Comparison of Low- and High-Cost Infrared Thermal Imaging Devices for the Detection of Lameness in Dairy Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Lameness
1.2. Lameness Detection
1.3. Thermography
2. Materials and Methods
2.1. Experiment Design
2.1.1. Data Collection
2.1.2. Data Processing
2.2. Statistical Analysis
2.2.1. Environmental Factor Analysis
2.2.2. Adjusted Temperature Analysis
2.2.3. Temperature Difference Analysis
2.2.4. Threshold Analysis
3. Results
3.1. Environmental Factor Results
3.2. Adjusted Temperature Results
3.3. Temperature Difference Results
3.4. Threshold Results
4. Discussion
4.1. Findings
4.1.1. Environmental Factor Findings
4.1.2. Adjusted Temperature Findings
4.1.3. Temperature Difference Findings
4.1.4. Threshold Findings
4.2. Implications
4.3. Limitations
4.4. Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



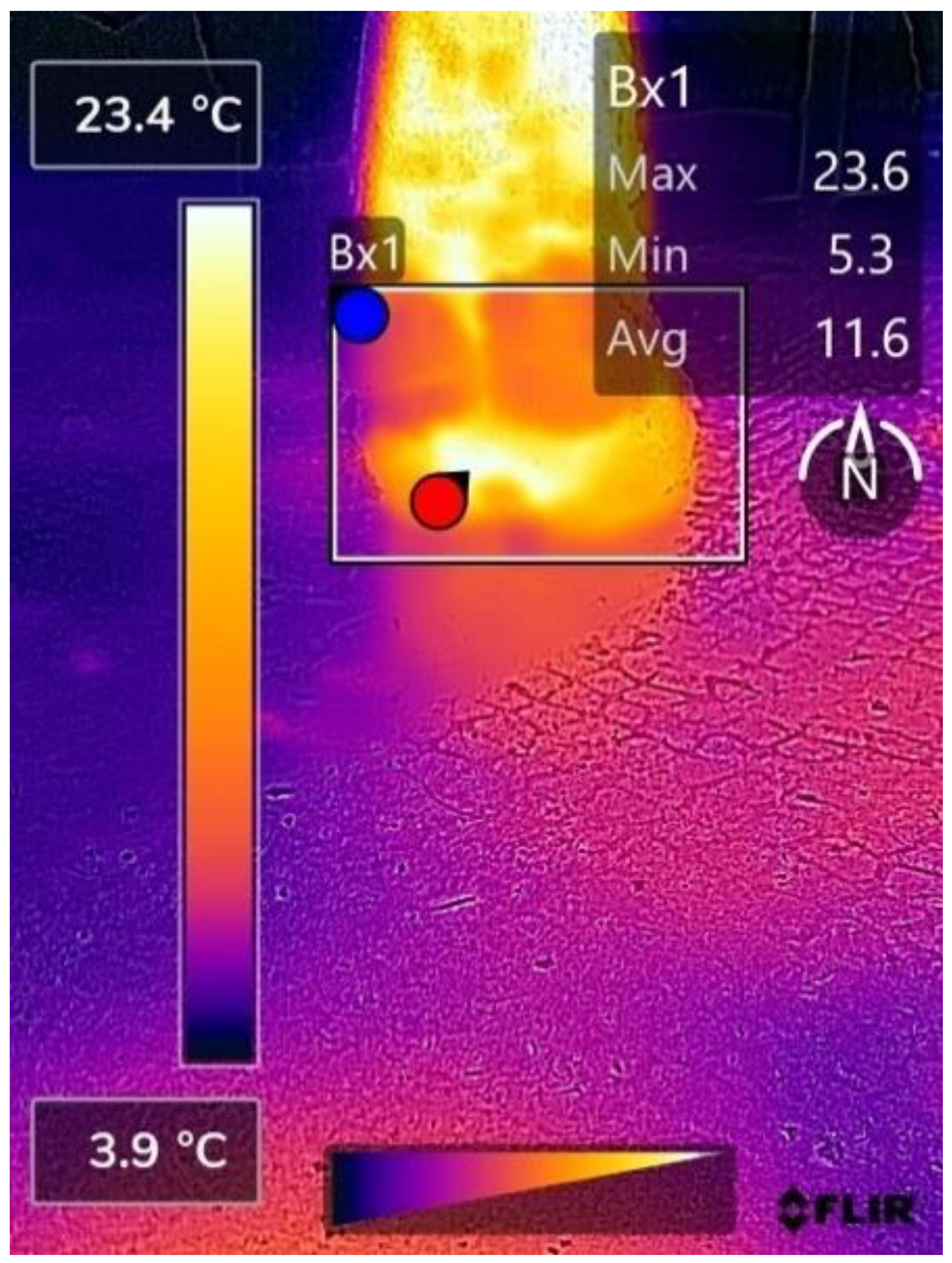


References
- Griffiths, B.E.; White, D.G.; Oikonomou, G. A Cross-Sectional Study into the Prevalence of Dairy Cattle Lameness and Associated Herd-Level Risk Factors in England and Wales. Front. Vet. Sci. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Flower, F.C.; Weary, D.M. Gait Assessment in Dairy Cattle. Animal 2009, 3, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.S.; Bruce, M.; Keating, P.; Raboisson, D.; Clough, H.; Oikonomou, G.; Rushton, J. Profiling Detection and Classification of Lameness Methods in British Dairy Cattle Research: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2020, 7, 542. [Google Scholar] [CrossRef]
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. Assessment of the Welfare of Dairy Caftle Using Animal-Based Measurements: Direct Observations and Investigation of Farm Records. Vet. Rec. 2003, 153, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, B.W.; Chamorro, M.F. Distribution of Lameness Lesions in Beef Cattle: A Retrospective Analysis of 745 Cases. Can. Vet. J. 2016, 57, 401. [Google Scholar]
