Abstract
This study aims to compare soluble (pro)renin receptor [s(P)RR] levels between black and white adults and to explore the associations of left ventricular (LV) structure and function with s(P)RR in the total and ethnicity-stratified groups. The study sample included 1172 apparently healthy black (n = 587) and white (n = 585) participants of the African-PREDICT study aged 20–30 years. Echocardiography was performed to determine relative wall thickness (RWT), LV mass index, LV ejection fraction and stroke volume index (SVi). s(P)RR was analyzed from serum samples, while plasma renin activity-surrogate (PRA-S) and eq angiotensin II were determined using the RAS™ Fingerprint. s(P)RR was higher in the white participants compared to the black participants (p < 0.001). In multivariable-adjusted linear regression analyses, we observed a positive association between RWT and s(P)RR (β = 0.141; p = 0.005) and negative associations of LV ejection fraction (β = −0.123; p = 0.016) and SVi (β = −0.144; p = 0.004) with s(P)RR only in white adults. Higher s(P)RR observed in white vs. black participants was associated with higher RWT and poorer LV function only in young white adults but not in their black counterparts. These results suggest that s(P)RR may contribute to LV remodeling and dysfunction in white populations due to its role in volume–pressure regulation and its proinflammatory as well as profibrotic effects.
1. Introduction
The renin angiotensin system (RAS) is the major hormonal system involved in the regulation of fluid balance and blood pressure (BP) [1]. Consequently, this system forms the hallmark of therapeutic interventions in the treatment of hypertension and cardiovascular disease (CVD) [2,3]. Activation of RAS is initiated by the release of the enzyme renin from the juxtaglomerular apparatus in response to low perfusion pressure, among others [4,5]. Renin cleaves circulating angiotensinogen to form angiotensin I (AngI), which is then converted to angiotensin II (AngII) by angiotensin-converting enzyme (ACE) [4,5]. AngII directly and indirectly contributes to pathophysiological mechanisms leading to cardiac damage and remodeling, such as left ventricular (LV) remodeling, hypertrophy and associated complications [6,7,8]. The mechanisms, which are mediated by activation of the AngII receptor type 1 (AT1R) include chronic elevation of BP, inflammation, oxidative stress, tissue growth and cell proliferation [9,10].
(Pro)renin receptor [(P)RR], with an affinity for both prorenin and active renin, is expressed in various organs, including cardiac myocytes, the kidney and brain [11,12,13]. When activated, (P)RR elicits proinflammatory and profibrotic effects through the AngII-AT1R axis and intracellular signaling pathways such as mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase 1/2 (ERK1/2) [13,14]. In earlier studies on the role of (P)RR as a biomarker, it was shown that binding of prorenin to (P)RR resulted in activation of MAPK p44/42 and tissue-growth factor-β (TGF-β), leading to increased contractility, hypertrophy and fibrosis [11,15]. Soluble (P)RR [s(P)RR], a product of (P)RR cleavage, is adversely linked to lipid and glucose metabolism and BP in mice [16]. In human studies, patients with heart failure presented with higher plasma s(P)RR levels as compared to healthy controls [17]. Observations on (P)RR and s(P)RR in relation to target organ damage have prompted suggestions that blockade of renin receptors may serve as a target for tissue protection [5]. However, evidence on the use of s(P)RR as a clinical marker is inconclusive, possibly due to limited data in population studies, especially on circulating s(P)RR. Nguyen et al. [18] showed that s(P)RR differed by ethnicity, being lower in black (n = 9) as compared to white men (n = 10). For the s(P)RR comparison, the study included a small sample of black and white participants, highlighting the need for further studies in human populations to confirm the ethnic differences in s(P)RR. Ethnic differences are important when investigating RAS due to the known lower levels of classical RAS components in black populations as compared to their white counterparts, and the impact on response to some classes of antihypertensive drugs [19,20,21].
Previous studies that included data from the African Prospective study on Early Detection and Identification of Cardiovascular disease and HyperTension study (African-PREDICT study) [22] have identified some of the biomarkers that may contribute to early cardiac deterioration. These markers include some of the well-known RAS components (renin, AngII, aldosterone excess) and sodium excretion [23,24,25,26]. Exploring the possible contribution of s(P)RR to LV structure and function will add to the knowledge and identification of potential targets for therapeutic intervention, given the scarcity of human data on s(P)RR. This study therefore compared s(P)RR levels between apparently healthy young black and white adults and explored whether LV structure and function are associated with s(P)RR in the total and ethnicity-stratified populations.
2. Materials and Methods
2.1. Study Design and Population
This study utilized data from participants of the African-PREDICT study. The study sample included 1202 young adults prospectively followed over time to identify novel and early markers of cardiovascular risk [22]. To be eligible, participants were required to be self-reported black or white men and women, aged 20–30 years, with screening office BP <140/90 mmHg. Pregnant or lactating women, individuals who were on chronic medication or those who had been previously diagnosed with a chronic health condition were excluded. The present substudy used cross-sectional baseline data from 1172 participants with complete data for s(P)RR and echocardiographic measurements.
