When Does the Human Embryonic Heart Start Beating? A Review of Contemporary and Historical Sources of Knowledge about the Onset of Blood Circulation in Man
Abstract
1. Introduction
2. How Can We Clarify the Timing of the First Heartbeats in Human Embryos?
3. Contemporary Sources of Knowledge
4. Historical Sources of Knowledge
4.1. Knowledge about the Developmental Stage of Human Embryos at the Onset of Heart Beating Can Be Traced Back in Time up to the Late 19th Century
4.2. Historical Changes in the Statements on the Age of Human Embryos
4.3. Extrapolating Observations on Animal Embryos to the Human Species
5. A Search for Published but Unknown Ex Utero Observations on Human Embryonic Heart Activity
5.1. The Embryological Literature (Journals and Books on Embryology, Anatomy, Teratology)
5.1.1. Reports on CS-10 Embryos
5.1.2. Reports and Studies on Collections of Human Embryos
5.1.3. Embryological Studies Focusing on Human Embryonic Hearts
5.2. The Non-Embryological Literature
6. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Santoro, G.; Wood, M.D.; Merlo, L.; Anastasi, G.P.; Tomasello, F.; Germano, A. The anatomic location of the soul from the heart, through the brain, to the whole body, and beyond: A journey through western history, science, and philosophy. Neurosurgery 2009, 65, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Powner, D.J.; Ackerman, B.M.; Grenvil, A. Medical diagnosis of death in adults: Historical contributions to current controversies. Lancet 1996, 348, 1219–1223. [Google Scholar] [CrossRef]
- Smolensky, K. Defining life from the perspective of death: An introduction to the forced symmetry approach. Univ. Chic. Leg. Forum 2006, 41–86. [Google Scholar]
- English, J. Abortion evolution: How Roe v. Wade has come to support a pro-life & pro-choice position. Creighton Law Rev. 2019, 53, 157–210. [Google Scholar]
- Evans, D.P.; Narasimhan, S. A narrative analysis of anti-abortion testimony and legislative debate related to Georgia’s fetal “heartbeat” abortion ban. Sex. Reprod. Health Matters 2020, 28, 1686201. [Google Scholar] [CrossRef]
- Carlson, B.M. Human Embryology and Developmental Biology, 6th ed.; Elsevier: St. Louis, MI, USA, 2019. [Google Scholar]
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Larsen’s Human Embryology, 5th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The Developing Human. Clinically Oriented Embryology, 11th ed.; Elsevier: Edinburgh, UK; London, UK; New York, NY, USA; Oxford, UK; Philidelphia, PA, USA; St. Louis, MI, USA; Sidney, Australia, 2020. [Google Scholar]
- O’Rahilly, R.; Müller, F. Developmental Stages in Human Embryos; Carnegie Institution Washington Publication 673: Washington DC, USA, 1987. [Google Scholar]
- Davis, C.L. The development of the human heart from its first appearance to the stage found in embryos of twenty paired somites. Contr. Embryol. Carnegie Inst. 1927, 19, 245–284. [Google Scholar]
- Cambridge Advanced Learner’s Dictionary, 4th ed.; Cambridge University Press: Cambridge, UK, 2013.
- Männer, J.; Wessel, A.; Yelbuz, T.M. How does the tubular embryonic heart work? Looking for the physical mechanism driving unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 2010, 239, 1035–1046. [Google Scholar] [CrossRef]
- Minot, C.S. Human Embryology; William Wood and Company: New York, NY, USA, 1892. [Google Scholar]
- Heuser, C.H.; Corner, G.W. Developmental horizons in human embryos. Description of age group X, 4 to 12 somites. Contr. Embryol. Carnegie Inst. 1957, 36, 29–39. [Google Scholar]
- Tyser, R.C.V.; Miranda, A.M.A.; Chen, C.; Davidson, S.M.; Srinivas, S.; Riley, P.R. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLIFE 2016, 5, e17113. [Google Scholar] [CrossRef]
