Simple Summary
Pythium insidiosum is a waterborne fungus-like organism commonly present in tropical and subtropical areas that causes a disease named pythiosis in dogs and other animals. This disease can cause inflammatory lesions on the skin and gastrointestinal tract and, if left untreated, may be fatal. Because this mold lacks the distinctive features of true fungi, it requires specific treatment. Diagnosis of pythiosis is commonly achieved by microscope visualization after staining the tissue sections (histopathology). Although some stains highlight hyphae, these techniques are challenging to distinguish P. insidiosum from other fungi. Our study aimed to develop a molecular technique (nested PCR) to specifically detect P. insidiosum DNA in biopsy specimens to aid in diagnosing this organism. Archived biopsies from 26 dogs suspected of pythiosis were examined by histopathology with special stains and tested by the novel nested PCR. Agreement between histopathology and nested PCR occurred in 18/26 cases. The microscopic examination identified hyphae consistent with Pythium sp. in 57.7% of the samples, whereas the nested PCR detected P. insidiosum DNA in 76.9% of samples, aiding in the sensitivity of the diagnosis of pythiosis in dogs. Using this combination of techniques, we report 20 canine cases of pythiosis over 18 years in Indiana and Kentucky, an unexpectedly high incidence for temperate climatic regions. Thus, we recommend using our nested PCR test in addition to the microscopic examination to increase the sensitivity of the diagnosis.
Abstract
Pythium insidiosum is an infectious oomycete affecting dogs that develop the cutaneous or gastrointestinal form of pythiosis with a poor prognosis. If left untreated, pythiosis may be fatal. This organism is not a true fungus because its cell wall and cell membrane lack chitin and ergosterol, respectively, requiring specific treatment. Identifying the organism is challenging, as a hematoxylin and eosin (H&E) stain poorly stain the P. insidiosum hyphae and cannot be differentiated conclusively from other fungal or fungal-like organisms (such as Lagenidium sp.) morphologically. Our study aimed to develop a nested PCR to detect P. insidiosum and compare it with the traditional histopathologic detection of hyphae. Formalin-fixed, paraffin-embedded (FFPE) tissue scrolls from 26 dogs with lesions suggesting the P. insidiosum infection were assessed histologically, and DNA was extracted from the FFPE tissue sections for nested PCR. Agreement between the histologic stains, (H&E), periodic acid–Schiff (PAS), and/or Grocott methenamine silver (GMS) and the nested PCR occurred in 18/26 cases. Hyphae consistent with Pythium sp. were identified via histopathology in 57.7% of the samples, whereas the nested PCR detected P. insidiosum in 76.9% of samples, aiding in the sensitivity of the diagnosis of pythiosis in dogs. Using this combination of techniques, we report 20 canine cases of pythiosis over 18 years in Indiana and Kentucky, an unexpectedly high incidence for temperate climatic regions. Using a combination of histopathology evaluation and nested PCR is recommended to aid in the accurate diagnosis of pythiosis.
1. Introduction
Pythium insidiosum is an oomycte that infects dogs and horses [1] and, infrequently, humans and other vertebrates [2,3,4,5,6,7]. Pythium sp. infections typically occur in tropical/subtropical regions; however, a few cases are reported in other areas considered abnormal locations to find this oomycete [4,6,8]. The cutaneous or gastrointestinal forms of pythiosis are the most common types to develop in infected animals, and both forms often have poor prognoses [4] unless diagnosed in an early stage of infection when the complete excision of lesions is possible [4]. This organism is not an actual fungus since its cell wall and cell membrane lack chitin and ergosterol, respectively [4,6,8]. The antifungal treatment of P. insidiosum cases is usually not successful due to this unique characteristic of this organism. Therefore, recent studies have suggested that novel drug combinations may be associated with better outcomes [6,9,10,11,12,13]. Morphologically, P. insidiosum is typically ~3–10 µm wide, with thick walls, few septa, and close to right-angle branches. The organism identification is challenging, as a hematoxylin and eosin (H&E) stain poorly stains the P. insidiosum hyphae. When visualized, it cannot always be definitively distinguished from other fungal or fungal-like organisms (such as Lagenidium sp.) based on morphology [3,6,14].
Due to the challenges in differentiating pythiosis infection from fungal and other infections that result in similar inflammatory lesions, molecular diagnosis-based PCR assays were developed to detect the genomic DNA of P. insidiosum extracted from cultured organisms or infected tissues [8,15,16,17,18,19]. Ribosomal DNA (rDNA) [20] is the widely used target for detecting fungal DNA [21]. The rDNA of the 18S rRNA gene, inter transcriptional space 1 (ITS1), ITS2, 28SrRNA, and 5.8S rRNA are the most commonly used targets for detecting P. insidiosum DNA using universal pan fungal primers [7,22]. The use of pan fungal primers must be followed by sequencing the amplified DNA region to confirm P. insidiosum. Specific primers for P. insidiosum rDNA detection have been developed to avoid the subsequent DNA sequencing step [15,16,21]. Unfortunately, these primers failed to detect some P. insidiosum due to a sequence mismatch with some P. insidiosum strains [23,24]. Another study used P. insidiosum-specific primers targeting the exo-1,3-b-glucanase gene [25]. In this study, the PCR product was 550 bp, and the authors considered this assay efficient in detecting the P. insidiosum strains [3].
Since the therapeutic response is unfortunately poor and patients in late stages succumb to the disease, pythiosis must be accurately diagnosed and treated as early as possible. A faster and more sensitive diagnostic test is a continuously evolving need. Therefore, this study intended to assess the efficiency of using a nested PCR method. In this method, we combine the pan-fungal primers ITS3 and ITS4, followed by species-specific P. insidiosum primers to detect the genomic DNA of P. insidiosum extracted from formalin-fixed, paraffin-embedded (FFPE) scrolls.
2. Materials and Methods
2.1. Samples
Twenty-six FFPE samples of different tissues from dogs with lesions suggestive of P. insidiosum infection submitted to the Indiana Animal Disease Diagnostic Laboratory (ADDL) between 2001 to 2019 were evaluated (Table 1). The lesions were pyogranulomatous to eosinophilic inflammation in the skin, gastrointestinal tract, and lymph nodes with either a lack of etiologic agent identified or an identification of hyphae consistent morphologically with Pythium sp. An H&E stain, GMS stain and PAS stain were also used to aid in identification. All the slides were examined for the presence of hyphae.

Table 1.
Summary of the results of each case selected and comparisons between the microscopic observation of hyphae in the H&E, GMS, and PAS stains to the nested PCR assay developed in this study.