- Holzhauer, M.; Hardenberg, C.; Bartels, C.J.M.; Frankena, K. Herd- and Cow-Level Prevalence of Digital Dermatitis in the Netherlands and Associated Risk Factors. J. Dairy Sci. 2006, 89, 580–588. [Google Scholar] [CrossRef]
- Underwood, W.J.; Blauwiekel, R.; Delano, M.L.; Gillesby, R.; Mischler, S.A.; Schoell, A. Biology and Diseases of Ruminants (Sheep, Goats, and Cattle), 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124095274. [Google Scholar]
- Amory, J.R.; Barker, Z.E.; Wright, J.L.; Mason, S.A.; Blowey, R.W.; Green, L.E. Associations between Sole Ulcer, White Line Disease and Digital Dermatitis and the Milk Yield of 1824 Dairy Cows on 30 Dairy Cow Farms in England and Wales from February 2003–November 2004. Prev. Vet. Med. 2008, 83, 381–391. [Google Scholar] [CrossRef]
- Shearer, J.K.; Plummer, P.J.; Schleining, J.A. Perspectives on the Treatment of Claw Lesions in Cattle. Vet. Med. Res. Rep. 2015, 6, 273. [Google Scholar] [CrossRef]
- Barker, Z.E.; Amory, J.R.; Wright, J.L.; Mason, S.A.; Blowey, R.W.; Green, L.E. Risk Factors for Increased Rates of Sole Ulcers, White Line Disease, and Digital Dermatitis in Dairy Cattle from Twenty-Seven Farms in England and Wales. J. Dairy Sci. 2009, 92, 1971–1978. [Google Scholar] [CrossRef]
- Palmer, M.A.; O’Connell, N.E. Digital Dermatitis in Dairy Cows: A Review of Risk Factors and Potential Sources of Between-Animal Variation in Susceptibility. Animals 2015, 5, 512. [Google Scholar] [CrossRef]
- Kofler, J.; Fiedler, A.; Charfeddine, N.; Capion, N.; Fjeldaas, T.; Cramer, G.; Bell, N.J.; Müller, K.E.; Christen, A.-M.; Thomas, G.; et al. ICAR Claw Health Atlas-Appendix 1 Digital Dermatitis Stages (M-Stages), 1st ed.; ICAR: New Delhi, India, 2020; ISBN 9295014146. [Google Scholar]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. The Reliability of Detecting Digital Dermatitis in the Milking Parlour. Vet. J. 2012, 193, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, A.; Barden, M.; Tulloch, J.; Williams, K.; Griffiths, B.; Bedford, C.; Rudd, M.; Psifidi, A.; Banos, G.; Oikonomou, G. A Study on the Use of Thermal Imaging as a Diagnostic Tool for the Detection of Digital Dermatitis in Dairy Cattle. J. Dairy Sci. 2021, 104, 10194–10202. [Google Scholar] [CrossRef] [PubMed]