- Tyser, R.C.V.; Srinivas, S. The first heartbeat—Origin of cardiac contractile activity. Cold Spring Harb. Perspect. Biol. 2020, 12, a037135. [Google Scholar] [CrossRef]
- Eternod, A.C.F. Sur un œuf humain de 16.3 mm avec embryon de 2.1 mm (uterus et adnexes). Soc Helvet Sci Nat. 1896, 79, 164–169. [Google Scholar]
- Eternod, A.C.F. Il y a un canal notochordal dans l’embryon humain. Anat. Anz. 1899, 16, 131–143. [Google Scholar]
- Naegele, F.C. Erfahrungen und Abhandlungen aus dem Gebiethe der Krankheiten des Weiblichen Geschlechtes. Nebst Grundzügen einer Methodenlehre der Geburtshülfe; Löffler: Mannheim, Germany, 1812. [Google Scholar]
- Johnson, M.H. A short history of in vitro fertilization. Int. J. Dev. Biol. 2019, 63, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Doubilet, P.M. Ultrasound evaluation of the first trimester. Radiol. Clin. N. Am. 2014, 52, 1191–1199. [Google Scholar] [CrossRef]
- van Heeswijk, M.; Nijhuis, J.G.; Hollander, H.M.G. Fetal heart rate in early pregnancy. Early Hum. Dev. 1990, 22, 151–156. [Google Scholar] [CrossRef]
- Ouyang, Y.; Qin, J.; Lin, G.; Xiang, S.; Li, X. Reference intervals of gestational sac, yolk sac, embryonic length, embryonic heart rate at 6–10 weeks after in vitro fertilization-embryo transfer. BMC Pregnancy Childbirth 2020, 20, 533. [Google Scholar] [CrossRef]
- Schats, R.; Jansen, C.A.; Wladimiroff, J.W. Embryonic heart activity: Appearance and development in early human pregnancy. Br. J. Obs. Gynaecol. 1990, 97, 989–994. [Google Scholar] [CrossRef]
- van Os, H.C.; Hout, J.I.T.; Hermans, J.; Jansen, C.A.M. Embryonic length, crown-rump length and fetal heart activity in early human pregnancy determination by transvaginal ultrasound. BMUS Bull. 1993, 1, 18–23. [Google Scholar] [CrossRef]
- Howe, R.S.; Isaacson, K.J.; Albert, J.L.; Coutifaris, C.B. Embryonic heart rate in human pregnancy. J. Ultrasound Med. 1991, 10, 367–371. [Google Scholar] [CrossRef]
- Elnekheli, M.; Kahles, G.; Khoshyomn, S.; Kirisits, R.; Troger, P.; Boldizsar, A.; Feichtinger, W. Transvaginale Doppleruntersuchung der embryonalen Herzaktion in der Frühschwangerschaft. (Transvaginal doppler sonography of embryonic cardiac activity in early human pregnancy). Ultraschall Med. 1992, 13, 12–14. [Google Scholar] [CrossRef]
- Dickey, R.P.; Gasser, R.F. Ultrasound evidence for variability in the size and development of normal human embryos before the tenth post-insemination week after assisted reproductive technologies. Hum. Reprod. 1993, 8, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rotsztejn, D.; Rana, N.; Dmowski, W.P. Correlation between fetal heart rate, crown-rump length, and ß-human chorionic gonadotropin levels during the first trimester of well-timed conceptions resulting from infertility treatment. Fertil. Steril. 1993, 59, 1169–1173. [Google Scholar] [CrossRef]
- Wisser, J.; Dirschedl, P. Embryonic heart rate in dated human embryos. Early Hum. Dev. 1994, 37, 107–115. [Google Scholar] [CrossRef]
- Britten, S.; Soenksen, D.M.; Bustillo, M.; Coulam, C.B. Very early (24–56 days from the last menstrual period) embryonic heart rate in normal pregnancies. Hum. Reprod. 1994, 9, 2424–2426. [Google Scholar] [CrossRef]
- Coulam, C.B.; Britten, S.; Soenksen, D.M. Early (34–56 days from last menstrual period) ultrasonographic measurements in normal pregnancies. Hum. Reprod. 1996, 11, 1771–1774. [Google Scholar] [CrossRef]
- Tezuka, N.; Sato, S.; Banzai, M.; Saito, H.; Hiroi, M. Development and sexual differences in embryonic heart rate in pregnancies resulting from in vitro fertilization. Gynaecol. Obstet. Investig. 1998, 46, 217–219. [Google Scholar] [CrossRef]