2.2. DNA Extraction
Four scrolls (20 µm thick) from the FFPE tissue were used for DNA isolation as previously described with some modifications [26]. Briefly, 1 mL of a deparaffinization reagent (d-limonene, QIAGEN® Inc., Valencia, CA, USA) was added to the scrolls to dissolve the paraffin of each sample. The samples were washed with ethanol (1 mL) and digested with an ATL buffer (QIAGEN) and proteinase K (QIAGEN) at 56 °C for 1 h, followed by another 1 h of incubation at 90 °C. The FastPrep-24™ Classic Instrument (MP Biomedicals, Irvine, CA, USA) was used to homogenize the samples for 1 min five times at 5 min intervals to avoid overheating and degradation of the sample. The downstream DNA extraction was performed using the Quick-DNA™ Fungal/Bacterial Miniprep Kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s instructions. Cultured Aspergillus fumigatus was used as the DNA extraction positive control for the kit, and DNase-free water was used as the negative extraction control. The quantity and quality of DNA were assessed by UV spectrophotometry (NanoDrop, ThermoFisher Scientific Waltham, MA USA).
2.3. Pan-Fungal Conventional PCR Assay
Conventional PCR was performed using the pan-fungal protocol [27] with the ITS3 forward primer (5’-GCA TCG ATG AAG AAC GCA GC-3’) and ITS4 reverse primer (5’-TCC TCC GCT TAT TGA TAT GC-3’). These pan-fungal primers amplify a DNA fragment of approximately 623 bp of the ITS2 non-coding region, located between the coding regions for 5.8S and the small rRNA gene subunits conserved in virtually all fungal genomes [21,24]. The PCR assay was performed by using the GoTaq® Colorless Master Mix (Promega® Corporation, Madison, WI, USA) and consisted of 12.5 μL of the GoTaq® Colorless Master Mix, 1 μL of each primer ITS3 (10 mM) and ITS4 (10 Μm), 5 μL of nuclease-free water, and 5 μL of the assigned DNA sample. The thermoprofile consisted of enzyme activation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 1 min, and extension at 72 °C for 1 min, and then one cycle of final extension at 72 °C for 5 min. Cultured A.fumigatus DNA was used as the positive PCR control. Nuclease-free water was used as the negative PCR control.
2.4. Nested PCR
The PCR product from the pan-fungal PCR was then used as the template for a second (nested) PCR assay using the newly designed primers NE Fw: 5’-ATG CCT GGA AGT ATG CCT GT-3’ and NE Rev: 5’-TCA CTG CGT TCG AGC ATT AC-3’. These primers were designed to specifically amplify a product size of 191 bp of P. insidiosum rDNA. This protocol used 12.5 μL of the GoTaq® Colorless Master Mix, 1 μL of each primer NE Fw (10 Μm) and NE Rv (10 Μm), 9.5 μL of nuclease-free water, and 1 μL of the assigned sample. The thermoprofile consisted of enzyme activation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 1 min, and extension at 72 °C for 1 min. One cycle of final extension at 72 °C for 5 min followed. P. insidiosum DNA isolated from a known positive clinical sample was used as the positive PCR control and nuclease-free water as the negative control. After amplification, the PCR products and molecular weight markers (GeneRuler 1 kb Plus DNA Ladder, ThermoFisher Scientific) were loaded into a 1.5% agarose gel with 0.5 μg/mL ethidium bromide and separated by electrophoresis for 1 h at 100 V. Visualization and documentation was achieved by exposing the gel to 312 nm UV light (G: BOX XT4: Chemiluminescence and Fluorescence Imaging System, Syngene, Frederick, MD, USA).
2.5. Conventional PCR for the GAPDH Gene
Negative nested PCR samples were subject to a conventional PCR targeting glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the housekeeping gene, to verify the presence of amplifiable DNA [27].