- Orman, A.; Endres, M.I. Use of Thermal Imaging for Identification of Foot Lesions in Dairy Cattle. Acta Agric. Scand. A Anim. Sci. 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Green, L.E.; Hedges, V.J.; Schukken, Y.H.; Blowey, R.W.; Packington, A.J. The Impact of Clinical Lameness on the Milk Yield of Dairy Cows. J. Dairy Sci. 2002, 85, 2250–2256. [Google Scholar] [CrossRef]
- Alsaaod, M.; Fadul, M.; Steiner, A. Automatic Lameness Detection in Cattle. Vet. J. 2019, 246, 35–44. [Google Scholar] [CrossRef]
- Van De Gucht, T.; Saeys, W.; Van Nuffel, A.; Pluym, L.; Piccart, K.; Lauwers, L.; Vangeyte, J.; Van Weyenberg, S. Farmers’ Preferences for Automatic Lameness-Detection Systems in Dairy Cattle. J. Dairy Sci. 2017, 100, 5746–5757. [Google Scholar] [CrossRef]
- Schlageter-Tello, A.; Van Hertem, T.; Bokkers, E.A.M.; Viazzi, S.; Bahr, C.; Lokhorst, K. Performance of Human Observers and an Automatic 3-Dimensional Computer-Vision-Based Locomotion Scoring Method to Detect Lameness and Hoof Lesions in Dairy Cows. J. Dairy Sci. 2018, 101, 6322–6335. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.A.; Cripps, P.J.; Downham, Y.; Murray, R.D. Subjective and Objective Assessment of Pain and Discomfort Due to Lameness in Dairy Cattle. Anim. Welf. 2003, 12, 605–610. [Google Scholar]
- Scoley, G.E.; Gordon, A.W.; Morrison, S.J. Use of Thermal Imaging in Dairy Calves: Exploring the Repeatability and Accuracy of Measures Taken from Different Anatomical Regions. Transl. Anim. Sci. 2019, 3, 564–576. [Google Scholar] [CrossRef]
- Williams, C. The Use of Thermal Imaging Technology to Enhance Livestock Production. Available online: https://businesswales.gov.wales/farmingconnect/news-and-events/technical-articles/use-thermal-imaging-technology-enhance-livestock-production (accessed on 13 February 2022).
