Vericiguat: The Fifth Harmony of Heart Failure with Reduced Ejection Fraction
Abstract
:1. Introduction
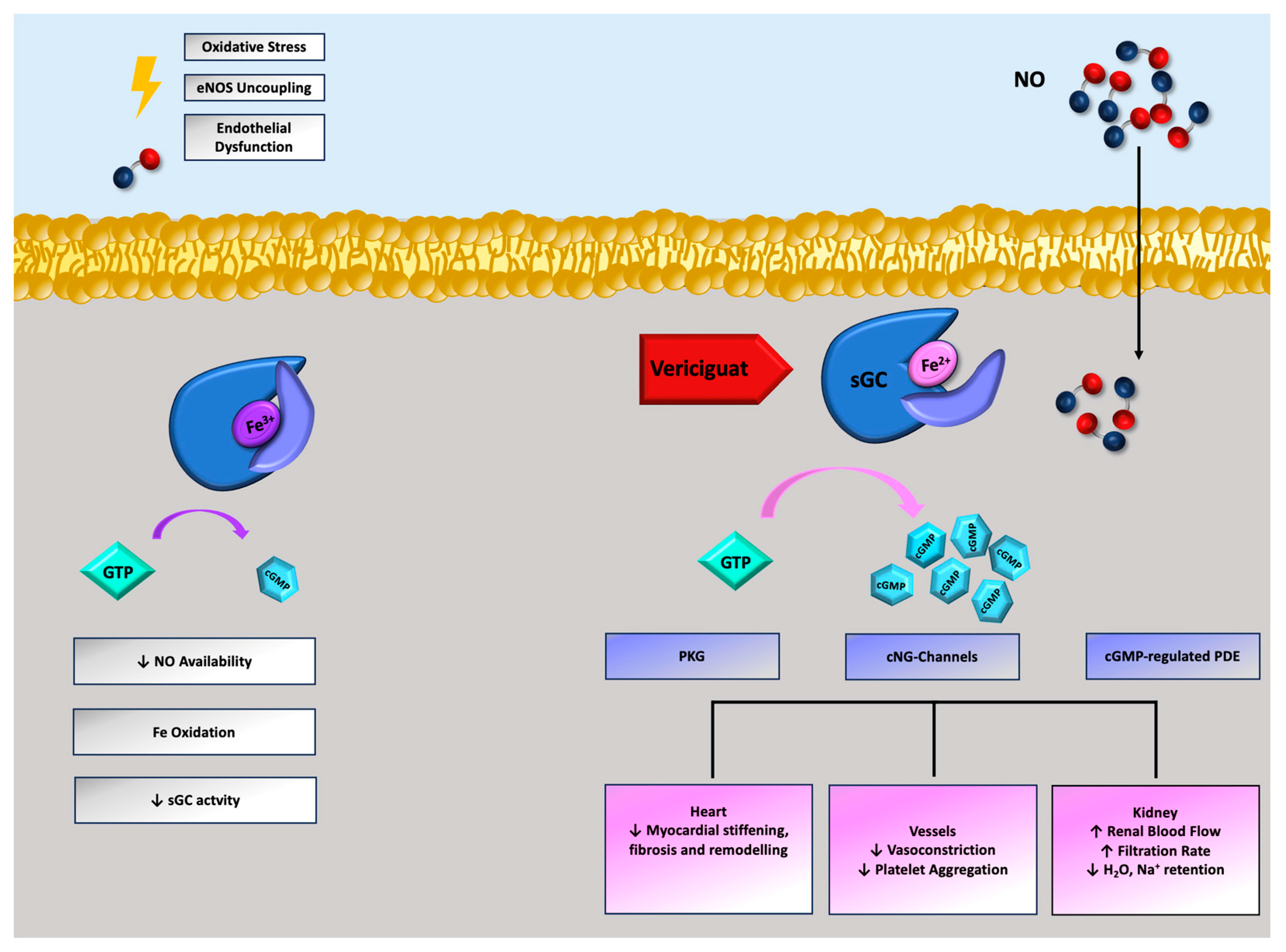
2. Pathophysiology of Nitric Oxide-sGC-Cyclic Guanosine Monophosphate Cascade in HFrEF
3. Clinical Pharmacology of Vericiguat
4. Clinical Evidence Supporting the Use of Vericiguat in HFrEF
4.1. SOCRATES-Reduced
4.2. VICTORIA Trial
5. Place of Vericiguat
6. Real-World Experience
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ACE | Angiotensin-Converting Enzyme |
| AE | Adverse event |
| AHA | American Heart Association |
| ARB | Angiotensin Receptor Blocker |
| ARNI | Angiotensin Receptor Neprilysin Inhibitor |
| BNP | B-type natriuretic peptide |
| cGMP | Cyclic guanosine monophosphate |
| CAD | Coronary artery disease |
| CCS | Canadian Cardiovascular Society |
| CHFS | Canadian Heart Failure Society |
| CI | Confidence Interval |
| cNG | Cyclic nucleotide-gated |
| COPD | Chronic obstructive pulmonary disease |
| CRT | Cardiac Resynchronization Therapy |
| CYP | Cytochrome |
| DAPA-HF | Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure |
| eGFR | Estimated glomerular filtration rate |
| EMPEROR-Reduced | Empagliflozin Outcome Trial in Patients With Chronic Heart Failure with Reduced Ejection Fraction |
| eNOS | Endothelial nitric oxide synthase |
| ESC | European Society of Cardiology |
| FDA | Food and Drug Administration |
| GDMT | Guideline-directed medical therapy |
| HF | Heart failure |
| HFA | Heart Failure Association |
| HFrEF | Heart failure with reduced ejection fraction |
| HFSA | Heart Failure Society of America |
| HR | Hazard Ratio |
| KorAHF | Korean Acute Heart Failure |
| ICD | Implantable Cardioverter Defibrillator |
| LVEF | Left ventricular ejection fraction |
| MAGGIC | Meta-Analysis Global Group in Chronic Heart Failure |
| MRA | Mineralocorticoid Receptor Antagonist |
| NYHA | New York Heart Association |
| NO | Nitric oxide |
| NT-proBNP | N terminal pro B-type natriuretic peptide |
| PAH | Pulmonary arterial hypertension |
| PARADIGM-HF | Safety and Tolerability During Open-label Treatment with LCZ696 in Patients with CHF and Reduced Ejection Fraction |
| PDE | Phosphodiesterase |
| PINNACLE | Practice Innovation and Clinical Excellence |
| PKG | Cyclic GMP-dependent protein kinase |
| Q | Quartile |
| QoL | Quality of life |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RCT | Randomized clinical trial |
| sGC | Soluble guanylate cyclase |
| SGLT2 | Sodium–Glucose Transporter 2 |