- Shiota, K. Variability in human embryonic development and its implications for the susceptibility to environmental agents. Birth Defects Res. 2009, 85, 661–666. [Google Scholar] [CrossRef]
- Rempen, A. Diagnosis of viability in early pregnancy with vaginal sonography. J. Ultrasound Med. 1990, 9, 711–716. [Google Scholar] [CrossRef]
- Yapar, E.G.; Ekici, E.; Gökmen, O. First trimester fetal heart rate measurements by transvaginal ultrasound combined with pulsed Doppler: An evaluation of 1331 cases. Europ. J. Obstet. Gynec. Reprod. Biol. 1995, 60, 133–137. [Google Scholar] [CrossRef]
- Robinson, H.P.; Shaw-Dunn, J. Fetal heart rate as determined by sonor in early pregnancy. J. Obstet. Gynecol. 1973, 80, 805–809. [Google Scholar]
- Papaioannou, G.I.; Syngelaki, A.; Poon, L.C.Y.; Ross, J.A.; Nicolaides, K.H. Normal ranges of embryonic length, embryonic heart rate, gestational sac diameter and yolk sac diameter at 6–10 weeks. Fetal. Diagn. Ther. 2010, 28, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Oosthoek, P.W.; Wenink, A.C.G.; Vrolijk, B.C.M.; Wisse, L.J.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Development of the atrioventricular valve tension apparatus in the human heart. Anat. Embryol. 1998, 198, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Gui, Y.; Cnota, W.; Huhta, J.; Wloch, A. Human embryonic cardiovascular function. Acta Obstet. Gynecol. Scand. 2016, 95, 621–628. [Google Scholar] [CrossRef] [PubMed]
- DuBose, T.J.; Cunyus, J.A.; Johnson, L.F. Embryonic heart rate and age. JDMS 1990, 6, 151–157. [Google Scholar] [CrossRef]
- DuBose, T.J. Embryonic heart rates. Fertil. Steril. 2009, 92, e57. [Google Scholar] [CrossRef]
- Romanoff, A.L. The heart beat of avian embryos. Anat. Rec. 1944, 89, 313–316. [Google Scholar] [CrossRef]
- Patten, B.M. Human Embryology, 1st ed.; J. & A. Churchill Ltd.: London, UK, 1947. [Google Scholar]
- Campbell, S. A short history of sonography in obstetrics and gynaecology. Facts Views Vis. Obgyn. 2013, 5, 213–229. [Google Scholar]
- O’Rahilly, R.; Müller, F. Mini-review: Prenatal ages and stages—Measures and errors. Teratology 2000, 61, 382–384. [Google Scholar] [CrossRef]
- O’Rahilly, R.; Müller, F. Developmental stages in human embryos: Revised and new measurements. Cells Tissues Organs 2010, 192, 73–84. [Google Scholar] [CrossRef]
- Kollmann, J. Lehrbuch der Entwicklungsgeschichte des Menschen; Verlag Gustav Fischer: Jena, Germany, 1898. [Google Scholar]
- His, W. Anatomie Menschlicher Embryonen I. Embryonen des Ersten Monats; F.C.W. Vogel: Leipzig, Germany, 1880. [Google Scholar]
- Müller, F.; O’Rahilly, R. Wilhelm His und 100 Jahre Embryologie des Menschen. Acta Anat. 1986, 125, 73–75. [Google Scholar] [CrossRef]
- Reichert, C.B. Beschreibung einer frühzeitigen menschlichen Frucht im bläschenförmigen Bildungszustande nebst vergleichenden Untersuchungen über die bläschenförmigen Früchte der Säugethiere und des Menschen. Abhandl Königl Akad Wissensch 1873, 1–92. [Google Scholar]
- Kollmann, J. Handatlas der Entwicklungsgeschichte des Menschen, Erster Teil (Vol. 1); Verlag Gustav Fischer: Jena, Germany, 1907. [Google Scholar]
- Kollmann, J. Handatlas der Entwicklungsgeschichte des Menschen, Zweiter Teil (Vol. 2); Verlag Gustav Fischer: Jena, Germany, 1907. [Google Scholar]
- Keibel, F.; Mall, F.P. Handbuch der Entwicklungsgeschichte des Menschen. 1. Band; Verlag S. Hirzel: Leipzig, Germany, 1910. [Google Scholar]
- Bryce, T.H.; Teacher, J.H. Contribution to the Study of the Early Development and Imbedding of the Human Ovum. I. An Early Ovum Imbedded in the Decidua; James Maclehose and Sons: Glasgow, UK, 1908. [Google Scholar]