All participants gave written informed consent. The African-PREDICT study was approved by the Health Research Ethics Committee of the North-West University (NWU-00001-12-A1) and complied with the Declaration of Helsinki criteria for human research. The study is registered on ClinicalTrials.gov (NCT03292094).
2.2. Demographic, Anthropometric and Physical Activity Measurements
Participant’s age, sex, ethnicity, alcohol, tobacco and medication use, and family history were obtained using a demographic and health questionnaire. The socioeconomic status (SES) of each participant was obtained using the Kuppuswamy’s Socioeconomic Status Scale 2010 adapted to the South African context [27]. The socioeconomic score was calculated based on skill level, education and household income by using a point system from the scale.
All anthropometric measurements were performed using the International Standards for Anthropometric Assessment [28] and included weight (kg) (SECA electronic scales, SECA, Birmingham, UK), height (m) (SECA stadiometer, SECA, Birmingham, UK) and waist circumference (WC) (Holtain, Crymych, UK). Body mass index (BMI) was calculated using the standard weight (kg)/height (m2) calculation. Body surface area (BSA) (m2) was additionally calculated using the Mosteller formula [29].
The ActiHeart device (CamNtech Ltd., England, UK), which was worn for a maximum of 7 days, was used to calculate average daily activity-energy expenditure (AEE) [30].
2.3. Cardiovascular Measurements
Ambulatory BP data was collected over a 24 h period using CardXplore devices (MediTech, Budapest, Hungary), programmed to take recordings every 30 min during the day (0600 to 2200 h) and every hour during the night (22:00 to 06:00 h). The device was fitted to each participant at approximately the same time every day (late morning), using an appropriately sized cuff as specified by the manufacturer. The mean inflation rate for this study population was calculated as 88% (standard deviation ± 12.3).
A standard transthoracic echocardiography procedure was followed while each participant was in a partial left decubitus position with the head of the examining table moderately elevated. The General Electric Vivid E9 device (GE Vingmed Ultrasound A/S, Hearten, Norway) was used along with the 2.5 to 3.5 MHz phased-array transducer and a three-lead electrocardiograph for timing purposes. Standardized methods were employed to obtain high-quality recordings according to the latest guidelines of the American Society of Echocardiography. LV mass was indexed for BSA (LVMi) and end-diastolic volume (EDVi), end-systolic volume (ESVi), LV internal diameter and posterior wall thickness at diastole for body height and stroke volume for height to the power of 2.04 (SVi) [31,32,33].
2.4. Biological Sampling and Biochemical Analyses
Participants were required not to eat or drink anything except water overnight for at least 8 h prior to undertaking the research measurements. Blood samples were collected early in the morning by a qualified nurse. The samples were then prepared according to standardized protocols and stored at −80 °C until the time of analysis. Serum samples were analyzed for creatinine, C-reactive protein (CRP), total and low- and high-density lipoprotein cholesterol (TC, LDL-c, HDL-c), glucose and gamma-glutamyl transferase (GGT) (Cobas Integra 400plus, Roche, Basel, Switzerland).
Components of the RAS including PRA-s, ACE-s (eq AngII/eq AngI) and eq AngII were analyzed using the RAS-Fingerprint® (Attoquant Diagnostics, Vienna, Austria). Angiotensin peptides were quantified based on a liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) multiplex assay in equilibrated serum volumes of 350 μL. Following a solid-phase-based and internal-standard-controlled extraction procedure, the LC-MS/MS quantification was performed using highly specific and simultaneous multiple reaction monitoring (MRM) detection of endogenous angiotensin peptides and internal standards [34,35]. Equilibrium angiotensin levels were further used to calculate a surrogate marker of renin enzyme activity, PRA-s (eq AngI + eq AngII). We measured serum s(P)RR (Immuno-Biological Laboratories Co., Ltd. (IBL-Japan)) and plasma prorenin (Human Prorenin ELISA Kit (Biovendor Laboratorni Medicina, Karasek, Czech Republic)). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology (CKD-EPI) formula without the race factor [36,37].
Each participant self-collected a 24 h urine sample on a day that was convenient for them, as recommended by the Pan American Health Organization/World Health Organization (PAHO/WHO) [38]. Urine samples were aliquoted and placed in a −20 °C freezer until analyses. Urinary creatinine, sodium and potassium were then measured using ion-selective electrode potentiometry on the Cobas Integra® 400 plus (Roche, Basel, Switzerland) and were then used to calculate the 24 h urinary sodium:potassium ratio (Na+/K+).