| SOCRATES-Reduced | Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients with Worsening Chronic Heart Failure and Reduced Ejection Fraction |
| STRONG-HF | Safety: tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure |
| SwedeHF | Swedish Heart Failure |
| VICTOR | A Study of Vericiguat (MK-1242) in Participants with Chronic Heart Failure with Reduced Ejection Fraction (HFrEF) (MK-1242-035) |
| VICTORIA | Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction |
| WHF | Worsening heart failure |
References
- Hessel, F.P. Overview of the socio-economic consequences of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 254–262. [Google Scholar] [CrossRef]
- Iacoviello, M.; Palazzuoli, A.; Gronda, E. Recent advances in pharmacological treatment of heart failure. Eur. J. Clin. Investig. 2021, 51, e13624. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Docherty, K.F. Insights into foundational therapies for heart failure with reduced ejection fraction. Clin. Cardiol. 2022, 45, S26–S30. [Google Scholar] [CrossRef]
- Fudim, M.; Abraham, W.T.; von Bardeleben, R.S.; Lindenfeld, J.; Ponikowski, P.P.; Salah, H.M.; Khan, M.S.; Sievert, H.; Stone, G.W.; Anker, S.D.; et al. Device Therapy in Chronic Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 931–956. [Google Scholar] [CrossRef]
- Metra, M.; Tomasoni, D.; Adamo, M.; Bayes-Genis, A.; Filippatos, G.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Antohi, L.; Böhm, M.; et al. Worsening of chronic heart failure: Definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 2, 776–791. [Google Scholar]
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef]
- Vannuccini, F.; Campora, A.; Barilli, M.; Palazzuoli, A. Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications. Biomedicines 2022, 10, 2471. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.J.; Kass, D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009, 122, 216–238. [Google Scholar] [PubMed]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 3, 577–596. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [PubMed]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the role of nitric oxide and its clinical applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, P.; Schyvens, C.; Winlaw, D. The role of nitric oxide in heart failure. Potential. Pharmacol. Interv. Drugs Aging 1996, 8, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef]