- Fraenkel, L. Das zeitliche Verhalten von Ovulation und Menstruation. Zentralbl. F. Gyn. 1911, 35, 1591–1599. [Google Scholar]
- Meyer, R. Ueber Corpus luteum-Bildung beim Menschen. Arch. F. Gyn. 1911, 93, 354–404. [Google Scholar] [CrossRef]
- Mall, F.P. Die Altersbestimmung von menschlichen Embryonen und Feten. In Handbuch der Entwicklungsgeschichte des Menschen. 1. Band; Keibel, F., Mall, F.P., Eds.; Verlag S. Hirzel: Leipzig, Germany, 1910; pp. 185–207. [Google Scholar]
- Mall, F.P. On the age of human embryos. Am. J. Anat. 1918, 23, 397–422. [Google Scholar] [CrossRef]
- Corner, G.W. Oestrus, ovulation and menstruation. Physiol. Rev. 1923, 3, 457–482. [Google Scholar] [CrossRef]
- Knaus, H.H. Über den Zeitpunkt der Konzeptionsfähigkeit des Weibes im Intermenstruum. Münch. Med. Wochenschr. 1929, 76, 1157–1160. [Google Scholar]
- Ogino, K. Ovulationstermin und Konzeptionstermin. Zentralbl. F. Gyn. 1930, 54, 464–479. [Google Scholar]
- Hamilton, W.J.; Boyd, J.D.; Mossman, H.W. Human Embryology. In Prenatal Development of Form and Function, 1st ed.; Heffer & Sons: Cambridge, UK, 1945. [Google Scholar]
- Nishimura, H.; Takano, K.; Tanimura, T.; Yasuda, M. Normal and abnormal development of human embryos: First report of the analysis of 1,213 intact embryos. Teratology 1968, 1, 281–290. [Google Scholar] [CrossRef]
- Heuser, C.H.; Streeter, G.L. Development of the macaque embryo. Contr. Embryol. Carnegie Inst. 1941, 29, 15–56. [Google Scholar]
- Streeter, G.L. Developmental Horizons in Human Embryos: Age Groups XI to XXIII; Carnegie Institution of Washington: Washington DC, USA, 1951. [Google Scholar]
- Dandy, W.E. A human embryo with seven pairs of somites measuring about 2 mm in length. Am. J. Anat. 1910, 10, 85–111. [Google Scholar] [CrossRef]
- Evans, H.M.; Bartelmez, G.W. A human embryo of seven to eight somites. Anat. Rec. 1917, 11, 355–356. [Google Scholar]
- Goss, C.M. The first contractions of the heart in rat embryos. Anat. Rec. 1938, 70, 505–524. [Google Scholar] [CrossRef]
- De Vries, P.A.; Saunders, J.A.d.G.M. Development of the ventricles and spiral outflow tract of the human heart. Contr. Embryol. Carnegie Inst. 1962, 37, 87–114. [Google Scholar]
- DeHaan, R.L. Morphogenesis of the vertebrate heart. In Organogenesis; DeHaan, R.L., Ursprung, H., Eds.; Holt, Rinehart & Winston: New York, NY, USA, 1965; pp. 377–419. [Google Scholar]
- Preyer, W. Specielle Physiologie des Embryo—Untersuchungen ueber die Lebenserscheinungen vor der Geburt; Th. Grieben: Leipzig, Germany, 1885. [Google Scholar]
- Tarantal, A.F.; Hendrickx, A.G. Prenatal growth in the Cynomolgus and Rhesus macaque (Macaca fascicularis and Macaca mulatta): A comparison by ultrasonography. Am. J. Primatol. 1988, 15, 309–323. [Google Scholar] [CrossRef]
- Sissman, N.J. Developmental landmarks in cardiac morphogenesis: Comparative chronology. Am. J. Cardiol. 1970, 25, 141–148. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Christoffels, V.M.; Anderson, R.H.; van den Hoff, M.J.B. The heart-forming fields: One or multiple. Phil. Trans. R. Soc. B 2007, 362, 1257–1265. [Google Scholar] [CrossRef]
- Dwinnell, L.A. Physiological contraction of double hearts in rabbit embryos. Exp. Biol. Med. 1939, 42, 264–267. [Google Scholar] [CrossRef]
- Hiruma, T.; Hirakow, R. An ultrastructural topographical study on myofibrillogenesis in the heart of the chick embryo during pulsation onset period. Anat. Embryol. 1985, 172, 325–329. [Google Scholar] [CrossRef]
- DeRuiter, M.C.; Poelmann, R.E.; VanderPlas-de Vries, I.; Mentink, M.M.T.; Gittenberger-de Groot, A.C. The development of the myocardium and endocardium in mouse embryos. Fusion of two heart tubes? Anat. Embryol. 1992, 185, 461–473. [Google Scholar] [CrossRef]