2.6. Sequencing
Amplicons with the expected sizes (623 bp or 191 bp) were excised from the gel, and DNA was purified using a Zymoclean™ Gel DNA Recovery Kit (Zymo Research, Irvine, CA, USA). Sequencing was performed by the Sanger method [28] at the Genomics Core Facility at Purdue University.
2.7. Cloning of the PCR Amplicons
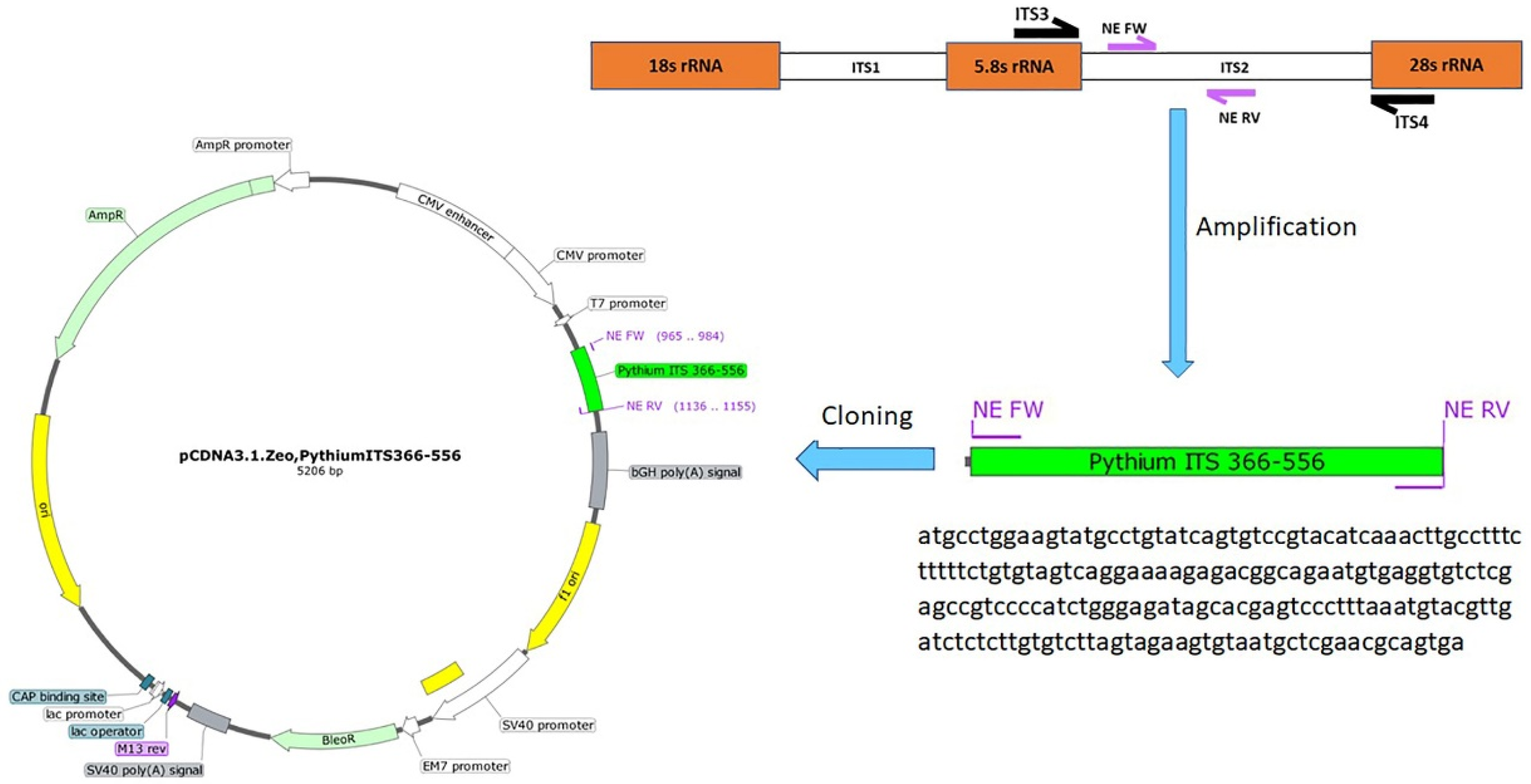
The NE primers were used to amplify the 191 bp amplicon using a Phusion Hot Start II DNA Polymerase (ThermoFisher Scientific, Waltham, MA, USA). Gel electrophoresis was used to purify the PCR product, and bands with the expected size were purified (Invitrogen™ PureLink™ Quick Gel Extraction Kit #K210012, Waltham, MA, USA). A Fast DNA End Repair Kit (ThermoFisher Scientific, Waltham, MA, USA) was used to add the phosphate groups required for ligation to the amplicons. The EcoRV restriction enzyme was used to linearize the pCDNA3.1 plasmid, followed by using the quick CIP dephosphorylation kit (New England Biolabs, Ipswich, MA, USA). The phosphorylated amplified PCR product was ligated to the linearized pCDNA3.1 plasmid using the rapid ligation kit (ThermoFisher Scientific) to create the pCDNA3.1-Pythium-ITS2 366-556 plasmid (Figure 1). The ligation mixture was used to transform Stellar™ Competent Cells (Takara Bio USA, Mountain View, CA, USA). The correct clone was identified, and the cloned plasmid was purified from a 200 mL bacterial culture using a Maxi Fast-Ion Plasmid Kit (IBI Scientific, Road Dubuque, IA, USA). The DNA yield was 1.28 mg, and the 260/280 ratio was 1.85.
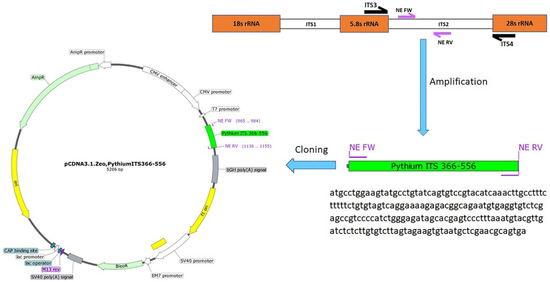
Figure 1.
Cloning steps of the pCDNA3.1-Pythium-ITS2 366-556 plasmid were developed to estimate the detection limit of the nested PCR assay, including a diagram showing the location of the primers used for the development of a nested PCR. The ITS2 and ITS4 primers have been previously described. The NE Fw and NE Rv primers were designed in this study. The size of the products is based on the P. Insidiosum sequence (GenBank ID: GQ260125.1).
2.8. Determination of PCR Sensitivity
The minimum copy number needed for detection was determined using the pCDNA3.1-Pythium-ITS2 366-556 plasmid diluted in nuclease-free water to 20 ng/µL. The genome copy number was calculated for pCDNA3.1-Pythium-ITS2 366-556 using the plasmid size (5206 bp) and the DNA concentration using the DNA copy number and dilution calculator [29] and the calculator for determining the template of the copy number [30]. Ten-fold dilutions of the plasmid were then used to calculate the minimum detection limit of the copies by the nested PCR assay.
3. Results
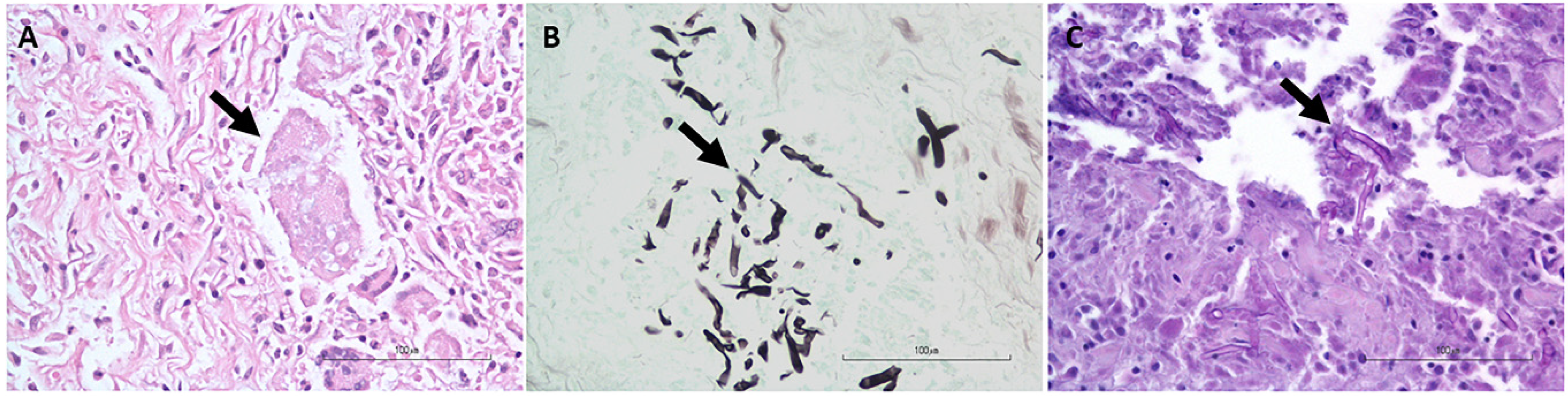
The histopathology and nested PCR were in agreement in 18/26 (69.2%) of the cases: 15/26 (57.7%) of the cases had hyphae identified histologically via the H&E, GMS, and/or PAS stains (Figure 2) and were also positive for P. insidiosum by the nested PCR (Figure 3 and Supplementary Figure S1), and 3/26 (11.5%, cases 6, 10, and 18) of the cases were negative histologically for hyphae identification and by the nested PCR and, therefore, considered negative for the P. insidiosum infection. Interestingly, case 18 was positive for the pan-fungal PCR (ITS3 and ITS4 primers), and the sequencing revealed the presence of A. fumigatus.
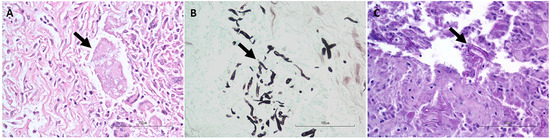
Figure 2.
Representative photomicrographs for the histopathologic identification of hyphae morphologically consistent with Pythium sp. (arrows). (A). H&E stain; (B). GMS stain; and (C). PAS stain.
