is Better than Cure, Volume 2. Proceedings of the 14th International Congress of the International Society for Animal Hygiene (ISAH), Vechta, Germany, 19–23 July 2009; Tribun EU: Brno-střed, Czech Republic, 2009; pp. 1085–1088. [Google Scholar]
- Sliwinsky, H.; Krabisch, P.; Rosenberger, E.; Schwarz, F. Hygienic quality of different forages and concentrates for horses. Pferdeheilkunde 2005, 21, 26. [Google Scholar] [CrossRef]
- Kamphues, J. A systematic approach to evaluate the hygienic quality of feedstuffs for horses. Pferdeheilkunde 2005, 21, 15–18. [Google Scholar] [CrossRef]
- Stickdorn, T.; Ellis, A.; Kienzle, E. Horse feed hygiene evaluation with microbial and sensory examination. In Forages and Grazing in Horse Nutrition; Springer: Berlin/Heidelberg, Germany, 2012; pp. 255–262. [Google Scholar]
- Schmidt, H. Mikrobiologische Richtwerte für die Futtermittelbeurteilung. In Proceedings of the VII Int Kongreß für Tierhygiene, Leipzig, Germany, 23–28 June 1991; pp. 923–928. [Google Scholar]
- Kaya, G.; Sommerfeld-Stur, I.; Iben, C. Risk factors of colic in horses in Austria. J. Anim. Physiol. Anim. Nutr. 2009, 93, 339–349. [Google Scholar] [CrossRef]
- Meyer, H.; Kamphues, J.; Schneider, D.; Leibetseder, J. Supplemente zu Vorlesungen und Übungen in der Tierernährung; 9 überarbeitete Auflage; Schaper: Salzgitter, Germany, 1999. [Google Scholar]
- VDLUFA. VDLUFA Methodenbuch, Band III—Die Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2017; Volume 8. [Google Scholar]
- Felšöciová, S.; Kowalczewski, P.Ł.; Krajčovič, T.; Dráb, Š.; Kačániová, M. Effect of Long-Term Storage on Mycobiota of Barley Grain and Malt. Plants 2021, 10, 1655. [Google Scholar] [CrossRef]
- EU. Regulation (EC) No 183/2005 OF The European Parliament and of the Council laying down requirements for feed hygiene. Off. J. Eur. Union 2005, L35, 1–22. [Google Scholar]
- Kamphues, J. Feed Hygiene and Related Disorders in Horses. In Equine Applied and Clinical Nutrition; Geor, R.J., Harris, P.A., Coenen, M., Eds.; Saunders: Philadelphia, PA, USA, 2013; pp. 367–380. [Google Scholar]
- Boroojeni, F.G.; Svihus, B.; von Reichenbach, H.G.; Zentek, J. The effects of hydrothermal processing on feed hygiene, nutrient availability, intestinal microbiota and morphology in poultry—A review. Anim. Feed. Sci. Technol. 2016, 220, 187–215. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. 2010, 27, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Harris, P.A.; Ellis, M.; Fradinho, J.; Jansson, J.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal 2017, 11, 958–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, A. Qualität von Futterkonserven und Mikrobielle Kontamination. In Proceedings of the 8th Alpine Expert Forum, Irdning, Austria, 9–11 April 2002; pp. 17–26. [Google Scholar]
- Metzler, A.; Bauer, J.; Hörmansdorfer, S.; Pfirrmann, A.; Böhm, R.; Schneweis, I.; Barth, D.; Kamphues, J.; Reichmuth, C. Schadorganismen und deren Stoffwechselprodukte. Potentielle Schadorganismen und Stoffe Futterm. Sowie Tier. Fäkalien: Sachstandsbericht Mitt. 2000, 4, 5–284. [Google Scholar]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Benbrook, C. Breaking the Mold–Impacts of Organic and Conventional Farming Systems on Mycotoxins in Food and Livestock Feed; The Organic Center: Washington, DC, USA, 2005. [Google Scholar]
- Matthäus, K.; Dänicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.; Meyer, K.; Strumpf, A.; Ziesenib, H.; Flachowsky, G. Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch. Anim. Nutr. 2004, 58, 19–35. [Google Scholar] [CrossRef]