- Alsaaod, M.; Schaefer, A.L.; Büscher, W.; Steiner, A. The Role of Infrared Thermography as a Non-Invasive Tool for the Detection of Lameness in Cattle. Sensors 2015, 15, 14513–14525. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An Investigation into the Use of Infrared Thermography (IRT) as a Rapid Diagnostic Tool for Foot Lesions in Dairy Cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef]
- Renn, N.; Onyango, J.; Mccormick, W. Digital Infrared Thermal Imaging and Manual Lameness Scoring as a Means for Lameness Detection in Cattle. Vet. Clin. Sci. 2014, 2, 16–23. [Google Scholar]
- Alsaaod, M.; Büscher, W. Detection of Hoof Lesions Using Digital Infrared Thermography in Dairy Cows. J. Dairy Sci. 2012, 95, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Lokesh Babu, D.S.; Vasant, P.J.; Jeyakumar, S.; Manimaran, A.; Kumaresan, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Kataktalware, M.A.; Siddaramanna. Monitoring Foot Surface Temperature Using Infrared Thermal Imaging for Assessment of Hoof Health Status in Cattle: A Review. J. Therm. Biol. 2018, 78, 10–21. [Google Scholar] [CrossRef]
- Turner, T.A. Thermography as an Aid to the Clinical Lameness Evaluation. Vet. Clin. N. Am. Equine Pract. 1991, 7, 311–338. [Google Scholar] [CrossRef]
- Çetinkaya, M.A.; Demirutku, A. Thermography in the Assessment of Equine Lameness. Turk. J. Vet. Anim. Sci. 2012, 36, 43–48. [Google Scholar] [CrossRef]
- Soroko, M.; Jodkowska, E. Usefulness of Thermography Applied to Horse Diagnosis and in the Equine Sports Industry. Med. Weter. 2011, 67, 397–401. [Google Scholar]
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96. [Google Scholar] [CrossRef]
- Kříž, P.; Horčičková, M.; Bumbálek, R.; Bartoš, P.; Smutný, L.; Stehlík, R.; Zoubek, T.; Černý, P.; Vochozka, V.; Kuneš, R. Application of the Machine Vision Technology and Infrared Thermography to the Detection of Hoof Diseases in Dairy Cows: A Review. Appl. Sci. 2021, 11, 11045. [Google Scholar] [CrossRef]
- Mangus, D.L.; Sharda, A.; Zhang, N. Development and Evaluation of Thermal Infrared Imaging System for High Spatial and Temporal Resolution Crop Water Stress Monitoring of Corn within a Greenhouse. Comput. Electron. Agric. 2016, 121, 149–159. [Google Scholar] [CrossRef]
- Clinton, M.; Manullang, T.; Lin, Y.-H.; Lai, S.-J.; Chou, N.-K.; Manullang, M.C.T.; Lin, Y.-H.; Lai, S.-J.; Chou, N.-K.; Lepore, M. Implementation of Thermal Camera for Non-Contact Physiological Measurement: A Systematic Review. Sensors 2021, 21, 7777. [Google Scholar] [CrossRef]
- Obinah, M.P.B.; Nielsen, M.; Hölmich, L.R. High-End versus Low-End Thermal Imaging for Detection of Arterial Perforators. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3175. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, P.D.; Henson, F.M.D.; Parker, C. Thermography of a Septic Metatarsophalangeal Joint in a Heifer. Vet. Rec. 2000, 146, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, A.; Plaizier, J.C.; Einarson, M.S.; Berry, R.J.; Scott, S.L.; Kennedy, A.D. Short Communication: Infrared Thermography and Visual Examination of Hooves of Dairy Cows in Two Stages of Lactation. J. Dairy Sci. 2005, 88, 2749–2753. [Google Scholar] [CrossRef]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A Field Trial of Infrared Thermography as a Non-Invasive Diagnostic Tool for Early Detection of Digital Dermatitis in Dairy Cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef]