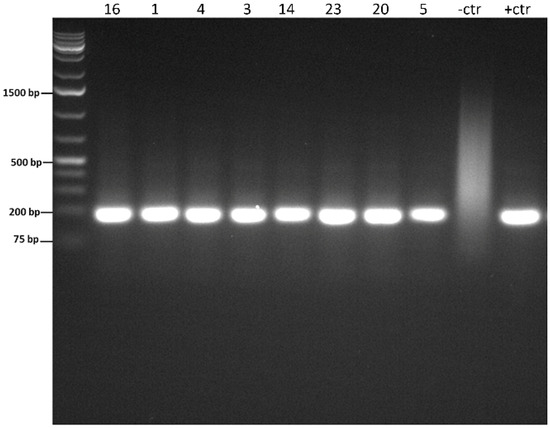
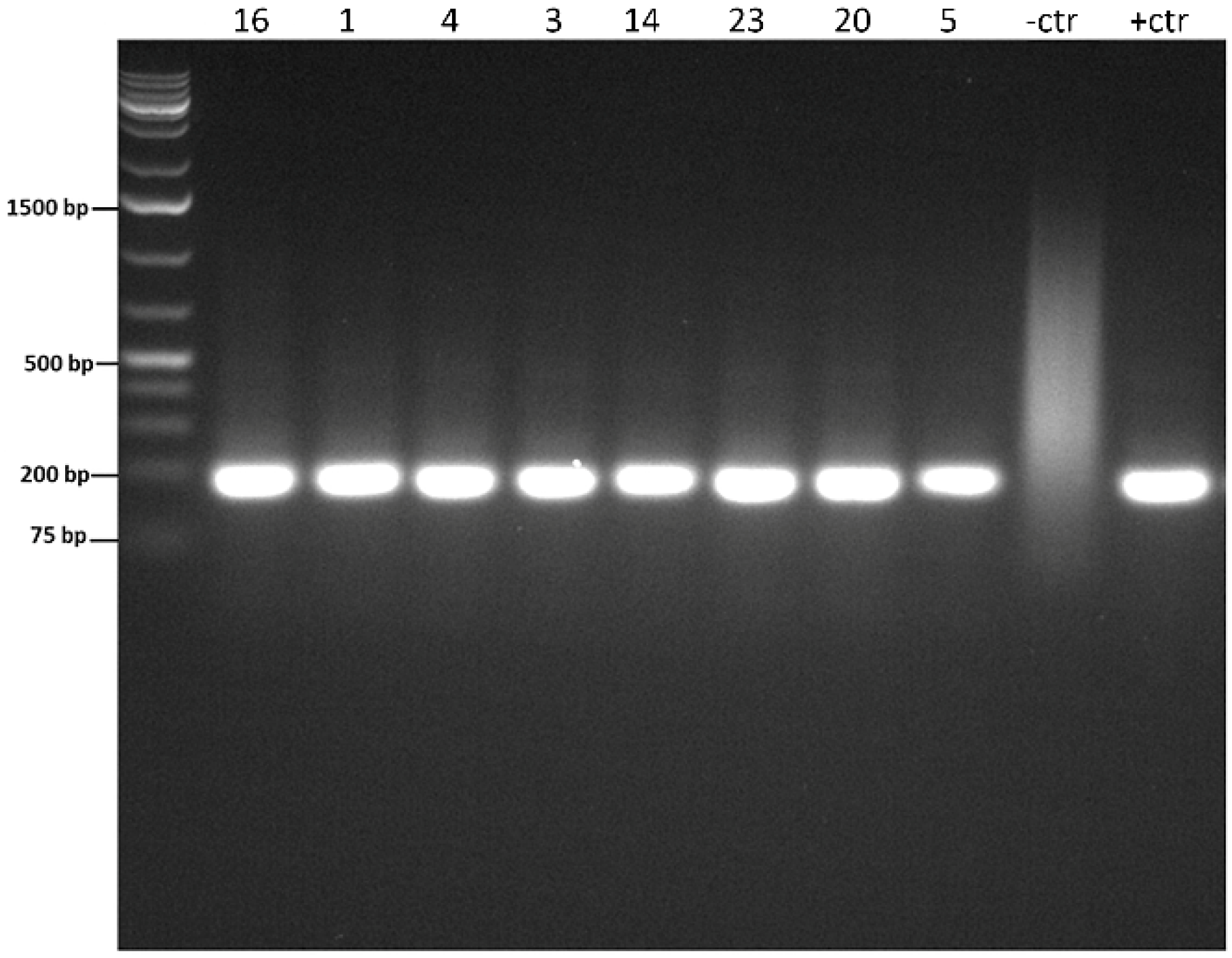
Figure 3.
Representative picture of the nested PCR gel’s electrophoresis, showing positive bands with approximately 191 bp correspondent to P. insidiosum. The negative control was nuclease-free water, and the positive control was P. insidiosum DNA. DNA Ladder (L), base pair size markers; numbers refer to clinical cases; negative control (-ctr); positive control (+ctr).
Disagreement between the histopathology and nested PCR occurred in 8/26 cases: in 5/26 (19.2%, cases 14, 15, 20, 22, and 24), hyphae were not identified histologically via the H &E, GMS, and/or PAS stains but were positive by the nested PCR. On the other hand, 3/26 cases (11.5%, cases 8, 9, and 12) were positive by the histopathology but negative by the nested PCR. A summary of the case results is shown in Table 1.
Seven PCR products from the nested PCR were sent for sequencing to verify the specificity of the nested PCR assay: the seven sequences were 100% identical to the P. insidiosum sequences (Sequence IDs: GQ260125.1, GU137331.1, GU137328.1, and EF016914.1) deposited in the Genbank® (Bethesda, MD, USA) nucleotide database [31]. A representative sequence was then submitted to the Genbank® database under accession number: OM282097.