- Gabal, M.; Awad, Y.; Morcos, M.; Barakat, A.; Malik, G. Fusariotoxicoses of farm animals and mycotoxic leucoencephalomalacia of the equine associated with the finding of trichothecenes in feedstuffs. Vet. Hum. Toxicol. 1986, 28, 207–212. [Google Scholar]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.J.; Casteel, S.W.; Messer, N.T. Effect of feeding deoxynivalenol (vomitoxin)-contaminated barley to horses. J. Vet. Diagn. Investig. 1997, 9, 219–221. [Google Scholar] [CrossRef] [Green Version]
- VDLUFA. Vorschlag: Orientierungswertschema zur Auswertung der Ergebnisse Mikrobiologischer Untersuchungen Zwecks Beurteilung von Futtermitteln Nach §7 (3) Futtermittelgesetz/Arbeitskreis Futtermittelmikrobiologie der Fachgruppe VI (Futtermittel) des Verbandes Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten; 2002. Available online: https://www.lfl.bayern.de/mam/cms07/ite/dateien/31386_mikrobielle_beurteilung_von_futtermitteln_-_orientierungswertschema.pdf (accessed on 6 June 2022).
- Anacker, G. Mikrobiologische Belastung von HauptfutterkomponentenUrsache für Gesundheitsprobleme in Milchviehherden; Thüringer Landesanstalt für Landwirtschaft: Jena, Germany, 2006. [Google Scholar]
- Kellerman, T.S.; Marasas, W.F.O.; Pienaar, J.; Naudé, T. A Mycotoxicosis of Equidae Caused by Fusarium Moniliforme Sheldon. A Preliminary Communication. Onderstepoort J. Vet. Res. 1972, 39, 205–208. [Google Scholar]
- Anacker, G. Mikrobiologische Futterqualität—Ursache für Gesundheitsprobleme in Milchviehherden? Viehwirtsch. Fachtag. 2010, 40, 93. [Google Scholar]
- Leblond, A.; Villard, I.; Leblond, L.; Sabatier, P.; Sasco, A. A retrospective evaluation of the causes of death of 448 insured French horses in 1995. Vet. Res. Commun. 2000, 24, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Tinker, M.K.; White, N.; Lessard, P.; Thatcher, C.; Pelzer, K.; Davis, B.; Carmel, D. Prospective study of equine colic risk factors. Equine Vet. J. 1997, 29, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Saulez, M.N.; Donnellan, C.; Gummow, B. Causes of gastrointestinal colic at an equine referral hospital in South Africa (1998–2007). J. South Afr. Vet. Assoc. 2009, 80, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Hudson, J.M.; Cohen, N.D.; Gibbs, P.G.; Thompson, J.A. Feeding practices associated with colic in horses. J. Am. Vet. Med. Assoc. 2001, 219, 1419–1425. [Google Scholar] [CrossRef]
- Meyer, H.; Ahlswede, L.; Pferdekamp, M. Untersuchungen uber Magenentleerung und Zusammensetzung des Mageninhaltes beim Pferd. DTW Dtsch. Tierarztl. Wochenschr. 1980, 87, 43–47. [Google Scholar]
- Meyer, H. Krampfkolik beim Pferd–Vorstellungen zu einer alimentären Genese. Pferdeheilkunde 2001, 17, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Gieselmann, A. Nutritive Anamnese bei Kolikfällen des Pferdes; University of Vetreinary Medicine, Foundation: Hanover, Germany, 1994. [Google Scholar]
- Coenen, M. Fütterung und Kolik. Pferdeheilkunde 2013, 29, 176–182. [Google Scholar]
- Kamphues, J.; Böhm, K. Krampfkoliken bei Pferden nach Fütterung von verdorbenem Hafer. Dtsch. Tierärztl. Wschr 1990, 97, 367–368. [Google Scholar]
- Kamphues, J. Lipopolysaccharide in Futtermitteln–mögliche Bedeutung, Bestimmung und Gehalte. Übers Tierernährg 1986, 14, 131–156. [Google Scholar]
- Clarke, A. Environmental monitoring in relation to equine respiratory disease. Current Therapy in Equine Medicine. 1992; pp. 310–315. Available online: https://www.ivis.org/library/equine-respiratory-diseases/environmental-control-of-respiratory-disease (accessed on 6 June 2022).