2.5. Statistical Analyses
Data analysis was performed with Statistica v13.3 (TIBCO software, Palo Alto, CA, USA). Prior to statistical analyses, normality assumption for quantitative variables was assessed using the Kolmogorov–Smirnov test and visual inspection of histograms. Normally distributed data were reported as the mean and standard deviation, and natural-logarithmically transformed data were presented by the geometric mean with 5th and 95th percentiles. Means across quartiles of s(P)RR were compared using ANOVA, followed by post hoc analyses where indicated, to compare pairwise means between quartiles. Chi-squared tests were used to compare quartiles of s(P)RR with qualitative variables. Independent sample t-tests were used to compare black and white participants. Ethnicity-pooled and ethnicity-specific regression analyses were performed to account for the well-known ethnic heterogeneity in RAS activity [19,25]. Candidate independent variables included age, ethnicity (in ethnicity-pooled analysis), sex, waist circumference, socioeconomic score, 24 h diastolic blood pressure, eGFR, Na+/K+, glucose, LDL-c, CRP, smoking, alcohol use and AEE as independent variables. Variables considered for entry in the multivariable linear regression analysis were chosen based on clinical, exploratory bivariate analysis, and partial correlations. Linear relationships were summarized using Pearson correlation coefficients and linear regression slopes and 95% confidence intervals. The proportion of variation in s(P)RR accounted for by regression models was summarized using adjusted R2. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Descriptive and Linear Regression Analyses
3.1.1. Characteristics of the Study Sample
Ethnicity-pooled quartiles for s(P)RR were <20.40, 20.4–22.69, 22.70–25.31, and ≥25.31 ng/mL for Quartiles 1, 2, 3 and 4, respectively. Table 1 presents the profile of the participants stratified according to quartiles of s(P)RR. The proportion of women (p-trend = 0.008) and black participants (p-trend < 0.001) decreased with increasing quartiles of s(P)RR. All measures of body composition increased with s(P)RR (all p-trend < 0.001). 24 h systolic and diastolic BP increased with increasing s(P)RR (p-trend ≤ 0.001), SBP: 114 mmHg in quartile 1 vs. 120 mmHg in quartile 4, p < 0.001). End diastolic and systolic volume indices increased with s(P)RR quartiles (both p-trend < 0.001). LV internal diameter and posterior wall thickness at diastole and left atrial diameter to aortic root ratio (LA/Ao ratio) linearly increased across quartiles s(P)RR (all p-trend ≤ 0.022). Components of RAS increased across s(P)RR quartiles (all p-trend ≤ 0.032), while 24 h urinary Na+/K+ and eGFR showed a decreasing trend across s(P)RR quartiles (both p-trend ≤ 0.035; eGFR: quartile 1 vs. quartile 4, p ≤ 0.001). Metabolic variables and all inflammatory markers increased across the s(P)RR quartiles, with significantly higher levels in quartile 4 as compared to quartile 1 (all p-trend < 0.001, quartile 1 vs. quartile 4, all p < 0.001). A measure of energy expenditure decreased across s(P)RR quartiles (p-trend < 0.001) and quartile 4 had lower levels as compared to quartile 1 (5.11 vs. 6.31; p < 0.001).

Table 1.
Characteristics of the population across quartiles of soluble (pro)renin receptor.
Figure 1 represents differences in s(P)RR and other RAS components between black and white participants. s(P)RR, prorenin, PRA and eq AngII were higher in white participants as compared to their black counterparts (all p ≤ 0.001) and Na+/K+ was higher in the black compared to white participants (p < 0.001). Further comparisons between black and white participants are presented in Table 2. White participants had substantially higher values of measures of adiposity compared to their black counterparts (all p ≤ 0.005), whereas black participants had a greater proportion of women with waist circumference greater than the cut-off recommended by WHO in combination with the South African population-based guidelines (p < 0.001). In terms of echocardiographic data, the white participants had lower relative wall thickness; LV posterior wall thickness at diastole, ejection fraction and E/e’ ratio (all p ≤ 0.014); and higher LV internal diameter at diastole, end systolic and diastolic volume, stroke volume, E/A ratio and E/e’ ratio (all p ≤ 0.010).
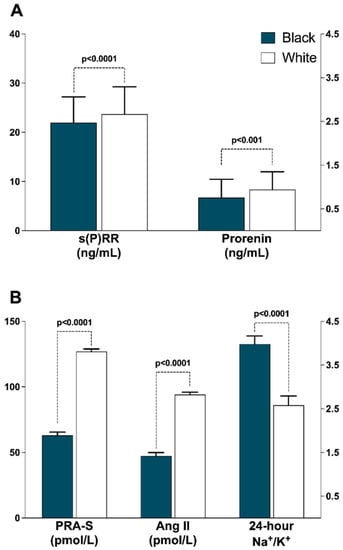
Figure 1.
Ethnic comparisons of renin-angiotensin system profiles. (A) Soluble (pro)renin receptor and prorenin; (B) Plasma renin activity surrogate, Angiotensin II and Na+/K+. Abbreviations: s(P)RR, soluble (pro)renin receptor; PRA-S, angiotensin-based plasma renin activity, AngII, Angiotensin II.

Table 2.
Comparisons between black and white adults.