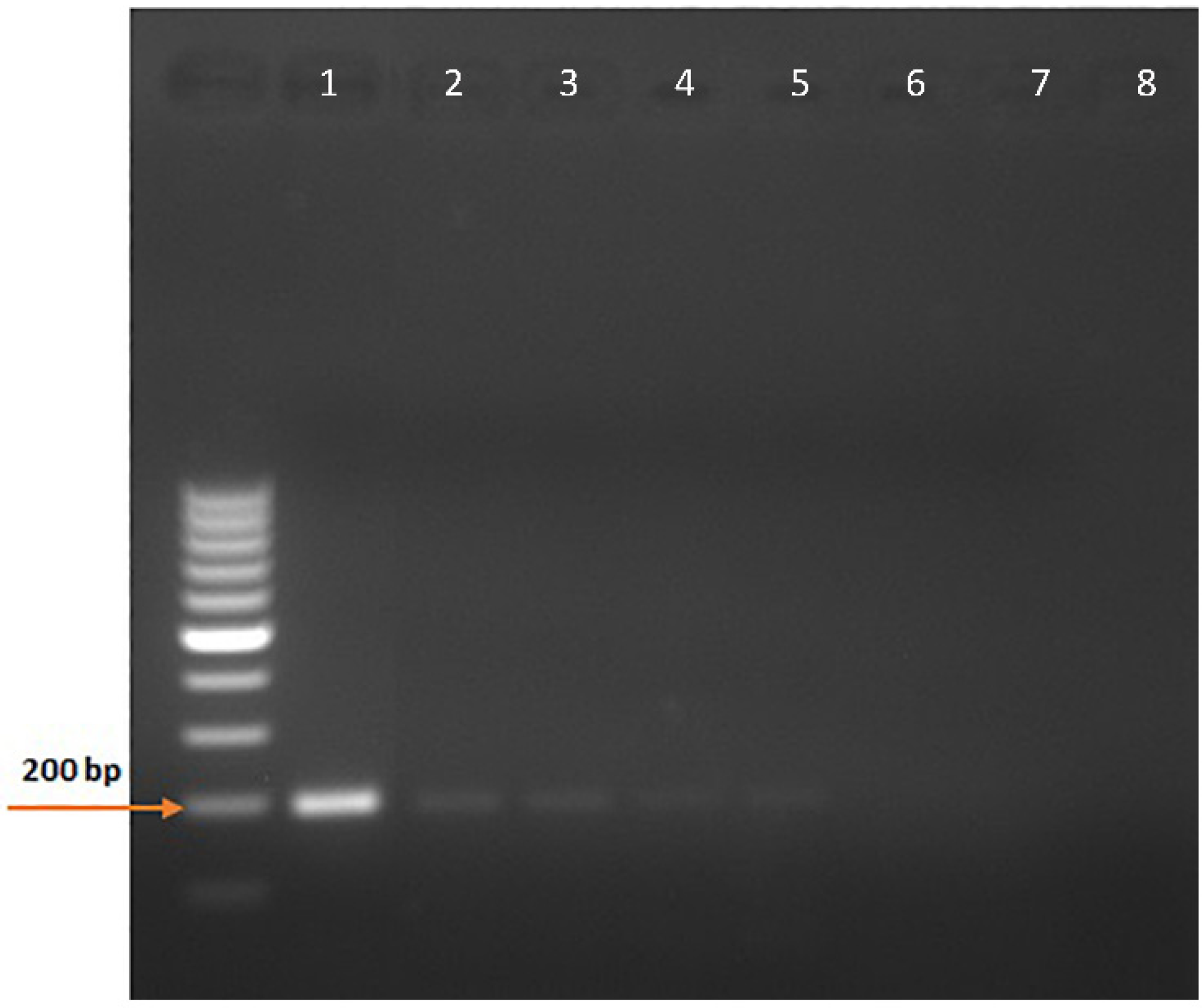
The cloning of pCDNA3.1-Pythium-ITS2 366-556 was successful, and the large-scale plasmid preparation provided a DNA yield of 1.28 mg with a 260/280 ratio of 1.85. The minimum copy number detection using the NE primers for the nested PCR was 0.05, corresponding to five copies in 100 µL (Figure 4 and Supplementary Figure S1).
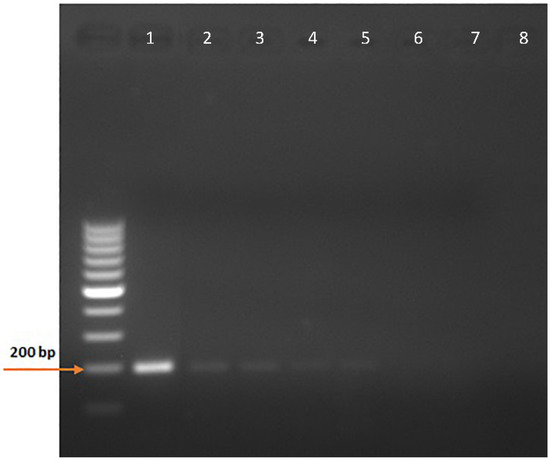
Figure 4.
Minimum copy number detection for the nested PCR assay. Ten-fold dilutions of the pCDNA3.1-Pythium-ITS2 366-556 plasmid were used to determine the sensitivity of the nested PCR assay detecting the ITS2 366-556 sequences of P. insidiosum rDNA. 1, 5 × 102 copies; 2, 5 × 101 copies; 3, 5 × 100 copies; 4, 5 × 10−1 copies; 5, 5 × 10−2 copies; 6, 5 × 10−3 copies; 7, 5 × 10−4 copies; 8, No template.
4. Discussion
P. insidiosum is a fungal-like organism that can infect dogs, mostly in tropical and subtropical regions [32]. The clinicopathological findings of this infection are characterized by pyogranulomatous inflammation in the skin or the gastrointestinal tract. Due to the complexities associated with identifying or isolating P. insidiosum, a conclusive diagnosis is hard to attain. Diagnosing P. insidiosum by morphology alone is presumptive given the similarities with other phycomycotic or zygomycotic organisms, usually resulting in erroneous diagnosis and delayed treatment [32]. Similarly, immunohistochemistry using anti-P. insidiosum antibodies may cross-react with other related fungal species, preventing a definitive diagnosis [33]. The molecular diagnosis is of great importance in identifying the organisms’ genome, especially if other diagnostic tools are inconclusive. Thus, we have developed a novel nested PCR assay to help identify P. insidiosum in FFPE sections from different organs from dogs suspected of pythiosis based on the presence of pyogranulomatous to eosinophilic inflammation and a consistent history or location of lesions.
When choosing the clinical cases for this study, we were interested in cases with granulomatous to eosinophilic dermatitis, enteritis, colitis, mesenteritis, pancreatitis, and lymphadenitis, with either visualized hyphal structures in the sections consistent with P. insidiosum or the suspicion of pythiosis based on the location and nature of the inflammatory reaction. Most of the cases positive by H& E [34] staining (13/26) were also positive with either GMS [35] or PAS staining; however, a few cases were negative by H&E staining but positive for PAS (case 4) or GMS (cases 7, 9, 26) staining. These results are in agreement with Mendoza and collaborators [34], who described the challenges in detecting the P. insidiosum hyphae using an H &E stain but increased sensitivity using a GMS stain, showing the advantage of using special stains to help identify fungal organisms [34]. Regarding the PAS stain, Mittal and collaborators [36] reported the variability or poor staining of P. insidiosum hyphae due to the presence of cellulose in their cell walls. Thus, the PAS stain requires a longer exposure time and a higher concentration of periodic acid to generate the magenta color [36,37]. In the same study, the authors proposed a new staining technique for diagnosing P. insidiosum using potassium iodide–sulfuric acid (IKI-H2SO4). Although more sensitive and cost-effective than the PAS stain, using the IKI-H2SO4 stain entails caution in handling highly concentrated sulfuric acid (65%) [36]. Also, this technique requires well-trained professionals to operate a florescent microscope that is not always used or accessed in many clinics [38].