- Emdin, M.; Aimo, A.; Castiglione, V.; Vergaro, G.; Georgiopoulos, G.; Saccaro, L.F.; Lombardi, C.M.; Passino, C.; Cerbai, E.; Metra, M.; et al. Targeting cyclic guanosine monophosphate to treat heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 76, 1795–1807. [Google Scholar] [CrossRef]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C., Jr.; Roessig, L.; Stasch, J.P.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef]
- Kukovetz, W.R.; Holzmann, S.; Romanin, C. Mechanism of vasodilation by nitrates: Role of cyclic GMP. Cardiology 1987, 74, 12–19. [Google Scholar] [CrossRef]
- Petraina, A.; Nogales, C.; Krahn, T.; Mucke, H.; Lüscher, T.F.; Fischmeister, R.; Kass, D.A.; Burnett, J.C.; Hobbs, A.J.; Schmidt, H.H.H.W. Cyclic GMP modulating drugs in cardiovascular diseases: Mechanism-based network pharmacology. Cardiovasc. Res. 2022, 118, 2085–2102. [Google Scholar] [CrossRef]
- Lapp, H.; Mitrovic, V.; Franz, N.; Heuer, H.; Buerke, M.; Wolfertz, J.; Mueck, W.; Unger, S.; Wensing, G.; Frey, R. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation 2009, 119, 2781–2788. [Google Scholar] [CrossRef]
- Erdmann, E.; Semigran, M.J.; Nieminen, M.S.; Gheorghiade, M.; Agrawal, R.; Mitrovic, V.; Mebazaa, A. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur. Heart J. 2013, 34, 57–67. [Google Scholar] [CrossRef]
- Halank, M.; Tausche, K.; Grünig, E.; Ewert, R.; Preston, I.R. Practical management of riociguat in patients with pulmonary arterial hypertension. Ther. Adv. Respir. Dis. 2019, 13, 1753466619868938. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Mulder, H.; Lopatin, Y.; Vazquez-Tanus, J.B.; Siu, D.; Ezekowitz, J.; Pieske, B.; O’ Connor, C.M.; Roessig, L.; Patel, M.J.; et al. Blood Pressure and Safety Events With Vericiguat in the VICTORIA Trial. J. Am. Heart Assoc. 2021, 10, e021094. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.R.; Chakinala, M.M.; Langleben, D.; Rosenkranz, S.; Sitbon, O. Riociguat: Clinical research and evolving role in therapy. Br. J. Clin. Pharmacol. 2021, 87, 2645–2662. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Thomas, D.; Mueck, W.; Loewen, S.; Arens, E.; Yoshikawa, K.; Becker, C. Safety, pharmacodynamic, and pharmacokinetic characterization of vericiguat: Results from six phase I studies in healthy subjects. Eur. J. Clin. Pharmacol. 2021, 77, 527–537. [Google Scholar] [CrossRef]
- Markham, A.; Duggan, S. Vericiguat: First Approval. Drugs 2021, 81, 721–726. [Google Scholar] [CrossRef]
- Merck & Co. VERQUVO (Vericiguat) Tablets, for Oral Use. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf (accessed on 6 August 2023).