3.1.2. Linear Regression Analyses
In crude correlations (Figure 2, Table S1) a statistically significant but weak positive association was observed between LVMi (r = 0.063; p = 0.030) and s(P)RR in the sample including both black and white participants. A similar pattern was observed in the black participants for LVMi (r = 0.085; p = 0.039). SVi was associated positively with s(P)RR only in black participants (r = 0.146; p < 0.001). In white participants, relative wall thickness was positively associated with s(P)RR (r = 0.191; p = 0.001), which was not evident in black participants. LV ejection fraction showed a negative association with s(P)RR only in white participants (r = −0.138; p = 0.001).
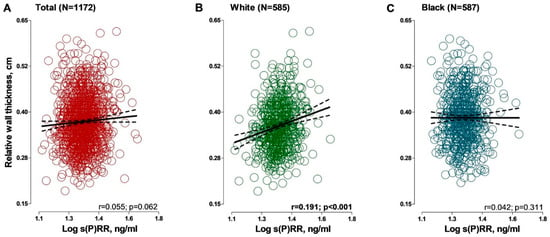
Figure 2.
Associations between relative wall thickness and soluble (pro)renin receptor in (A) total group; (B) black group and (C) white group. Solid and dashed lines represent the regression line and 95% CI boundaries, respectively. Abbreviations: s(P)RR, soluble (pro)renin receptor.
Analyses adjusted for sex, ethnicity and waist circumference in the total group (Table 3) revealed a statistically significant positive association between relative wall thickness and s(P)RR (r = 0.062; p = 0.036). In the participants as stratified by ethnicity, an additional adjustment was made for socioeconomic status score. In black participants, the positive association between SVi and s(P)RR persisted (r = 0.11; p = 0.008). In white participants, statistically significant partial-correlation coefficients were observed between relative wall thickness and LV ejection fraction and s(P)RR (all p ≤ 0.024), with an additional negative linear association observed between SVi and s(P)RR (r = −0.142; p = 0.001).

Table 3.
Partial correlations between left ventricular indices and soluble (pro)renin receptor.
In multivariable-adjusted linear regression analysis adjusted for ethnicity, sex, waist circumference, socioeconomic score and other covariates (Table 4), some of the associations between s(P)RR and measures of LV structure and function became attenuated and lost statistical significance, whereas new associations became evident. In ethnicity-pooled analysis, none of the associations remained statistically significant; however, a borderline-linear positive association was observed between s(P)RR and relative wall thickness (β = 0.073; p = 0.056). In white participants, there was a positive linear association between relative wall thickness and s(P)RR (β = 0.141; p = 0.005) and negative associations between LV ejection fraction (β = −0.123; p = 0.016) and SVi (β = −0.144; p = 0.004) and s(P)RR. The adjusted R-squared for LV ejection fraction was <0.10, but statistically significant (p < 0.001). Multivariable-adjusted linear regression models showing all covariates are shown in Tables S3–S5 and show s(P)RR as the main independent variables and the contribution of other covariates such as waist circumference, sex, ethnicity, glucose and Na/K to the models.

Table 4.
Associations between left ventricular structure and function and soluble (pro)renin receptor.
4. Discussion
This study investigated serum levels of s(P)RR in young black and white adults and associations with measures of LV structure and function due to the emerging role of s(P)RR in CVD and target organ damage. The main findings of the exploration were higher levels of s(P)RR in white as compared to black individuals and an independent positive association of relative wall thickness and s(P)RR, accompanied by negative associations of stroke volume and LV ejection fraction with s(P)RR only in white participants. To the best of our knowledge, this is the first investigation to show the difference in the presence of associations in a biethnic young population, with a relatively large sample size.
The higher levels of s(P)RR in the white population are consistent with a study by Nguyen et al., [18] which compared healthy men of black (n = 9) and white (n = 10) ethnicities aged 18–35 years. It was found that s(P)RR levels were lower in black men as compared to their white counterparts. Our study included apparently healthy black (n = 587) and white (n = 585) men and women with a comparable sex distribution and confirmed the influence of ethnicity on s(P)RR levels. As previously reported in this cohort [19], PRA-s, a measure of renin activity and eq AngII were two-fold higher in white participants as compared to black participants. Prorenin was higher in white participants which is inconsistent with recent relevant studies [18,41]. Tu et al. [41] found similar prorenin levels when comparing healthy black (n = 58) and white (n = 71) adults, a similar observation made by Nguyen et al. [18]. The discrepancies between the current study and previous observations could be partly due to the measurement technique and participant characteristics. In the current study, prorenin was measured directly in plasma, while in studies by Tu et al. [41] and Nguyen et al., [18], active renin was subtracted from total renin to obtain prorenin concentration values. Prorenin, when discovered, was initially regarded as an inactive precursor of the enzyme renin. However, data emerged showing that prorenin can bind with high affinity to (P)RR and result in various effects by activation of this receptor [11,42].