Several researchers have used molecular diagnostic tools for diagnosing P. insidiosum, including a pan-fungal primers’ PCR followed by sequencing [19,39,40]. However, this method requires highly purified samples and is expensive for regular clinical diagnosis [41]. Some studies have used P. insidiosum-specific primers, but they either used a small sample size (n = 6) [15] or fresh tissue or cultured organisms as the starting material [23,42]. Our study used a nested PCR assay to detect the P. insidiosum DNA from the FFPE blocks from different organs with pyogranulomatous to eosinophilic inflammation and a consistent history or location of pythiosis lesions. Our novel assay has also proven sensitive, with a detection limit of five copies in 100 µL. We have found that 20 samples out of the 26 are positive for P. insidiosum (20/26), with a nearly 77% positive rate in the suspected samples. Five of these samples were negative in the hyphae staining and positive nested PCR for P. insidiosum 19.2% (5/26), corroborating the limitations of the histopathological diagnosis of pythiosis. Another study has shown that the PCR successfully detected P. insidiosum in 6 of 21 (28.57%) clinical samples that failed to grow in culture and also emphasized the limitation of the microbiological diagnosis of pythiosis [42]. The addition of a nested PCR assay increased the sensitivity for P. insidiosum from 57.7% (15/26) to 76.9% (20/26 samples) positivity. Thus, the specific Pythium sp. nested PCR is more sensitive than histopathology alone and should be added to the diagnostic profile in cases where P. insidiosum is strongly suspected, but organisms are not seen using H&E, GMS, or PAS staining. This combinatory approach could more accurately direct the therapeutic approach.
A disagreement between histopathology and nested PCR occurred in 8/26 of the cases. Five cases (14, 15, 20, 22, and 24) were positive by the nested PCR, but hyphae were not identified histologically via H&E, GMS, and/or PAS staining. P. insidiosum is notoriously tricky to identify on H&E staining because it is non-staining and appears as a clearing in the shape of hyphae surrounded by an eosinophilic rim [2]. These organisms either stain poorly or do not stain with the PAS stain due to the lack of chitin within the cell wall; therefore, the lack of PAS staining cannot rule out infection with this organism. The GMS stain should stain these organisms well; however, it is possible that hyphae were not present in the examined histologic cut. Since PCR is generally more sensitive and able to detect the presence of DNA whether or not intact fungal hyphae are present, the lack of the identification of hyphae histologically is likely due to a combination of low numbers of organisms, difficulty visualizing the hyphae, or the absence of hyphae in the tissue cuts examined histologically. The characteristics of the lesions combined with a positive nested PCR and sequencing provide confidence for diagnosing pythiosis in all five of these cases.
The three cases with histopathology positive and nested PCR negative (8, 9, 12) had hyphae consistent with P. insidiosum identified on the histopathology, but the hyphae were fragmented. In cases 8 and 12, the hyphae were 7–10 µm wide, had roughly parallel walls, and with rare septa and rare right-angle branching, and in case 9, hyphae were 5–10 µm wide with few septa (the fragmentation prevented further characterization). It is possible that these hyphae are not P. insidiosum, and other etiologies should be considered. Lagenidium sp. has almost identical hyphae (6–20 µm wide, rare septa, and right-angle branching) and is, therefore, a top differential diagnosis in this case [4]. Additionally, zygomycosis, such as Basidiobolus sp. (5–20 µm wide, rare septa, and non-dichotomous branching) and Conidiobolus sp. (5–13 µm wide, rare septa, and non-dichotomous branching), can also appear similar to [4] and should be ruled out.
The occurrence of 20 cases of canine pythiosis in 18 years is higher than expected in Indiana and Kentucky, USA (the northern temperate climate zone), where the study was conducted, compared to four cases reported in a period of nine years in an epidemiological study in the same area [43]. A few clinical cases of pythiosis in dogs are reported in non-tropical regions, such as two cases of cutaneous pythiosis in a dog from Wisconsin, USA [44], ten canine cases reported in California, USA [45], and dogs in Arizona, USA [4,46,47]. The presence of P. insidiosum in temperate climate areas raises awareness for broader geographic distribution. It highlights the importance of recognizing the clinical and histopathological features of the disease, as well as the available diagnostic tools to identify this organism correctly. A study investigating the epidemiology of this disease in the midwestern region of the USA is ongoing.
As previously mentioned, P. insidiosum is exceptionally challenging to visualize histopathologically, even with special stains, so it is always possible to miss the etiologic agent using histopathology alone [4]. The novel nested PCR reported herein may be used as an ancillary tool to help confirm P. insidiosum from biopsy specimens providing a fast and accurate diagnosis paramount to initiating the most appropriate treatment. Given that P. insidiosum is an oomycete lacking ergosterol in the cell membrane and chitin in the cell wall [5,6], typical fungicides are ineffective because they target either ergosterol synthesis (i.e., the azoles) or alter membrane permeability (i.e., amphotericin B) [46], emphasizing the importance of an accurate diagnosis. Other medications, such as caspofungin (which inhibits B-glucan synthesis) and mefenoxam (which inhibits RNA polymerase), are promising therapies but have not been thoroughly evaluated to date [11]. Therapeutic options include surgical excision and immunotherapy, but the overall therapeutic response is low, and most patients do not survive [46]. Regardless of the treatment chosen, pythiosis must be correctly diagnosed and therapy started immediately. The developed P. insidiosum-specific nested PCR allows for a rapid and more sensitive and precise diagnosis that enables clinicians to initiate the correct treatment earlier and give the dog a better chance of surviving.
5. Conclusions
Infection caused by P. insidiosum is life-threatening to dogs if left untreated. Pythiosis may also affect humans and other animals, such as horses and cattle, and is more common in humid tropical and subtropical climatic zones. An accurate pythiosis diagnosis is essential for successful treatment, while a histopathological examination using multiple stains to detect the organism’s hyphae is not conclusive. Herein, we describe a novel nested PCR assay to detect the P. insidiosum infection from FFPE samples. Using this sensitive and accurate molecular diagnostic technique enabled us to record 20 cases over 18 years in Indiana and Kentucky, an unexpectedly high incidence for such temperate climatic regions. Our study raises awareness of the wider geographic distribution of this organism in addition to underscoring the significance of incorporating the nested PCR assay with the histologic assessment to improve the diagnostic sensitivity of pythiosis in dogs.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vetsci9080444/s1, Figure S1: Detection of P. insidiousm by PCR and the minimum genome copy number detection limit
Author Contributions
Conceptualization, A.P.S., L.F.G., and Y.L.J.-H.; methodology, A.P.S., L.F.G., and Y.L.J.-H.; validation A.P.S., L.F.G., N.O.E., E.E.S., J.H., M.M., and Y.L.J.-H.; formal analysis, N.O.E., E.E.S., A.P.S., and J.H.; investigation, A.P.S., L.F.G., N.O.E., E.E.S., J.H., M.M., and Y.L.J.-H.; resources, A.P.S. and L.F.G.; data curation, A.P.S., L.F.G., N.O.E., E.E.S., J.H., and Y.L.J.-H.; writing—original draft preparation, N.O.E., E.E.S., A.P.S., and J.H.; writing—review and editing, A.P.S., L.F.G., N.O.E., E.E.S., J.H., M.M., and Y.L.J.-H.; visualization; supervision, A.P.S., L.F.G., and Y.L.J.-H.; project administration, A.P.S., L.F.G., and Y.L.J.-H.; funding acquisition, A.P.S., and L.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
Purdue Research Foundation (PRF) 2018–2019 of Purdue University provided salary support for Nelly O. Elshafie.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All owners have signed a release form approving the use of excess animal samples for research purposes. The samples used were archived samples belonging to Purdue University’s Animal Disease Diagnostic Laboratory.