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Mülsch, A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005, 97, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Marti, C.N.; Sabbah, H.N.; Roessig, L.; Greene, S.J.; Böhm, M.; Burnett, J.C.; Campia, U.; Cleland, J.G.; Collins, S.P.; et al. Soluble guanylate cyclase: A potential therapeutic target for heart failure. Heart Fail. Rev. 2013, 18, 123–134. [Google Scholar] [CrossRef]
- Krüger, M.; Kötter, S.; Grützner, A.; Lang, P.; Andresen, C.; Redfield, M.M.; Butt, E.; dos Remedios, C.G.; Linke, W.A. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ. Res. 2009, 104, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chiles, R.; Al-Horani, R.A. Vericiguat: A New Hope for Heart Failure Patients. Cardiovasc. Ther. 2022, 2022, 1554875. [Google Scholar] [CrossRef] [PubMed]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Haskó, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Butler, J.; Filippatos, G.; Lam, C.; Maggioni, A.P.; Ponikowski, P.; Shah, S.; Solomon, S.; Kraigher-Krainer, E.; Samano, E.T.; et al. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur. J. Heart Fail. 2014, 16, 1026–1038. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 2015, 314, 2251–2262. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; O’Connor, C.M.; Troughton, R.W.; Alemayehu, W.G.; Westerhout, C.M.; Voors, A.A.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Emdin, M.; et al. N-Terminal Pro-B-Type Natriuretic Peptide and Clinical Outcomes: Vericiguat Heart Failure With Reduced Ejection Fraction Study. JACC Heart Fail. 2020, 8, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Senni, M.; Lopez-Sendon, J.; Cohen-Solal, A.; Ponikowski, P.; Nkulikiyinka, R.; Freitas, C.; Vlajnic, V.M.; Roessig, L.; Pieske, B. Vericiguat and NT-proBNP in patients with heart failure with reduced ejection fraction: Analyses from the VICTORIA trial. ESC Heart Fail. 2022, 9, 3791–3803. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Zheng, Y.; Cohen-Solal, A.; Melenovský, V.; Escobedo, J.; Butler, J.; Hernandez, A.F.; Lam, C.S.P.; O’ Connor, C.M.; Pieske, B.; et al. Hemoglobin and Clinical Outcomes in the Vericiguat Global Study in Patients With Heart Failure and Reduced Ejection Fraction (VICTORIA). Circulation 2021, 144, 1489–1499. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Alemayehu, W.; Oto, A.; Bahit, M.C.; Noori, E.; Patel, M.J.; Butler, J.; Ezekowitz, J.A.; Hernandez, A.F.; Lam, C.S.P.; et al. Vericiguat in patients with atrial fibrillation and heart failure with reduced ejection fraction: Insights from the VICTORIA trial. Eur. J. Heart Fail. 2021, 23, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Senni, M.; Alemayehu, W.G.; Sim, D.; Edelmann, F.; Butler, J.; Ezekowitz, J.; Hernandez, A.F.; Lam, C.S.P.; O’Connor, C.M.; Pieske, B.; et al. Efficacy and safety of vericiguat in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan: Insights from the VICTORIA trial. Eur. J. Heart Fail. 2022, 24, 1614–1622. [Google Scholar] [CrossRef]
- Saldarriaga, C.; Atar, D.; Stebbins, A.; Lewis, B.S.; Abidin, I.Z.; Blaustein, R.O.; Butler, J.; Ezekowitz, J.A.; Hernandez, A.F.; Lam, C.S.P.; et al. Vericiguat in patients with coronary artery disease and heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2022, 24, 782–790. [Google Scholar] [CrossRef]
- Butler, J.; Stebbins, A.; Melenovský, V.; Sweitzer, N.K.; Cowie, M.R.; Stehlik, J.; Khan, M.S.; Blaustein, R.O.; Ezekowitz, J.A.; Hernandez, A.F.; et al. Vericiguat and Health-Related Quality of Life in Patients With Heart Failure With Reduced Ejection Fraction: Insights From the VICTORIA Trial. Circ. Heart Fail. 2022, 15, e009337. [Google Scholar] [CrossRef]
- Greene, S.J.; Mentz, R.J.; Felker, G.M. Outpatient Worsening Heart Failure as a Target for Therapy: A Review. JAMA Cardiol. 2018, 3, 252–259. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event: Insights From the VICTORIA Trial. JAMA Cardiol. 2021, 6, 706–712. [Google Scholar] [CrossRef]