In the current study, BP and EDVi tended to increase as s(P)RR levels increased when investigated across s(P)RR quartiles, indicating involvement of s(P)RR in pressure and volume regulation. Kidney function (eGFR) declined, while glucose and LDL cholesterol increased across the s(P)RR quartiles. These observations represent the overall cardiovascular risk presented by high levels of s(P)RR, which may to some extent explain the adverse associations with LV structure and function. Previous observations have linked (P)RR polymorphisms to BP levels in Japanese men (mean age 61.1 ± 9.6 years) [43] and white men (mean age, 45.4 ± 14 years) [44], while in animals s(P)RR was positively associated with systolic BP [16]. Recently, Amari et al. [45] showed that s(P)RR is associated with cardiovascular events and mortality, serving as a biomarker to identify patients that require intensive care. The study population was older, with a number of CVDs and undergoing hemodialysis [45]. Our study already points to the usefulness of s(P)RR as a biomarker even in a healthy, young population (aged 20–30 years) with no current or historic CVD.
Previous studies have shown the detrimental effect of s(P)RR on kidney function and BP [46,47,48], with limited research on the role of s(P)RR in cardiac deterioration, particularly in young and healthy populations. To the best of our knowledge, the current study is the first to show the positive association between relative wall thickness in a healthy population, which may indicate the potential role of s(P)RR in early LV-structure alterations. The negative associations of two measures of LV function (LV ejection fraction and stroke volume) with s(P)RR further support its potential role in the early phases of LV remodeling and compromised functioning. The adverse association of LV structure and function were observed only in white participants and not in their black counterparts, which may be explained by the higher levels of s(P)RR and both its ligands, prorenin and active renin (represented by PRA) in white participants as compared to black participants. In Japanese women, a +1513A>G polymorphism of (P)RR was independently associated with the risk of LV hypertrophy and lacunar infarction, supporting the role of the renin receptor in target organ damage [49]. The mean age of women was 65.5 ± 5.6 years. When compared to men, 25.2% of the women presented with lacunar infarction, 13.5% presented with LV hypertrophy and 52.7% were classified as hypertensive [49]. In another study, plasma s(P)RR was found to be higher in patients with heart failure and associated with kidney damage in that group [17]. In our white participants with positive associations between relative wall thickness and s(P)RR, there was a comparable distribution of men and women, and sex was accounted for in multiple regression analyses. An important observation from our study was the substantially higher values of measures of adiposity in the white participants. This is despite a higher proportion of women with waist circumference greater than the cut-off point in the black participants, in which there were no statistically significant associations between s(P)RR and echocardiographic measures. The participants were normotensive at screening (clinic BP < 140/90 mmHg) and did not present with any known CVD. This may explain the absence of associations between s(P)RR and LVMi and only between relative wall thickness and s(P)RR in our study. Activation of s(P)RR by renin and/or renin can activate pathways via Ang II, leading to LV remodeling by promoting volume expansion and overload, inflammation and fibrosis as part of circulating RAS activation [50]. In addition to contributing to AngII formation, s(P)RR activation is involved in proinflammatory and profibrotic pathways that can set the stage for cardiac damage [11,15]. The absence of significant associations between LVMi and s(P)RR may be due to young age and healthy status and may be apparent when the study population is followed up over time with advancing age.
Our findings should be interpreted within the context of strengths and limitations. The cross-sectional nature of the investigation prevents us from making assumptions regarding the causal relationship between s(P)RR and markers of LV structure and function. The study sample was from one area of the North West Province of South Africa and cannot be regarded as representative of black and white populations in general. In the presence of these limitations, the current study confirmed some findings by earlier studies which included very small sample sizes and showed for the first time the positive association between relative wall thickness and s(P)RR in young healthy white participants.
In conclusion, we found higher levels of s(P)RR in healthy white young adults as compared to their black counterparts. s(P)RR was associated with an increase in relative wall thickness and adversely with LV function, an observation made only in white participants. These results suggest that s(P)RR may play a role in LV structural deterioration and compromised function in this study group. Our findings further support the notion that s(P)RR could serve as a cardiovascular risk marker, already in young populations without overt CVD; however, confirmation with follow-up data is required.
Implications
In this study we found higher levels of s(P)RR in young healthy white adults as compared to black adults. The independent positive association between s(P)RR and relative wall thickness, a marker of LV remodeling, accompanied by a negative association of s(P)RR with measures of LV function in a young healthy cohort without overt CVD suggest that s(P)RR may have the potential to serve as a biomarker of cardiovascular risk. Further research is required to understand the absence of relationships between s(P)RR and markers of LV remodeling in young black adults—a population known to be predisposed to hypertension-mediated organ damage and CVD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcdd9050130/s1, Table S1: Pearson correlation coefficients between left ventricular indices and soluble (pro)renin receptor. Table S2: Multivariable-adjusted linear regression analysis of relative wall thickness with soluble (pro)renin receptor as main outcome variable. Table S3: Multivariable-adjusted linear regression analysis of left ventricular mass index with soluble (pro)renin receptor as main outcome variable. Table S4: Multivariable-adjusted linear regression analysis of left ventricular ejection fraction with soluble (pro)renin receptor as main outcome variable. Table S5: Multivariable-adjusted linear regression analysis of stroke volume with soluble (pro)renin receptor as main outcome variable.