Data Availability Statement
The data are contained within the article or supplementary material. A representative sequence of a Pythium insidiosum 5.8S ribosomal RNA gene and internal transcribed spacer 2 was submitted to the Genbank® database under accession number: OM282097.
Acknowledgments
We would like to thank Peg Miller for completing the ADDL case search.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paz, G.S.D.; Camargo, G.G.; Cury, J.E.; Apolonio, E.V.P.; Garces, H.G.; Prado, A.C.D.; Chechi, J.L.; Oliveira, A.L.; Watanabe, M.J.; Bagagli, E.; et al. Outbreak of equine pythiosis in a southeastern region of Brazil: Environmental isolation and phylogeny. Transbound. Emerg. Dis. 2022, 69, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A. (Ed.) Pythiosis. In Clinical Veterinary Advisor; W.B. Saunders: Saint Louis, MO, USA, 2012; pp. 485–487. [Google Scholar]
- Mendoza, L.; Arias, M.; Colmenarez, V.; Perazzo, Y. Intestinal canine pythiosis in Venezuela confirmed by serological and sequencing analysis. Mycopathologia 2005, 159, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Grooters, A.M. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 695–720. [Google Scholar] [CrossRef]
- Gaastra, W.; Lipman, L.J.; De Cock, A.W.; Exel, T.K.; Pegge, R.B.; Scheurwater, J.; Vilela, R.; Mendoza, L. Pythium insidiosum: An overview. Vet. Microbiol. 2010, 146, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Kaur, K.; Venugopal, A.; Srinivasan, B.; Bagga, B.; Iyer, G.; Christy, J.; Prajna, L.; Vanathi, M.; Garg, P.; et al. Pythium insidiosum keratitis—A review. Indian J. Ophthalmol. 2022, 70, 1107–1120. [Google Scholar] [CrossRef]
- Reis, J.L., Jr.; de Carvalho, E.C.; Nogueira, R.H.; Lemos, L.S.; Mendoza, L. Disseminated pythiosis in three horses. Vet. Microbiol. 2003, 96, 289–295. [Google Scholar] [CrossRef]
- Lohnoo, T.; Jongruja, N.; Rujirawat, T.; Yingyon, W.; Lerksuthirat, T.; Nampoon, U.; Kumsang, Y.; Onpaew, P.; Chongtrakool, P.; Keeratijarut, A.; et al. Efficiency comparison of three methods for extracting genomic DNA of the pathogenic oomycete Pythium insidiosum. J. Med. Assoc. Thai. 2014, 97, 342–348. [Google Scholar]
- Cridge, H.; Hughes, S.M.; Langston, V.C.; Mackin, A.J. Mefenoxam, Itraconazole, and Terbinafine Combination Therapy for Management of Pythiosis in Dogs (Six Cases). J. Am. Anim. Hosp. Assoc. 2020, 56, 307. [Google Scholar] [CrossRef]
- Salt, C.; Morris, P.J.; Wilson, D.; Lund, E.M.; German, A.J. Association between life span and body condition in neutered client-owned dogs. J. Vet. Intern. Med. 2019, 33, 89–99. [Google Scholar] [CrossRef]
- Hummel, J.; Grooters, A.; Davidson, G.; Jennings, S.; Nicklas, J.; Birkenheuer, A. Successful management of gastrointestinal pythiosis in a dog using itraconazole, terbinafine, and mefenoxam. Med. Mycol. 2011, 49, 539–542. [Google Scholar] [CrossRef]
- Denardi, L.B.; Weiblen, C.; Ianiski, L.B.; Stibbe, P.C.; Pinto, S.C.; Santurio, J.M. Anti-Pythium insidiosum activity of MSI-78, LL-37, and magainin-2 antimicrobial peptides. Braz. J. Microbiol. 2022, 53, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Medhasi, S.; Chindamporn, A.; Worasilchai, N. A Review: Antimicrobial Therapy for Human Pythiosis. Antibiotics 2022, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Ianiski, L.B.; Stibbe, P.C.; Denardi, L.B.; Weiblen, C.; Soares, M.P.; Valente, J.S.S.; Sangioni, L.A.; Pereira, D.I.B.; Santurio, J.M.; Botton, S.A. In vitro anti-Pythium insidiosum activity of amorolfine hydrochloride and azithromycin, alone and in combination. Med. Mycol. 2021, 59, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Grooters, A.M.; Gee, M.K. Development of a nested polymerase chain reaction assay for the detection and identification of Pythium insidiosum. J. Vet. Intern. Med. 2002, 16, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Vanittanakom, N.; Supabandhu, J.; Khamwan, C.; Praparattanapan, J.; Thirach, S.; Prasertwitayakij, N.; Louthrenoo, W.; Chiewchanvit, S.; Tananuvat, N. Identification of emerging human-pathogenic Pythium insidiosum by serological and molecular assay-based methods. J. Clin. Microbiol. 2004, 42, 3970–3974. [Google Scholar] [CrossRef] [PubMed]
- Bosco Sde, M.; Bagagli, E.; Araújo, J.P., Jr.; Candeias, J.M.; de Franco, M.F.; Alencar Marques, M.E.; Mendoza, L.; de Camargo, R.P.; Alencar Marques, S. Human pythiosis, Brazil. Emerg. Infect. Dis. 2005, 11, 715–718. [Google Scholar] [CrossRef]
- Badenoch, P.R.; Coster, D.J.; Wetherall, B.L.; Brettig, H.T.; Rozenbilds, M.A.; Drenth, A.; Wagels, G. Pythium insidiosum keratitis confirmed by DNA sequence analysis. Br. J. Ophthalmol. 2001, 85, 502–503. [Google Scholar] [CrossRef]
- Znajda, N.R.; Grooters, A.M.; Marsella, R. PCR-based detection of Pythium and Lagendium DNA in frozen and ethanol-fixed animal tissues. Vet. Dermatol. 2002, 13, 187–194. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Botton, S.A.; Pereira, D.I.; Robe, L.J.; Jesus, F.P.; Mahl, C.D.; Costa, M.M.; Alves, S.H.; Santurio, J.M. Phylogenetic relationships of Brazilian isolates of Pythium insidiosum based on ITS rDNA and cytochrome oxidase II gene sequences. Vet. Microbiol. 2012, 159, 141–148. [Google Scholar] [CrossRef]
- Mar Htun, Z.; Laikul, A.; Pathomsakulwong, W.; Yurayart, C.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Payattikul, P.; Sae-Chew, P.; Rujirawat, T.; et al. Identification and Biotyping of Pythium insidiosum Isolated from Urban and Rural Areas of Thailand by Multiplex PCR, DNA Barcode, and Proteomic Analyses. J. Fungi 2021, 7, 242. [Google Scholar] [CrossRef]