- Pirie, R.S.; Couëtil, L.L.; Robinson, N.E.; Lavoie, J.-P. Equine asthma: An appropriate, translational and comprehendible terminology? Equine Vet. J. 2016, 48, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.P. Heaves, an asthma-like disease of horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Bracher, V.; Von Fellenberg, R.; Winder, C.N.; GRUENIG, G.; Hermann, M.; Kraehenmann, A. An investigation of the incidence of chronic obstructive pulmonary disease (COPD) in random populations of Swiss horses. Equine Vet. J. 1991, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Pirie, R.S. Severe equine asthma—an overview. Livestock 2017, 22, 208–215. [Google Scholar] [CrossRef]
- Vandenput, S.; Istasse, L.; Nicks, B.; Lekeux, P. Airborne dust and aeroallergen concentrations in different sources of feed and bedding for horses. Vet. Q. 1997, 19, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, S.; Duvivier, D.H.; Votion, D.; Art, T.; Lekeux, P. Environmental control to maintain stabled COPD horses in clinical remission: Effects on pulmonary function. Equine Vet. J. 1998, 30, 93–96. [Google Scholar] [CrossRef]
- Vesonder, R.; Haliburton, J.; Stubblefield, R.; Gilmore, W.; Peterson, S. Aspergillus flavus and aflatoxins B1, B2, and M1 in corn associated with equine death. Arch. Environ. Contam. Toxicol. 1991, 20, 151–153. [Google Scholar] [CrossRef]
- Casteel, S.W.; Rottinghaus, G.E.; Johnson, G.C.; Wicklow, D.T. Liver disease in cattle induced by consumption of moldy hay. Vet. Hum. Toxicol. 1995, 37, 248–251. [Google Scholar]
- Anacker, G. Mikrobiologische Belastung von Hauptfutterkomponenten—Ursache für Gesundheitsprobleme in Milchviehherden; TLL: Jena, Germany, 2007. [Google Scholar]
- Kamphues, J.; Wolf, P.; Coenen, M.; Eder, K.; Iben, C.; Kienzle, E.; Liesegang, A.; Männer, K.; Zebeli, Q.; Zentek, J. Beurteilung von Futtermitteln. In Supplemente zur Tierernährung für Studium und Praxis; 12 überarbeitete Auflage ed.; Schaper: Salzgitter, Germany, 2014; pp. 181–188. Available online: https://www.amazon.de/Supplemente-zur-Tierern%C3%A4hrung-Studium-Praxis/dp/3794402405 (accessed on 6 June 2022).
- Visscher, C.; Mischok, J.; Sander, S.; Schmicke, M.; Peitzmeier, E.-U.; von dem Busche, I.; Rohn, K.; Kamphues, J. Nutrient digestibility, organ morphometry and performance in vaccinated or non-vaccinated Lawsonia intracellularis infected piglets. BMC Vet. Res. 2018, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Commission, E. Regulation No. 767/2009/EC of 13 July 2009 on the placing on the market and use of feed, amending European Parliament and Council Regulation (EC) No. 1831/2003 and repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. Off. J. 2009, L 229, 1–28. [Google Scholar]
- Klötzer, P. Der Mikrobiologisch-Hygienische Status Eingesandter Futtermittel für Pferde. 2013. Available online: https://elib.tiho-hannover.de/servlets/MCRFileNodeServlet/etd_derivate_00000778/kloetzerp_ss13.pdf (accessed on 6 June 2022).
- ADLER, A. Orientierungswerte für Keimzahlen in Heu. In Proceedings of the ALVA-Tagung, St. Virgil, Salzburg, Austria, 18–19 May 2009; p. 193. [Google Scholar]
- Meyer, H. Supplemente zu Vorlesungen und Übungen in der Tierernährung. Schlütersche. 2004. Available online: https://www.amazon.de/Supplemente-zu-Vorlesungen-%C3%9Cbungen-Tierern%C3%A4hrung/dp/3794402235 (accessed on 6 June 2022).