Author Contributions
Conceptualization, L.F.G.-M.; methodology, L.F.G.-M., R.K. and P.N.G.; validation, R.K., A.E.S., J.M.V.R. and P.N.G.; formal analysis, L.F.G.-M.; investigation, L.F.G.-M.; resources, L.F.G.-M. and R.K.; data curation, A.E.S.; writing—original draft preparation, L.F.G.-M.; writing—review and editing, R.K., A.E.S., J.M.V.R. and P.N.G.; visualization, L.F.G.-M. and R.K.; supervision, R.K. and A.E.S.; project administration, A.E.S.; funding acquisition, A.E.S. and L.F.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the South African Medical Research Council (SAMRC) with funds from National Treasury under its Economic Competitiveness and Support Package; the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology and National Research Foundation (NRF) of South Africa; the SAMRC with funds received from the South African National Department of Health, GlaxoSmithKline R&D (Africa Non-Communicable Disease Open Lab grant); the UK Medical Research Council and with funds from the UK Government’s Newton Fund; as well as corporate social investment grants from Pfizer (South Africa); Boehringer-Ingelheim (South Africa); Novartis (South Africa); the MediClinic Hospital Group (South Africa); and in kind contributions from Roche Diagnostics (South Africa). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore, the NRF does not accept any liability in this regard. Research reported in this publication was supported by the South African Medical Research Council under Self-Initiated Research Grant. The views and opinions expressed are those of the author(s) and do not necessarily represent the official views of the SAMRC. P.N.G. was supported through 2021/2022 Fulbright Scholar Grant number PS00305928.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Health Research Ethics Committee of the NORTH-WEST UNIVERSITY (protocol code NWU-00001-12-A1 and 12 April 2012).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data supporting the findings of this study are available upon request from the principal investigator (A.E.S.) upon reasonable request.
Acknowledgments
The study would not have been possible without the effort, commitment and dedication of voluntary participants, research staff, postdoctoral fellows, postgraduate students and interns at the Hypertension Research and Training Clinic and Laboratory at the North-West University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Skeggs, L.T.; Dorer, F.E.; Kahn, J.R.; Lentz, K.E.; Levine, M. The biochemistry of the renin-angiotensin system and its role in hypertension. Am. J. Med. 1976, 60, 737–748. [Google Scholar] [CrossRef]
- Volpe, M.; Savoia, C.; De Paolis, P.; Ostrowska, B.; Tarasi, D.; Rubattu, S. The renin-angiotensin system as a risk factor and therapeutic target for cardiovascular and renal disease. J. Am. Soc. Nephrol. 2002, 13, S173–S178. [Google Scholar] [CrossRef]
- Ma, T.K.; Kam, K.K.; Yan, B.P.; Lam, Y.Y. Renin–angiotensin–aldosterone system blockade for cardiovascular diseases: Current status. Br. J. Pharmacol. 2010, 160, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M. Newly discovered components and actions of the renin–angiotensin system. Hypertension 2013, 62, 818–822. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fyhrquist, F.; Saijonmaa, O. Renin-angiotensin system revisited. J. Intern. Med. 2008, 264, 224–236. [Google Scholar] [CrossRef]
- Schmieder, R.E.; Langenfeld, M.R.; Friedrich, A.; Schobel, H.P.; Gatzka, C.D.; Weihprecht, H. Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation 1996, 94, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Klingbeil, A.U.; Schobel, H.; Langenfeld, M.R.; Hilgers, K.; Schäufele, T.; Schmieder, R.E. Hyper-responsiveness to angiotensin II is related to cardiac structural adaptation in hypertensive subjects. J. Hypertens. 1999, 17, 825–833. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Schobel, H.P.; Langenfeld, M.R.; Hilgers, K.; Schmieder, R.E. Inadequate suppression of angiotensin II modulates left ventricular structure in humans. Clin. Nephrol. 1998, 49, 153–159. [Google Scholar]
- Varagic, J.; Ahmad, S.; Nagata, S.; Ferrario, C.M. ACE2: Angiotensin II/Angiotensin-(1–7) Balance in Cardiac and Renal Injury. Curr. Hypertens. Rep. 2014, 16, 420. [Google Scholar] [CrossRef]
- Brunner, H.R.; Gavras, H. Angiotensin blockade for hypertension: A promise fulfilled. Lancet 2002, 359, 990–992. [Google Scholar] [CrossRef]