- Taylor, A.L.; Ziesche, S.; Yancy, C.; Carson, P.; D’Agostino, R., Jr.; Ferdinand, K.; Taylor, M.; Adams, K.; Sabolinski, M.; Worcel, M.; et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med. 2004, 351, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; LeWinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.L.; et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef]
- Cuffe, M.S.; Califf, R.M.; Adams, K.F., Jr.; Benza, R.; Bourge, R.; Colucci, W.S.; Massie, B.M.; O’Connor, C.M.; Pina, I.; Quigg, R.; et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002, 287, 1541–1547. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.; et al. Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Pedro Ferreira, J.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Brunner-La Rocca, H.P.; Linssen, G.C.; Smeele, F.J.; van Drimmelen, A.A.; Schaafsma, H.J.; Westendorp, P.H.; Rademaker, P.C.; van de Kamp, H.J.; Hoes, A.W.; Brugts, J.J. Contemporary Drug Treatment of Chronic Heart Failure With Reduced Ejection Fraction: The CHECK-HF Registry. JACC Heart Fail. 2019, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsen, T.; Benson, L.; Dahlström, U.; Edner, M.; Lund, L.H. Use of evidence-based therapy and survival in heart failure in Sweden 2003–2012. Eur. J. Heart Fail. 2016, 18, 503–511. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Howlett, J.G.; Fine, N.M. A Novel Approach to Medical Management of Heart Failure With Reduced Ejection Fraction. Can. J. Cardiol. 2021, 37, 632–643. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation 2021, 143, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Fonarow, G.C. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure-Optimizing Therapy With the Need for Speed. JAMA Cardiol. 2021, 6, 743–744. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Virani, S.; Chan, M.; Ducharme, A.; Ezekowitz, J.A.; Giannetti, N.; Heckman, G.A.; Howlett, J.G.; Koshman, S.L.; Lepage, S.; et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can. J. Cardiol. 2021, 37, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, ehad195. [Google Scholar] [CrossRef] [PubMed]
- Alsumali, A.; Djatche, L.M.; Briggs, A.; Liu, R.; Diakite, I.; Patel, D.; Wang, Y.; Lautsch, D. Cost Effectiveness of Vericiguat for the Treatment of Chronic Heart Failure with Reduced Ejection Fraction Following a Worsening Heart Failure Event from a US Medicare Perspective. Pharmacoeconomics 2021, 39, 1343–1354. [Google Scholar] [CrossRef]
- Alsumali, A.; Lautsch, D.; Liu, R.; Patel, D.; Nanji, S.; Djatche, L.M. Budget Impact Analysis of Vericiguat for the Treatment of Chronic Heart Failure with Reduced Ejection Fraction Following a Worsening Event. Adv. Ther. 2021, 38, 2631–2643. [Google Scholar] [CrossRef]
- Voors, A.A.; Mulder, H.; Reyes, E.; Cowie, M.R.; Lassus, J.; Hernandez, A.F.; Ezekowitz, J.A.; Butler, J.; O’Connor, C.M.; Koglin, J.; et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: Insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur. J. Heart Fail. 2021, 23, 1313–1321. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Moura, B.; Metra, M.; Böhm, M.; Bauersachs, J.; Ben Gal, T.; Adamopoulos, S.; Abdelhamid, M.; Bistola, V.; Čelutkienė, J.; et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 872–881. [Google Scholar] [CrossRef]
- Ezekowitz, J.; Mentz, R.J.; Westerhout, C.M.; Sweitzer, N.K.; Givertz, M.M.; Piña, I.L.; O’Connor, C.M.; Greene, S.J.; McMullan, C.; Roessig, L.; et al. Participation in a Heart Failure Clinical Trial: Perspectives and Opportunities From the VICTORIA Trial and VICTORIA Simultaneous Registry. Circ. Heart Fail. 2021, 14, e008242. [Google Scholar] [CrossRef]
- Butler, J.; Yang, M.; Manzi, M.A.; Hess, G.P.; Patel, M.J.; Rhodes, T.; Givertz, M.M. Clinical Course of Patients With Worsening Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 73, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Djatche, L.M.; Lautsch, D.; Yang, L.; Patel, M.J.; Mentz, R.J. Representativeness of the VICTORIA Trial Population in Clinical Practice: Analysis of the PINNACLE Registry. J. Card. Fail. 2021, 27, 1374–1381. [Google Scholar] [CrossRef]