- Sakai, T.; Hirota, A.; Kamino, K. Video-imaging assessment of initial beating patterns of the early embryonic chick heart. Japan. J. Physiol. 1996, 46, 465–472. [Google Scholar] [CrossRef]
- Nishii, K.; Shibata, Y. Mode and determination of the initial contraction stage in the mouse embryo heart. Anat. Embryol. 2006, 211, 95–100. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.E.; Koniski, A.D.; Malik, J.; Palis, J.J.P. Circulation is established in a stepwise pattern in the mammalian embryo. Blood 2003, 101, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.V. The initiation of blood flow and flow induced events in early vascular development. Sem. Cell Dev. Biol. 2011, 22, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol. Rev. 1991, 71, 53–91. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T. Observations upon young human embryos. J. Anat. 1914, 48, 315–351. [Google Scholar]
- Corner, G.W. A well-preserved human embryo of 10 somites. Contr. Embryol. Carnegie Inst. 1929, 20, 81–102. [Google Scholar]
- Baxter, J.S.; Boyd, J.D. Observations on the neural crest of a ten-somite human embryo. J. Anat. 1939, 73, 318–326. [Google Scholar]
- Holmdahl, D.E. Beitrag zur Kenntnis der Entwicklung des Blutgefäßsystems und Blutes beim Menschen, nebst eines Exkurs über die Entstehung der Neuralleiste. Eine Studie mit Ausgangspunkt von einem frühen, operative gewonnenen, menschlichen Embryo G-dt, 1.7 mm, mit 11 (-15) Somitenpaaren. Z. Mikrosk. Anat. Forsch. 1944, 54, 261–295. [Google Scholar]
- Müller, F.; O’Rahilly, R. The first appearance of the neural tube and optic primordium in the human embryo at stage 10. Anat. Embryol. 1985, 172, 157–169. [Google Scholar] [CrossRef]
- von Spee, G.F. Ueber einen menschlichen Embryo von 2.69 mm längstem geraden Durchmesser. Mitth. Ver. Schleswig Holst. Ärzte 1887, 11, 174–176. [Google Scholar]
- Kroemer, P. Wachsmodell eines jungen menschlichen Embryo. Verh. Deutsch. Ges. Gynaekol. Würzburg 1903, 537–540. [Google Scholar]
- Veit, O.; Esch, P. Untersuchung eines in situ fixierten, operativ gewonnenen menschlichen Eies der vierten Woche. Z. Anat. Entwickl. Gesch. 1922, 63, 343–414. [Google Scholar] [CrossRef]
- Payne, F. General description of a seven somite embryo. Contr. Embryol. Carnegie Inst. 1925, 16, 115–124. [Google Scholar]
- Bartelmez, G.W.; Evans, H.M. Development of the human embryo during the period of somite formation, including embryos with 2 to 16 pairs of somites. Contr. Embryol. Carnegie Inst. 1926, 17, 1–67. [Google Scholar]
- Sternberg, H. Beschreibung eines menschlichen Embryos mit vier Ursegmentpaaren, nebst Bemerkungen über die Anlage und früheste Entwicklung einiger Organe beim Menschen. Z. Anat. Entwick.L Gesch. 1927, 82, 142–240. [Google Scholar] [CrossRef]
- Ludwig, E. Embryon humain avec dix paires de somites mesoblastiques. C. R. Ass. Anat. 1929, 580–585. [Google Scholar]
- Studnicka, F.K. Über den Zusammenhang des Cytoplasmas bei jungen menschlichen Embryonen. Z. Mikrosk. Anat. Forsch. 1929, 18, 553–656. [Google Scholar]
- Politzer, G.; Sternberg, H. Über die Entwicklung der ventralen Körperwand und des Nabelstranges beim Menschen. Z. Anat. Entwickl. Gesch. 1930, 92, 279–379. [Google Scholar] [CrossRef]
- Politzer, G. Über einen menschlichen Embryo mit sieben Urwirbelpaaren. Z. Anat. Entwickl. Gesch. 1930, 93, 386–428. [Google Scholar] [CrossRef]
- West, C.M. Description of a human embryo of eight somites. Contr. Embryol. Carnegie Inst. 1930, 21, 25–35. [Google Scholar]
- Treutler, K. Über das wahre Alter junger menschlicher Embryonen. Anat. Anz. 1931, 71, 245–258. [Google Scholar]