- Oikonomou, G.; Trojacanec, P.; Ganda, E.K.; Bicalho, M.L.S.; Bicalho, R.C. Association of Digital Cushion Thickness with Sole Temperature Measured with the Use of Infrared Thermography. J. Dairy Sci. 2014, 97, 4208–4215. [Google Scholar] [CrossRef]
- Wilhelm, K.; Wilhelm, J.; Fürll, M. Use of Thermography to Monitor Sole Haemorrhages and Temperature Distribution over the Claws of Dairy Cattle. Vet. Rec. 2015, 176, 146. [Google Scholar] [CrossRef]
- Alsaaod, M.; Syring, C.; Luternauer, M.; Doherr, M.G.; Steiner, A. Effect of Routine Claw Trimming on Claw Temperature in Dairy Cows Measured by Infrared Thermography. J. Dairy Sci. 2015, 98, 2381–2388. [Google Scholar] [CrossRef]
- Montanholi, Y.R.; Lim, M.; Macdonald, A.; Smith, B.A.; Goldhawk, C.; Schwartzkopf-Genswein, K.; Miller, S.P. Technological, Environmental and Biological Factors: Referent Variance Values for Infrared Imaging of the Bovine. J. Anim. Sci. Biotechnol. 2015, 6, 27. [Google Scholar] [CrossRef]
- Harris-Bridge, G.; Young, L.; Handel, I.; Farish, M.; Mason, C.; Mitchell, M.A.; Haskell, M.J. The Use of Infrared Thermography for Detecting Digital Dermatitis in Dairy Cattle: What Is the Best Measure of Temperature and Foot Location to Use? Vet. J. 2018, 237, 26–33. [Google Scholar] [CrossRef]
- Lin, Y.C.; Mullan, S.; Main, D.C.J. Optimising Lameness Detection in Dairy Cattle by Using Handheld Infrared Thermometers. Vet. Med. Sci. 2018, 4, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Bobić, T.; Mijić, P.; Gregić, M.; Bagarić, A.; Gantner, V. Early Detection of the Hoof Diseases in Holstein Cows Using Thermovision Camera. Agric. Conspec. Sci. 2017, 82, 197–200. [Google Scholar]
- Main, D.C.J.; Stokes, J.E.; Reader, J.D.; Whay, H.R. Detecting Hoof Lesions in Dairy Cattle Using a Hand-Held Thermometer. Vet. Rec. 2012, 171, 504. [Google Scholar] [CrossRef]
- Caterpillar CAT S62-Pro. Available online: https://www.catphones.com/en-gb/cat-s62-pro-smartphone/ (accessed on 22 April 2022).
- FLIR Systems Inc. FLIR T620bx & T640bx. Available online: https://www.instrumart.com/assets/FLIR-T620bx-T640bx-Datasheet.pdf (accessed on 10 February 2022).
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared Thermography as a Non-Invasive Tool to Study Animal Welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar]
- AHDB Mobility Scoring: How to Score Your Cows. Available online: https://ahdb.org.uk/knowledge-library/mobility-scoring-how-to-score-your-cows (accessed on 10 February 2022).
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef]
- Wang, F.K.; Shih, J.Y.; Juan, P.H.; Su, Y.C.; Wang, Y.C. Non-Invasive Cattle Body Temperature Measurement Using Infrared Thermography and Auxiliary Sensors. Sensors 2021, 21, 2425. [Google Scholar] [CrossRef] [PubMed]
- Rainwater-Lovett, K.; Pacheco, J.M.; Packer, C.; Rodriguez, L.L. Detection of Foot-and-Mouth Disease Virus Infected Cattle Using Infrared Thermography. Vet. J. 2009, 180, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Gloster, J.; Ebert, K.; Gubbins, S.; Bashiruddin, J.; Paton, D.J. Normal Variation in Thermal Radiated Temperature in Cattle: Implications for Foot-and-Mouth Disease Detection. BMC Vet. Res. 2011, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Lin, Y.; Knowles, T.G.; Main, D.C.J. Infrared Thermometry for Lesion Monitoring in Cattle Lameness. Vet. Rec. 2015, 176, 308. [Google Scholar] [CrossRef]