- Nguyen, G.; Delarue, F.; Burcklé, C.; Bouzhir, L.; Giller, T.; Sraer, J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Investig. 2002, 109, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Gentile, G.; Reboldi, G. The renin angiotensin system in the development of cardiovascular disease: Role of aliskiren in risk reduction. Vasc. Health Risk Manag. 2008, 4, 971. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, F.; Liu, X.; Hu, J.; Su, J.; Lu, X.; Lu, A.; Cho, J.M.; Symons, J.D.; Zou, C.J.; et al. Soluble (pro) renin receptor induces endothelial dysfunction and hypertension in mice with diet-induced obesity via activation of angiotensin II type 1 receptor. Clin. Sci. 2021, 135, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Itoh, H. The (pro) renin receptor and the kidney. Semin. Nephrol. 2007, 27, 524–528. [Google Scholar] [CrossRef]
- Nguyen, G. The (pro) renin receptor: Pathophysiological roles in cardiovascular and renal pathology. Curr. Opin. Nephrol. Hypertens. 2007, 16, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Mohammadmoradi, S.; Thompson, J.; Su, W.; Gong, M.; Nguyen, G.; Yiannikouris, F. Adipocyte (pro) renin-receptor deficiency induces lipodystrophy, liver steatosis and increases blood pressure in male mice. Hypertension 2016, 68, 213–219. [Google Scholar] [CrossRef]
- Fukushima, A.; Kinugawa, S.; Matsushima, S.; Tsutsui, H.; Homma, T. Increased plasma soluble (pro) renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int. J. Cardiol. 2013, 168, 4313–4314. [Google Scholar] [CrossRef]
- Nguyen, G.; Blanchard, A.; Curis, E.; Bergerot, D.; Chambon, Y.; Hirose, T.; Caumont-Prim, A.; Tabard, S.B.; Baron, S.; Frank, M.; et al. Plasma soluble (pro) renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension 2014, 63, 297–302. [Google Scholar] [CrossRef]
- Gafane-Matemane, L.F.; Kruger, R.; Smith, W.; Mels, C.M.; Van Rooyen, J.M.; Mokwatsi, G.G.; Uys, A.S.; Brits, S.J.; Schutte, A.E. Characterization of the renin-angiotensin-aldosterone system in young healthy black adults: The african prospective study on the early detection and identification of hypertension and cardiovascular disease (African-PREDICT Study). Hypertension 2021, 78, 400–410. [Google Scholar] [CrossRef]
- Brewster, L.M.; Seedat, Y.K. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and β-adrenergic blockers? A systematic review. BMC Med. 2013, 11, 141. [Google Scholar] [CrossRef]
- Ojji, D.B.; Mayosi, B.; Francis, V.; Badri, M.; Cornelius, V.; Smythe, W.; Kramer, N.; Barasa, F.; Damasceno, A.; Dzudie, A.; et al. Comparison of dual therapies for lowering blood pressure in black Africans. N. Engl. J. Med. 2019, 380, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.E.; Gona, P.N.; Delles, C.; Uys, A.S.; Burger, A.; Mels, C.M.; Kruger, R.; Smith, W.; Fourie, C.M.; Botha, S.; et al. The African Prospective study on the Early Detection and Identification of Cardiovascular Disease and Hypertension (African-PREDICT): Design, recruitment and initial examination. Eur. J. Prev. Cardiol. 2019, 26, 458–470. [Google Scholar] [CrossRef] [PubMed]
- du Toit, W.L.; Schutte, A.E.; Gafane-Matemane, L.F.; Kruger, R.; Mels, C.M. The renin-angiotensin-system and left ventricular mass in young adults: The African-PREDICT study. Blood Press. 2021, 30, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gafane-Matemane, L.F.; Mokae, N.L.; Breet, Y.; Poglitsch, M.; Schutte, A.E. Associations of central and peripheral blood pressure with the renin-angiotensin-aldosterone system in healthy young adults: The African-PREDICT study. Hypertens. Res. 2021, 44, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, B.; Schutte, A.E.; Gafane-Matemane, L.F.; Kruger, R. Left ventricular mass independently associates with 24-hour sodium excretion in young masked hypertensive adults: The African-PREDICT study. Int. J. Cardiol. 2019, 276, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Strauss-Kruger, M.; Kruger, R.; Smith, W.; Gafane-Matemane, L.F.; Mokwatsi, G.; Wei, W.; Fedorova, O.V.; Schutte, A.E. The cardiotonic steroid marinobufagenin is a predictor of increased left ventricular mass in obesity: The African-PREDICT study. Nutrients 2020, 12, 3185. [Google Scholar] [CrossRef]
- Patro, B.K.; Jeyashree, K.; Gupta, P.K. Kuppuswamy’s socioeconomic status scale 2010—The need for periodic revision. Indian. J. Pediatr. 2012, 79, 395–396. [Google Scholar] [CrossRef]
- Marfell-Jones, T.; Stewart, A.; Olds, T. Kinanthropometry IX: Proceedings of the 9th International Conference of the International Society for the Advancement of Kinanthropometry; Routledge-Taylor & Francis: London, UK; New York, NY, USA, 2006. [Google Scholar]
- Mosteller, R. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Takken, T.; Stephens, S.; Balemans, A.; Tremblay, M.S.; Esliger, D.W.; Schneiderman, J.; Biggar, D.; Longmuir, P.; Wright, V.; McCrindle, B. Validation of the Actiheart activity monitor for measurement of activity energy expenditure in children and adolescents with chronic disease. Eur. J. Clin. Nutr. 2010, 64, 1494–1500. [Google Scholar] [CrossRef][Green Version]