- Litzenberg, J.C. A young human ovum of the early somite period. Am. J. Obs. Gynecol 1933, 26, 519–529. [Google Scholar] [CrossRef]
- Boyden, E.A. A volumetric analysis of young human embryos at the 10- and 12-somite stage. Contr. Embryol. Carnegie Inst. 1940, 28, 157–191. [Google Scholar]
- Orts Llorca, F. Beschreibung eines menschlichen Embryo mit 4 Urwirbelpaaren. Z. Anat. Entwickl. Gesch. 1934, 103, 765–792. [Google Scholar] [CrossRef]
- Arey, L.B.; Henderson, J.W. The Huber six somite human embryo (M 71). Anat. Rec. 1943, 85, 295. [Google Scholar]
- Streiter, A. Ein menschlicher Keimling mit 7 Urwirbelpaaren. Z. Mikrosk. Anat. Forsch. 1951, 57, 181–248. [Google Scholar]
- Schenk, R. Beschreibung eines menschlichen Keimlings mit 5 Ursegmentpaaren. Acta Anat. 1954, 22, 236–271. [Google Scholar] [CrossRef]
- Mori, T. Histochemical studies on the distribution of alkaline phosphatase in the early human embryo. I. Observations on two embryos in early somite stage, Streeter’s horizon X. Arch. Hist. Jap. 1959, 18, 169–173. [Google Scholar] [CrossRef]
- Mall, F.P. Report upon the collection of human embryos at the Johns Hopkins University. Anat. Rec. 1911, 5, 343–357. [Google Scholar] [CrossRef]
- Mall, F.P.; Meyer, A.W. Studies on abortuses: A survey of pathological ova in the Carnegie Embryological Collection. Carnegie Inst. Pub. 275 Contr. Embryol. 1921, 56. [Google Scholar]
- Cullen, T.S. Max Brödel, 1870–1941 director of the first department of art as applied to medicine in the world. Bull. Med. Libr. Assoc. 1945, 33, 4–29. [Google Scholar]
- Hutchins, G.M.; Kessler-Hanna, A.; Moore, G.W. Development of the coronary arteries in the embryonic human heart. Circulation 1988, 77, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Mall, F.P. On the development of the blood vessels of the brain in the human embryo. Am. J. Anat. 1905, 4, 1–18. [Google Scholar] [CrossRef]
- Wislocki, G.B. The meningeal relations of the hypophysis cerebri. II. An embryological study of the meninges and blood vessels of the human hypophysis. Am. J. Anat. 1937, 61, 95–129. [Google Scholar] [CrossRef]
- Evans, H.M. Development of the vascular system. In Manual of Human Embryology; Keibel, F., Mall, F.P., Eds.; Lippincott: Philadelphia, MI, USA; London, UK, 1912; Volume 2, pp. 570–709. [Google Scholar]
- Skidmore, F.D. An analysis of the age and size of 483 human embryos. Teratology 1977, 15, 97–103. [Google Scholar] [CrossRef]
- Nishimura, H.; Tanimura, T.; Semba, R.; Uwabe, C. Normal development of early human embryos: Observation of 90 specimens at Carnegie stages 7 to 13. Teratology 1974, 10, 1–7. [Google Scholar] [CrossRef]
- Shiota, K.; Uwabe, H.; Nishimura, H. High prevalence of defective human embryos at the early postimplantation period. Teratology 1987, 35, 309–316. [Google Scholar] [CrossRef]
- Harkness, L.M.; Baird, D.T. Morphological and molecular characteristics of living human fetuses between Carnegie stages 7 and 23: Developmental stages in the postimplantation embryo. Hum. Reprod. Update 1996, 3, 3–23. [Google Scholar] [CrossRef]
- Bullen, P.J.; Robson, S.C.; Strachan, T. Human post-implantation embryo collection: Medical and surgical techniques. Early Hum. Dev. 1998, 51, 213–221. [Google Scholar] [CrossRef]
- Moore, G.W.; Hutchins, G.M.; O’Rahilly, R. The estimated age of staged human embryos and early fetuses. Am. J. Obstet. Gynec. 1981, 139, 500–506. [Google Scholar] [CrossRef]
- Mall, F.P. On the development of the human heart. Am. J. Anat. 1912, 13, 249–298. [Google Scholar] [CrossRef]