- Rivierre, C.; Laprie, C.; Guiard-Marigny, O.; Bergeaud, P.; Berthelemy, M.; Guillot, J. Pythiosis in Africa. Emerg. Infect. Dis. 2005, 11, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Keeratijarut, A.; Lohnoo, T.; Yingyong, W.; Nampoon, U.; Lerksuthirat, T.; Onpaew, P.; Chongtrakool, P.; Krajaejun, T. PCR amplification of a putative gene for exo-1,3-β-glucanase to identify the pathogenic oomycete Pythium insidiosum. Asian Biomed. 2017, 8, 637–644. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. 38—Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Keeratijarut, A.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Srichunrusami, C.; Onpeaw, P.; Chongtrakool, P.; Brandhorst, T.T.; Krajaejun, T. Detection of the oomycete Pythium insidiosum by real-time PCR targeting the gene coding for exo-1,3-β-glucanase. J. Med. Microbiol. 2015, 64, 971–977. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Edwards, E.E.; Older, C.E.; Branco, M.; Bryan, L.K.; Lawhon, S.D.; Suchodolski, J.S.; Gomez, G.; Mansell, J.; Hoffmann, A.R. Panfungal Polymerase Chain Reaction for Identification of Fungal Pathogens in Formalin-Fixed Animal Tissues. Vet. Pathol. 2017, 54, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cadavid, C.; Rudd, S.; Zaki, S.R.; Patel, M.; Moser, S.A.; Brandt, M.E.; Gómez, B.L. Improving molecular detection of fungal DNA in formalin-fixed paraffin-embedded tissues: Comparison of five tissue DNA extraction methods using panfungal PCR. J. Clin. Microbiol. 2010, 48, 2147–2153. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Friehs, K. Plasmid copy number and plasmid stability. Adv. Biochem. Eng. Biotechnol. 2004, 86, 47–82. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Mittal, S.K. A potential approach for assessing the quality of human and nonhuman adenoviral vector preparations. Can. J. Vet. Res. 2020, 84, 314–318. [Google Scholar]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2005, 33, D34–D38. [Google Scholar] [CrossRef]
- Yolanda, H.; Krajaejun, T. Global Distribution and Clinical Features of Pythiosis in Humans and Animals. J. Fungi 2022, 8, 182. [Google Scholar] [CrossRef]
- Supabandhu, J.; Vanittanakom, P.; Laohapensang, K.; Vanittanakom, N. Application of immunoblot assay for rapid diagnosis of human pythiosis. J. Med. Assoc. Thai. 2009, 92, 1063–1071. [Google Scholar] [PubMed]
- Mendoza, L.; Prasla, S.H.; Ajello, L. Orbital pythiosis: A non-fungal disease mimicking orbital mycotic infections, with a retrospective review of the literature. Mycoses 2004, 47, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Bentinck-Smith, J.; Padhye, A.A.; Maslin, W.R.; Hamilton, C.; McDonald, R.K.; Woody, B.J. Canine pythiosis—Isolation and identification of Pythium insidiosum. J. Vet. Diagn. Investig. 1989, 1, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Jena, S.K.; Desai, A.; Agarwal, S. Pythium insidiosum Keratitis: Histopathology and Rapid Novel Diagnostic Staining Technique. Cornea 2017, 36, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.D. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch. Biochem. 1948, 16, 131–141. [Google Scholar]
- Bagga, B.; Vishwakarma, P.; Sharma, S.; Jospeh, J.; Mitra, S.; Mohamed, A. Sensitivity and specificity of potassium hydroxide and calcofluor white stain to differentiate between fungal and Pythium filaments in corneal scrapings from patients of Pythium keratitis. Indian J. Ophthalmol. 2022, 70, 542–545. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Barr, B.; Riggs, S.M.; Eigenheer, A.L.; Pamma, R.; Walker, R.L. Cutaneous pythiosis in a nestling white-faced ibis. Vet. Pathol. 2008, 45, 538–541. [Google Scholar] [CrossRef]
- Supabandhu, J.; Fisher, M.C.; Mendoza, L.; Vanittanakom, N. Isolation and identification of the human pathogen Pythium insidiosum from environmental samples collected in Thai agricultural areas. Med. Mycol. 2008, 46, 41–52. [Google Scholar] [CrossRef]
- Balows, A. Manual of clinical microbiology 8th edition: P. R. Murray, E. J. Baron, J. H. Jorgenson, M. A. Pfaller, and R. H. Yolken, eds., ASM Press, 2003, 2113 pages, 2 vol, 2003. Diagn. Microbiol. Infect. Dis. 2003, 47, 625–626. [Google Scholar] [CrossRef]
- Botton, S.A.; Pereira, D.I.; Costa, M.M.; Azevedo, M.I.; Argenta, J.S.; Jesus, F.P.; Alves, S.H.; Santurio, J.M. Identification of Pythium insidiosum by nested PCR in cutaneous lesions of Brazilian horses and rabbits. Curr. Microbiol. 2011, 62, 1225–1229. [Google Scholar] [CrossRef]
- Nguyen, D.; Vilela, R.; Miraglia, B.M.; Vilela, G.; Jasem-Alali, N.; Rohn, R.; Glass, R.; Hansen, R.D.; Mendoza, L. Geographic distribution of Pythium insidiosum infections in the United States. J. Am. Vet. Med. Assoc. 2021, 260, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Oldenhoff, W.; Grooters, A.; Pinkerton, M.E.; Knorr, J.; Trepanier, L. Cutaneous pythiosis in two dogs from Wisconsin, USA. Vet. Dermatol. 2014, 25, 52-e21. [Google Scholar] [CrossRef] [PubMed]
- Berryessa, N.A.; Marks, S.L.; Pesavento, P.A.; Krasnansky, T.; Yoshimoto, S.K.; Johnson, E.G.; Grooters, A.M. Gastrointestinal pythiosis in 10 dogs from California. J. Vet. Intern. Med. 2008, 22, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Yolanda, H.; Krajaejun, T. Review of methods and antimicrobial agents for susceptibility testing against Pythium insidiosum. Heliyon 2020, 6, e03737. [Google Scholar] [CrossRef]
- Duclos, D. Canine pododermatitis. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 57–87. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).