- de Simone, G.; Devereux, R.B.; Daniels, S.R.; Mureddu, G.; Roman, M.J.; Kimball, T.R.; Greco, R.; Witt, S.; Contaldo, F. Stroke volume and cardiac output in normotensive children and adults: Assessment of relations with body size and impact of overweight. Circulation 1997, 95, 1837–1843. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Ganau, A.; Hahn, R.T.; Saba, P.S.; Mureddu, G.F.; Roman, M.J.; Howard, B.V. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am. J. Cardiol. 1996, 78, 801–807. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Jankowski, J.; Zidek, W.; Jankowski, V. Absolute quantification of endogenous angiotensin II levels in human plasma using ESI-LC-MS/MS. Clin. Proteom. 2014, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Olkowicz, M.; Radulska, A.; Suraj, J.; Kij, A.; Walczak, M.; Chlopicki, S.; Smolenski, R.T. Development of a sensitive, accurate and robust liquid chromatography/mass spectrometric method for profiling of angiotensin peptides in plasma and its application for atherosclerotic mice. J. Chromatogr. A 2015, 1393, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Stevens, L.A.; Claybon, M.A.; Schmid, C.H.; Chen, J.; Horio, M.; Imai, E.; Nelson, R.G.; Van Deventer, M.; Wang, H.-Y.; Zuo, L.; et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011, 79, 555–562. [Google Scholar] [CrossRef]
- World Health Organization and the Pan American Health Organization Group for Cardiovascular Disease Prevention through Population-Wide Dietary Salt Reduction. Protocol for Population Level Sodium Determination in 24-h Urine Samples. 2010. Available online: https://www.paho.org/hq/dmdocuments/2013/24h-urine-Protocol-eng.pdf (accessed on 18 April 2022).
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000; p. 9. ISBN 9241208945. [Google Scholar]
- Ekoru, K.; Murphy, G.; Young, E.; Delisle, H.; Jerome, C.; Assah, F.; Longo-Mbenza, B.; Nzambi, J.; On’Kin, J.; Buntix, F.; et al. Deriving an optimal threshold of waist circumference for detecting cardiometabolic risk in Sub-Saharan Africa. Int. J. Obes. 2018, 42, 487–494. [Google Scholar] [CrossRef]
- Tu, W.; Eckert, G.J.; Pratt, J.H.; Jan Danser, A.H. Plasma levels of prorenin and renin in blacks and whites: Their relative abundance and associations with plasma aldosterone concentration. Am. J. Hypertens. 2012, 25, 1030–1034. [Google Scholar] [CrossRef]
- Danser, A.J.; Deinum, J. Renin, prorenin and the putative (pro) renin receptor. Hypertension 2005, 46, 1069–1076. [Google Scholar] [CrossRef]
- Hirose, T.; Hashimoto, M.; Totsune, K.; Metoki, H.; Asayama, K.; Kikuya, M.; Sugimoto, K.; Katsuya, T.; Ohkubo, T.; Hashimoto, J. Association of (pro) renin receptor gene polymorphism with blood pressure in Japanese men: The Ohasama study. Am. J. Hypertens. 2009, 22, 294–299. [Google Scholar] [CrossRef]
- Ott, C.; Schneider, M.P.; Delles, C.; Schlaich, M.P.; Hilgers, K.F.; Schmieder, R.E. Association of (pro) renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenet. Genom. 2011, 21, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Amari, Y.; Morimoto, S.; Suda, C.; Iida, T.; Okuda, H.; Yurugi, T.; Oyama, Y.; Aoyama, N.; Nakajima, F. Serum soluble (pro) renin receptor level as a prognostic factor in patients undergoing maintenance hemodialysis. Sci. Rep. 2021, 11, 17402. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Suzuki, F.; Nakagawa, T.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Nabi, A.N.; Nishiyama, A.; Sugaya, T.; Hayashi, M. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor–deficient mice. J. Am. Soc. Nephrol. 2006, 17, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Isobe, S.; Ishigaki, S.; Suzuki, T.; Iwakura, T.; Ono, M.; Fujikura, T.; Tsuji, T.; Otsuka, A.; Ishii, Y. Plasma soluble (pro) renin receptor reflects renal damage. PLoS ONE 2016, 11, e0156165. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Taniguchi, Y.; Shimamura, Y.; Inoue, K.; Ogata, K.; Ishihara, M.; Horino, T.; Fujimoto, S.; Ohguro, T.; Yoshimoto, Y. Serum level of soluble (pro) renin receptor is modulated in chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Hashimoto, M.; Totsune, K.; Metoki, H.; Hara, A.; Satoh, M.; Kikuya, M.; Ohkubo, T.; Asayama, K.; Kondo, T.; et al. Association of (pro) renin receptor gene polymorphisms with lacunar infarction and left ventricular hypertrophy in Japanese women: The Ohasama study. Hypertens. Res. 2011, 34, 530–535. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Aizaki, Y.; Kusano, K.I.; Kishi, F.; Susumu, T.; Iida, S.; Ishiura, S.; Nishimura, S.; Shichiri, M.; Senbonmatsu, T. The (pro) renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens. Res. 2011, 34, 599–605. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).