- Goor, D.A.; Edwards, J.E.; Lillehei, C.W. The development of the interventricular septum of the human heart; correlative morphogenetic study. Chest 1970, 58, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Anderson, R.H.; Christoffels, V.M.; Moorman, A.F.M. Three-dimensional and molecular analysis of the venous pole of the developing human heart. Circulation 2010, 122, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Li, H.R.; Cao, X.M.; Wang, Q.X.; Qiao, C.J.; Ya, J. Second heart field and the development of the outflow tract in the human embryonic heart. Develop. Growth Differ. 2013, 55, 359–367. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, J.; Peng, J.; Zhou, Y.; Song, W.; Chen, S.; Wang, Q.; Bai, K.; Sun, K. Dynamic transcriptome profiling towards understanding the development of the human embryonic heart during different Carnegie stages. FEBS Lett. 2020, 594, 4307–4319. [Google Scholar] [CrossRef]
- Chen, B.; Hou, A.; Zhao, L.; Liu, Y.; Shi, X.; Du, B.; Yu, Y.; Zhao, P.; Gao, Y. Next generation sequencing identify rare copy number variants in non-syndromic patent Ductus arteriosus. Front. Genet. 2020, 9, 559. [Google Scholar] [CrossRef]
- Pflüger, E. Die Lebenszähigkeit des menschlichen Fetus. Pflügers Arch. Physiol. 1877, 14, 628–629. [Google Scholar] [CrossRef]
- Zuntz, N. Ueber die Respiration des Säugethier-Foetus. Pflügers Arch. Physiol. 1877, 14, 605–627. [Google Scholar] [CrossRef]
- Veit, J. Die Eileiterschwangerschaft. Ein Beitrag zur Pathologie und Therapie derselben; Verlag Ferdinand Enke: Stuttgart, Germany, 1884; pp. 56–60. [Google Scholar]
- Neugebauer, F. Automatische Thätigkeit des Embryonalherzens bis 3 Stunden über den Tod hinaus. Centralbl. Gynäkol. 1898, 22, 1281–1286. [Google Scholar]
- Armann, W.F. Ueber einen Fall von Pulsationen, beobachtet am primitiven Herzschlauch des menschlichen Embryos aus der zweiten Woche. Arch. Gynäk. Geburtshilfe 1908, 85, 139–141. [Google Scholar] [CrossRef][Green Version]
- Armann, W.F. Pulsations observed in the primitive cardiac tube of a human embryo in the second week. Am. J. Obstet. Dis. Women Child. 1913, 67, 253–255. [Google Scholar]
- Lukinovic, J. Zur Frage der Herztätigkeit bei jungen menschlichen Embryonen. Zentralbl. Gynäkol. 1937, 61, 2912–2914. [Google Scholar]
- Heard, J.D.; Burkley, G.G.; Schaefer, C.R. Electrocardiograms derived from eleven fetuses through the medium of direct leads. Am. Heart J. 1936, 11, 41–48. [Google Scholar] [CrossRef]
- Marcel, M.P.; Exchaquet, J.P. L’electrocardiogramme du fœtus humain avec un cas du double rythme auriculaire vérifié. Arch. Mal. Cœur 1938, 31, 504–512. [Google Scholar]
- Straus, R.; Walker, R.H.; Cohen, M. Direct electrocardiographic recording of a twenty-three millimeter human embryo. Am. J. Cardiol. 1961, 8, 443–447. [Google Scholar] [CrossRef]
- Schubert, E.; Schwartze, H.; Schwartze, P. Vergleichende Untersuchungen zu Vektorkardiogramm sowie Lage und Aufbau am menschlichen fötalen Herzen. Arch. Kreislaufforsch. 1965, 49, 266–276. [Google Scholar] [CrossRef]
- Danielsson, C.; Brask, J.; Sköld, A.C.; Genead, R.; Andersson, U.; Stockling, K.; Pehrson, R.; Grinnemo, K.H.; Salari, S.; Hellmold, H.; et al. Exploration of human, rat and rabbit embryonic cardiomyocytes suggests K-channel block as a common teratogenic mechanism. Cardiovasc. Res. 2013, 97, 23–32. [Google Scholar] [CrossRef]
- Tuganowski, W.; Cekanski, A. Electric activity of a single fibre of the human embronic heart. Pflügers Arch. 1971, 323, 21–26. [Google Scholar] [CrossRef]
- Coltart, D.J.; Spilker, B.A.; Meldrum, S.J. An electrophysiological study of human foetal cardiac muscle. Experientia 1971, 27, 797–799. [Google Scholar] [CrossRef]
- Janse, M.J.; Anderson, R.H.; van Capelle, F.J.L.; Durrer, D. A combined electrophysiological and anatomical study of the fetal human heart. Am. Heart J. 1961, 91, 556–562. [Google Scholar] [CrossRef]