- Archer, S.; Bell, N.; Huxley, J. Lameness in UK Dairy Cows: A Review of the Current Status. Practice 2010, 32, 492–504. [Google Scholar] [CrossRef]
- Werema, C.W.; Laven, L.; Mueller, K.; Laven, R. Evaluating Alternatives to Locomotion Scoring for Lameness Detection in Pasture-Based Dairy Cows in New Zealand: Infra-Red Thermography. Animals 2021, 11, 3473. [Google Scholar] [CrossRef]
- Flower, F.C.; Sanderson, D.J.; Weary, D.M. Hoof Pathologies Influence Kinematic Measures of Dairy Cow Gait. J. Dairy Sci. 2005, 88, 3166–3173. [Google Scholar] [CrossRef]










| Camera | Resolution (Pixels) | Thermal Sensitivity (°C) | Temperature Range (°C) | Accuracy | Approximate Cost |
|---|---|---|---|---|---|
| CAT s62-Pro Smartphone | 160 × 120 | <0.05 | −20 to 400 | 3% or 3 °C | GBP 400 |
| FLIR T620bx | 640 × 480 | <0.04 | −40 to 650 | 2% or 2 °C | GBP 20,000 |
| AHDB Lameness Score | Criteria | Suggested Response |
|---|---|---|
| 0 | Locomotion with smooth and long steps. Weightbearing evenly across all 4 feet. The back is flat | Routine trim if needed |
| 1 | Uneven rhythm to gait or uneven weightbearing Shortened stride-length The affected limb is not identifiable | Routine trim when needed |
| 2 | Uneven weight bearing on an identifiable limb Shortened stride-length Arched back | Lift the foot to identify the issue as soon as possible |
| 3 | Very lame Unable to maintain pace speed of a human walking May be limping | Urgent medical attention needed; the animal should not be made to walk. |
| Data Collection Day | Ambient Temperature (°C) | Humidity (%) | LCD: Mean and Standard Deviation (±°C) | HCD: Mean and Standard Deviation (±°C) |
|---|---|---|---|---|
| 1 | 10 | 61 | 25.09 ± 4.479 | 26.07 ± 4.074 |
| 2 | 10 | 86 | 26.31 ± 3.819 | 26.72 ± 4.054 |
| 3 | 2 | 83 | 18.26 ± 5.249 | 21.82 ± 4.825 |
| 4 | 5 | 90 | 22.21 ± 4.089 | 23.40 ± 4.462 |
| Device Used | Test | Threshold Value (°C) | Sensitivity (%) | Specificity (%) | Positive Predictive value (PPV) (%) | Negative Predictive Value (NPV) (%) |
|---|---|---|---|---|---|---|
| LCD | Maximum adjusted temperature | 2.40 | 64.41 | 64.53 | 51.35 | 75.72 |
| HCD | Maximum adjusted temperature | 2.40 | 69.49 | 66.01 | 54.30 | 78.82 |
| LCD | Temperature difference | 1.85 | 66.95 | 61.08 | 50.00 | 76.07 |
| HCD | Temperature difference | 1.85 | 70.34 | 70.94 | 58.45 | 80.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coe, A.; Blackie, N. Comparison of Low- and High-Cost Infrared Thermal Imaging Devices for the Detection of Lameness in Dairy Cattle. Vet. Sci. 2022, 9, 414. https://doi.org/10.3390/vetsci9080414
Coe A, Blackie N. Comparison of Low- and High-Cost Infrared Thermal Imaging Devices for the Detection of Lameness in Dairy Cattle. Veterinary Sciences. 2022; 9(8):414. https://doi.org/10.3390/vetsci9080414
Chicago/Turabian StyleCoe, Aidan, and Nicola Blackie. 2022. "Comparison of Low- and High-Cost Infrared Thermal Imaging Devices for the Detection of Lameness in Dairy Cattle" Veterinary Sciences 9, no. 8: 414. https://doi.org/10.3390/vetsci9080414
APA StyleCoe, A., & Blackie, N. (2022). Comparison of Low- and High-Cost Infrared Thermal Imaging Devices for the Detection of Lameness in Dairy Cattle. Veterinary Sciences, 9(8), 414. https://doi.org/10.3390/vetsci9080414