- Valmor Ziegler and Ricardo Tadeu Paraginski and Cristiano Dietrich, F. Grain storage systems and effects of moisture, temperature and time on grain quality—A review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. In Epidemiology of Mycotoxin Producing Fungi; Springer: Berlin/Heidelberg, Germany, 2003; pp. 723–730. [Google Scholar]
- Garlipp, F.; Hessel, E.F.; Weghe, H. Airborne particle generation from horse feeds depending on type and processing. Landtechnik 2009, 64, 242–245. [Google Scholar]
- Barbara Kosiak and Mona Torp and Eystein Skjerve and Birgitte, A. Alternaria and Fusarium in Norwegian grains of reduced quality—A matched pair sample study. Int. J. Food Microbiol. 2004, 93, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Intemann, S.; Reckels, B.; Schubert, D.; Wolf, P.; Kamphues, J.; Visscher, C. The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports. Vet. Sci. 2022, 9, 226. [Google Scholar] [CrossRef]


| Parameter | Acceptable | Inadequate |
|---|---|---|
| Texture | Dry | Clammy, wet, sticky, clumping |
| Odor | Product-typical | Musty, vapid, moldy, roasted, yeasty, sweet, dusty, foreign smell |
| Content of dirt | Unremarkable | Abrasion, dust, husks, contamination with soil/sand, admixtures (husks, feces), storage pests (insects, mites) |
| Color | Typical color | Grey, black-brownish, green, reddish (grains only) |
| Size/Form | Unremarkable | Macroscopically visible deposits, incomplete evolution of grains, swollen pellets |
| Integrity (grains only) | Unremarkable | Broken grains, eroded, swollen |
| Loupe view | Unremarkable | Deficient integrity of grains, deposits of dirt/mold, fine particles (insects, mites, visible mold growth) |
| Type of Micro Organisms | Classification | Group No. 1 | Exemplary Species | Orientation Value for Colony Forming Units per Gram Feedstuff | ||
|---|---|---|---|---|---|---|
| Oats | Barley | Mixed feed | ||||
| Aerobic bacteria | Product-typical bacteria | 1 | Pantoea agglomerans, Pseudomonas, Enterobacteriaceae | 50 × 106 | 20 × 106 | 0.5 × 106 |
| Spoilage bacteria | 2 | Bacillus, Staphylococcus, Micrococcus | 1 × 106 | 1 × 106 | 0.5 × 106 | |
| 3 | Streptomyces | 0.05 × 106 | 0.05 × 106 | 0.01 × 106 | ||
| Molds | Product-typical molds | 4 | Alternaria, Acremonium, Fusarium | 200 × 103 | 40 × 103 | 2 × 103 |
| Spoilage molds | 5 | Aspergillus spp., Penicillium, Scopulariopsis | 50 × 103 | 30 × 103 | 6 × 103 | |
| 6 | Mucorales | 2 × 103 | 2 × 103 | 1 × 103 | ||
| Yeasts | 7 | All species | 200 × 103 | 100 × 103 | 5 × 103 | |
| Feed Type | n | DM (% < 86%) | Deviations Regarding Microbiological Quality (% cfu > n.c.) | |||
|---|---|---|---|---|---|---|
| Microbiology (Total) | Aerobic Bacteria | Molds | Yeasts | |||
| Concentrates (total) | 517 | 18.2 | 28.2 | 26.1 | 8.1 | 9.6 |
| Grains | 273 | 16.9 | 22.6 | 19.3 | 7.2 | 4.5 |
| Oats (intact) | 181 | 13.4 | 19.2 | 16.2 | 6.5 | 6.1 |
| Oats (rolled) | 56 | 26.1 | 28.9 | 25.6 | 4.7 | 2.3 |