- Oh, J.; Lee, C.J.; Park, J.J.; Lee, S.E.; Kim, M.S.; Cho, H.J.; Choi, J.O.; Lee, H.Y.; Hwang, K.K.; Kim, K.H.; et al. Real-world eligibility for vericiguat in decompensated heart failure with reduced ejection fraction. ESC Heart Fail. 2022, 9, 1492–1495. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Lindberg, F.; Benson, L.; Ferrannini, G.; Imbalzano, E.; Mol, P.G.M.; Dahlström, U.; Rosano, G.M.C.; Ezekowitz, J.; Butler, J.; et al. Eligibility for vericiguat in a real-world heart failure population according to trial, guideline and label criteria: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2023, 25, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Usman, M.S.; Anstrom, K.J.; Blaustein, R.O.; Bonaca, M.P.; Ezekowitz, J.A.; Freitas, C.; Lam, C.S.P.; Lewis, E.F.; Lindenfeld, J.; et al. Soluble guanylate cyclase stimulators in patients with heart failure with reduced ejection fraction across the risk spectrum. Eur. J. Heart Fail. 2022, 24, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
| Trial | PARADIGM-HF [37] | DAPA-HF [38] | VICTORIA [36] |
|---|---|---|---|
| Year | 2014 | 2019 | 2020 |
| Mean Age, years | 63.8 | 66.3 | 67.3 |
| Mean LVEF, % | 29 | 31.2 | 29 |
| Median NT-proBNP, pg/mL | 1631 | 1428 | 2816 |
| Median follow-up time, months | 27 | 18.2 | 10.8 |
| Mean eGFR, mL/min/m2 | 68 | 66 | 61 |
| eGFR < 60, % | 37 | 41 | 53 |
| NYHA class III–IV, % | 25 | 33 | 41 |
| ACE inhibitor/ARB, % | - | 83.2 | 73.4 |
| ARNI, % | - | 10.8 | 14.5 |
| Beta Blockers, % | 93 | 96 | 93.1 |
| MRA, % | 60 | 71 | 70.3 |
| ICD, % | 15 | 26 | 27.8 |
| CRT, % | 7 | 7 | 14.7 |
| HF Hospitalization < 6 months, % | 31 | 16 | 84 |
| Annualized Event Rate, n events per 100 patient-years at risk (placebo vs. treatment arm) | 13.2 10.5 | 15.6 11.6 | 37.8 33.6 |
| Primary Endpoint, HR (95% CI) | 0.80 (0.73–0.87) | 0.74 (0.65–0.85) | 0.90 (0.82–0.98) |
| Absolute Rate Reduction, n | 2.7 | 4 | 4.2 |
| Guideline | Year | Recommendation | Recommendation Strength | Evidence Quality |
|---|---|---|---|---|
| ESC/HFA [63] | 2021 | Vericiguat may be considered in patients in NYHA class II-IV who have had WHF despite treatment with an ACE-I (or ARNI), a beta-blocker and an MRA to reduce the risk of cardiovascular mortality or HF hospitalization. | IIb | B |
| CCS/CHFS [64] | 2021 | The authors recommend that vericiguat be considered in addition to optimal HF therapies for HFrEF patients with worsening symptoms and HF hospitalization in the past 6 months, to reduce the risk of subsequent HF hospitalization | Conditional Recommendation | Moderate-Quality Evidence |
| ACC/AHA/HFSA [65] | 2022 | In selected high-risk patients with HFrEF and recent WHF already on GDMT, vericiguat may be considered to reduce HF hospitalization and cardiovascular death. | IIb | B-R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, L.; Brescia, B.; Catapano, D.; Martucci, M.L.; Valente, F.; Gravino, R.; Contaldi, C.; Pacileo, G.; Masarone, D. Vericiguat: The Fifth Harmony of Heart Failure with Reduced Ejection Fraction. J. Cardiovasc. Dev. Dis. 2023, 10, 388. https://doi.org/10.3390/jcdd10090388
Falco L, Brescia B, Catapano D, Martucci ML, Valente F, Gravino R, Contaldi C, Pacileo G, Masarone D. Vericiguat: The Fifth Harmony of Heart Failure with Reduced Ejection Fraction. Journal of Cardiovascular Development and Disease. 2023; 10(9):388. https://doi.org/10.3390/jcdd10090388
Chicago/Turabian StyleFalco, Luigi, Benedetta Brescia, Dario Catapano, Maria Luigia Martucci, Fabio Valente, Rita Gravino, Carla Contaldi, Giuseppe Pacileo, and Daniele Masarone. 2023. "Vericiguat: The Fifth Harmony of Heart Failure with Reduced Ejection Fraction" Journal of Cardiovascular Development and Disease 10, no. 9: 388. https://doi.org/10.3390/jcdd10090388
APA StyleFalco, L., Brescia, B., Catapano, D., Martucci, M. L., Valente, F., Gravino, R., Contaldi, C., Pacileo, G., & Masarone, D. (2023). Vericiguat: The Fifth Harmony of Heart Failure with Reduced Ejection Fraction. Journal of Cardiovascular Development and Disease, 10(9), 388. https://doi.org/10.3390/jcdd10090388