| Other grains | 36 | 33.3 | 30.8 | 25.0 | 15.4 | 0 |
| Compound feed | 244 | 19.7 | 38.4 | 38.7 | 11.5 | 17.5 |
| Non- pelleted 1 | 151 | 24.3 | 45.1 | 44.6 | 11.8 | 22.6 |
| Pelleted 2 | 93 | 11.8 | 20.5 | 21.1 | 4.8 | 7.7 |
| Type of Micro Organism | n (Total) | Period 1 1 | Period 2 ² | Level of Significance (Period 1 vs. 2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n 1 | Log10 cfu (Mean) | % cfu >n.c. | n ² | Log10 cfu (Mean) | % cfu >n.c. | p (Mean cfu) | p (% cfu >n.c.) | ||
| Aerobic bacteria | 197 | 136 | 6.78 | 23.5% | 61 | 6.15 | 9.8% | 0.0003 | 0.0243 |
| Molds | 208 | 141 | 3.20 | 6.4% | 67 | 3.58 | 9.0% | 0.0360 | 0.5690 1 |
| Yeasts | 200 | 136 | 5.45 | 3.7% | 64 | 5.11 | 6.3% | 0.1580 | 0.4711 1 |
| Preliminary Report | n | Proportional Frequency of Deviations (%) | ||||
|---|---|---|---|---|---|---|
| Microbiology (Total) 1 | Aerobic Bacteria 1 | Molds 1 | Yeasts 1 | LPS ² | ||
| Routine examination | 47 | 27.8 | 29.4 | 2.8 | 5.7 | 33.3 |
| GIDs | 165 | 31.3 | 30.5 | 3.8 | 10.9 | 37.9 |
| Liver enzymes | 75 | 21.8 | 21.2 | 7.1 | 12.2 | 29.4 |
| Coughing | 32 | 16.7 | 15.8% | 4.2 | 0 | 30.0 |
| Preliminary Report | n | Mold Counts (log10 cfu g−1) | ||||
|---|---|---|---|---|---|---|
| Mean | s.d. | s.e. | Min | Max | ||
| Molds (total) | ||||||
| Routine examination | 37 | 2.40 a | 1.59 | 0.26 | 0.00 | 4.54 |
| Coughing | 24 | 3.29 b | 1.09 | 0.22 | 0.00 | 6.15 |
| Aspergillus sp. | ||||||
| Routine examination | 37 | 1.12 | 1.59 | 0.26 | 0.00 | 4.30 |
| Coughing | 24 | 1.85 | 1.54 | 0.31 | 0.00 | 4.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intemann, S.; Reckels, B.; Schubert, D.C.; Wolf, P.; Kamphues, J.; Visscher, C. The Microbiological Quality of Concentrates for Horses—A Retrospective Study on Influencing Factors and Associations with Clinical Symptoms Reported by Owners or Referring Vets. Vet. Sci. 2022, 9, 413. https://doi.org/10.3390/vetsci9080413
Intemann S, Reckels B, Schubert DC, Wolf P, Kamphues J, Visscher C. The Microbiological Quality of Concentrates for Horses—A Retrospective Study on Influencing Factors and Associations with Clinical Symptoms Reported by Owners or Referring Vets. Veterinary Sciences. 2022; 9(8):413. https://doi.org/10.3390/vetsci9080413
Chicago/Turabian StyleIntemann, Sandra, Bernd Reckels, Dana Carina Schubert, Petra Wolf, Josef Kamphues, and Christian Visscher. 2022. "The Microbiological Quality of Concentrates for Horses—A Retrospective Study on Influencing Factors and Associations with Clinical Symptoms Reported by Owners or Referring Vets" Veterinary Sciences 9, no. 8: 413. https://doi.org/10.3390/vetsci9080413
APA StyleIntemann, S., Reckels, B., Schubert, D. C., Wolf, P., Kamphues, J., & Visscher, C. (2022). The Microbiological Quality of Concentrates for Horses—A Retrospective Study on Influencing Factors and Associations with Clinical Symptoms Reported by Owners or Referring Vets. Veterinary Sciences, 9(8), 413. https://doi.org/10.3390/vetsci9080413