- Lloyd, W.D.M. The action of calcium on the isolated human foetal heart. J. Pharmacol. 1929, 36, 185–193. [Google Scholar]
- Garrey, W.E.; Townsend, S.E. Neural responses and reaction of the heart of a human embryo. Am. J. Physiol. 1948, 152, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.B.E. Some observations upon isolated perfused human foetal hearts. J. Physiol. 1953, 120, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gennser, G.; Nilsson, E. Response to Adrenaline, Acetylcholine and change of contraction frequency in early human foetal hearts. Experientia 1970, 10, 1105–1799. [Google Scholar] [CrossRef]
- Coltart, D.J.; Spilker, B.A. Development of human inotropic responses to catecholamines. Experientia 1972, 28, 525–526. [Google Scholar] [CrossRef]
- Resch, B.A.; Papp, J.G.; Szontagh, F.E.; Szekeres, L. Comparison of spontaneous contraction rates of in situ and isolated fetal hearts in early pregnancy. Am. J. Obstet. Gynecol. 1974, 118, 73–76. [Google Scholar] [CrossRef]
- Sontag, L.W.; Richards, T.W. Studies in fetal behavior: I. Fetal heart rate as a behavioral indicator. Monographs Soc. Res. Child Dev. 1938, 3. [Google Scholar] [CrossRef]
- Hooker, D. The Prenatal Origin of Behavior; University of Kansas Press: Lawrence, KS, USA, 1952. [Google Scholar]
- Kollmann, J. Die Körperform menschlicher normaler und pathologischer Embryonen. Arch. Anat. Physiol. Anat. Abth. 1889, 105–138. [Google Scholar]
- von Jaschke, R.T. Lehrbuch der Geburtshilfe, 4th ed.; Springer: Berlin/Heidelberg, Germany, 1935; p. 56. [Google Scholar]
- Hackelöer, B.J.; Hansmann, M. Ultraschalldiagnostik in der Frühschwangerschaft (Ultrasonic diagnosis in early—pregnancy). Gynäkologe 1976, 9, 108–122. [Google Scholar]
- Schillinger, H. Detection of heart action and movements of the human embryo by ultrasonic time motion techniques. Europ. J. Obstet. Gynec. Reprod. Biol. 1976, 6, 333–338. [Google Scholar] [CrossRef]
- Resch, B.A.; Papp, J.G.; Herczeg, J. Normalwerte der fetalen Herzfrequenz in der 5. bis 18. Schwangerschaftswoche (in vivo und in vitro Untersuchungen). Zentralbl. Gynäkol. 1979, 101, 29–34. [Google Scholar] [PubMed]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Oxygen and the development of the human placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef] [PubMed]

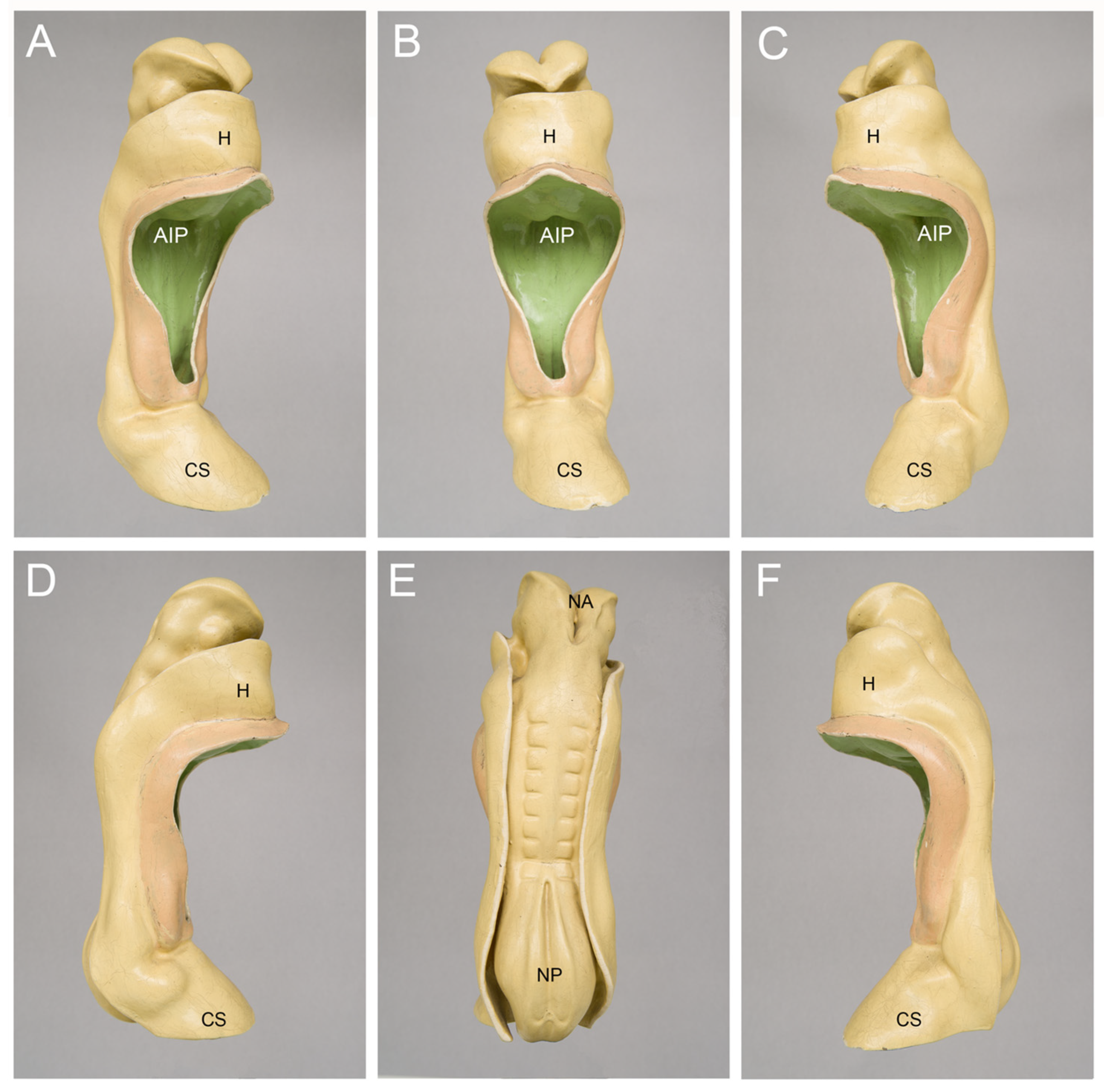

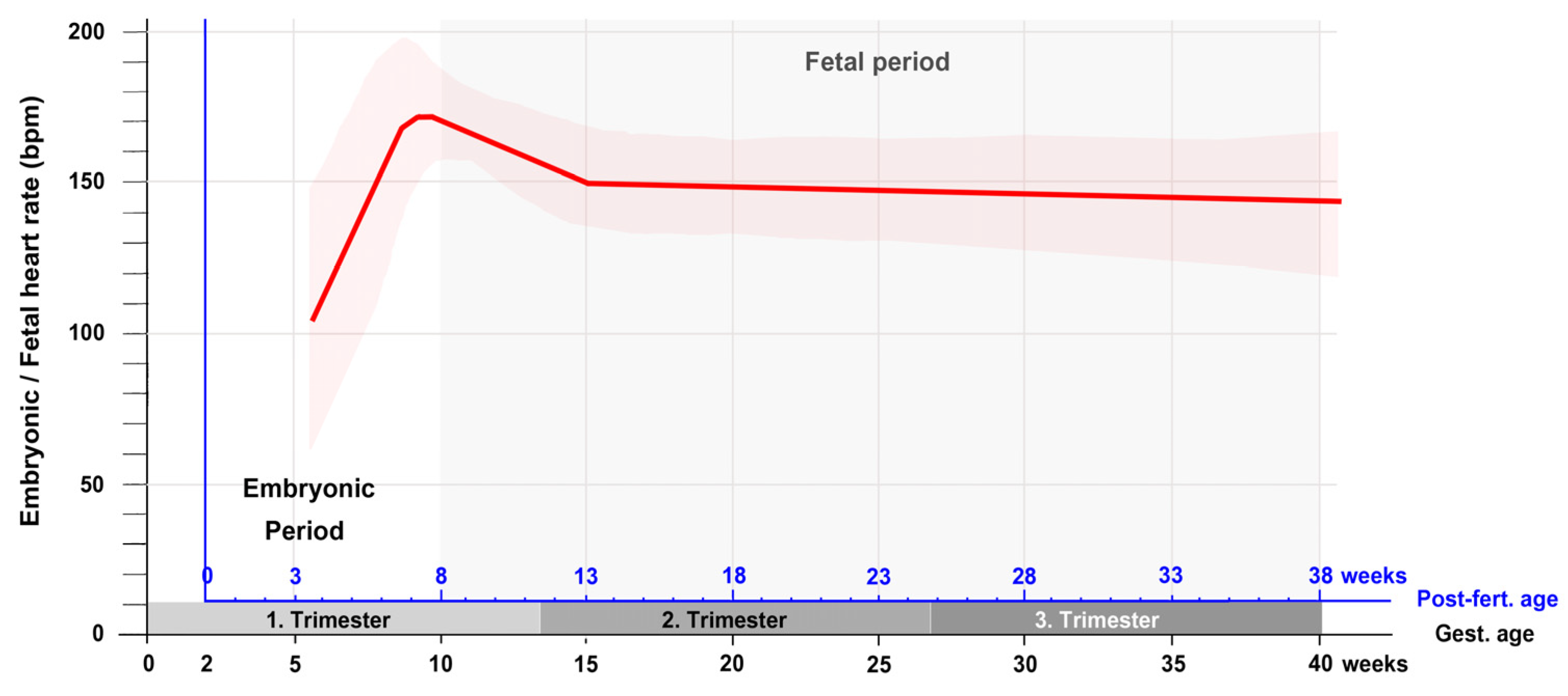
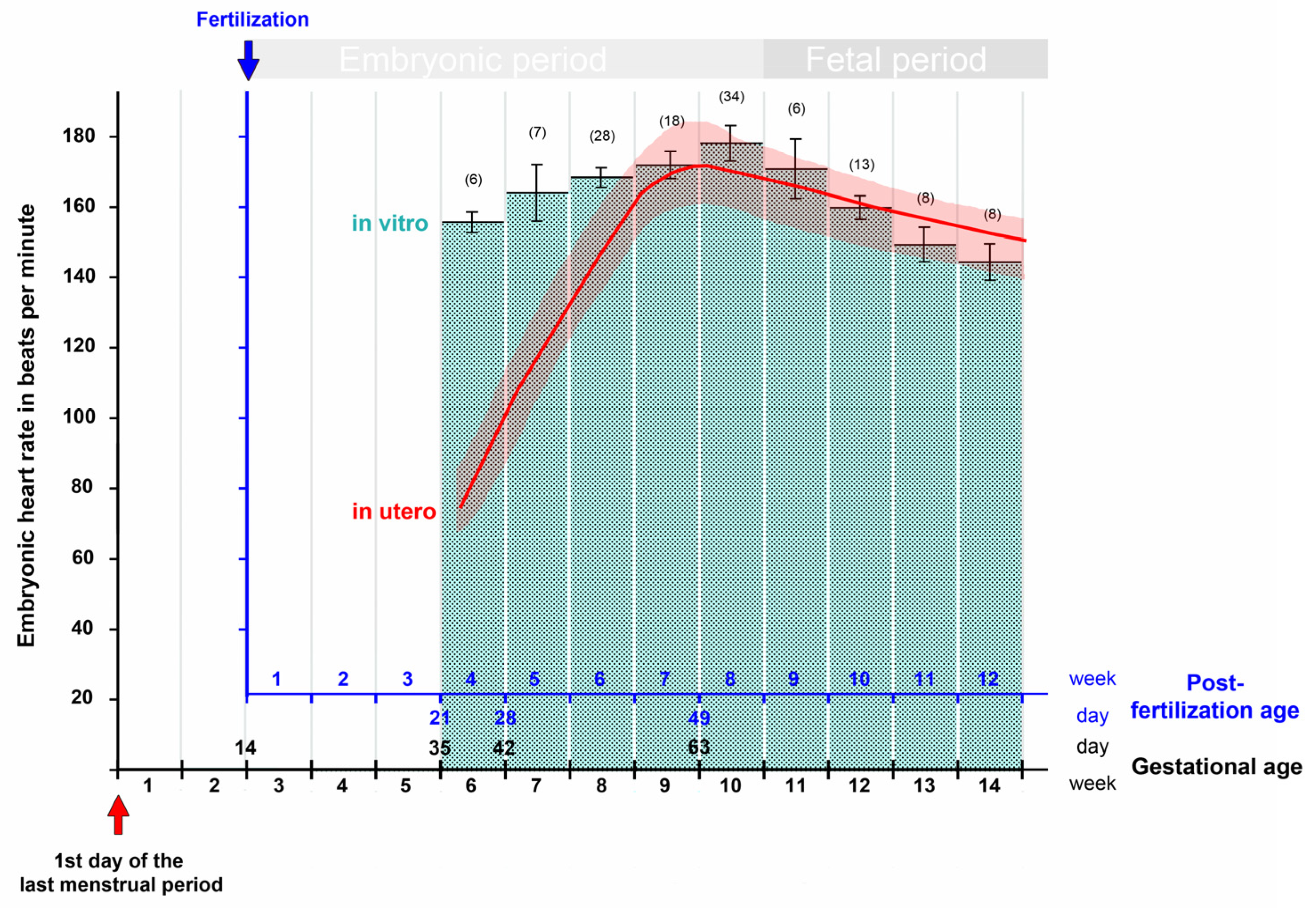
| Post-Fertil. Age (Days) | Estimated Gest. Age (Days) | Greatest Length (mm) | Heart Rate (bpm) | Reference |
|---|---|---|---|---|
| 25–30 | 39–44 | 2 | 65 | [24,25] |
| 26–32 | 40–46 | 4 | 80 | [26] |
| 28 | 42 | 6 | ~130 | [27] |
| 27–32 | 41–46 | 2 | No info. | [28] |
| 27–34 | 41–48 | 4 | 120 | [29] |
| 23 | 37 | 3 | 80 | [30] |
| 20–23 | 34–37 | 1.6 | 89–99 | [31,32] |
| 24 | 38 | No info. | 87 | [33] |
| Data/Methods Used for the Estimation of the Post-Fertil. Ages | Proposed Post-Fertil. Ages of CS-10 Embryos |
|---|---|
| Menstrual history/calculations according to the Reichert-His convention (~1880–1910) | 13–14 days [48,52,53] |
| Menstrual history/calculations according to concepts of intermenstrual ovulation (~1900–1940) | 21–24 days [67] 20–22 days [68] |
| Morphology-based staging/comparison with macaque embryos of known ovulation age (since~1940) | 21–23 days [9,14] |
| Menstrual history/calculations according to the concept of mid-cycle ovulation (since~1940) | 20–23 days [63] 26–28 days [64] |
| Greatest length of human embryos from the Carnegie Collection/ comparison with the embryonic growth curves obtained from first trimester sonographic examinations (since 2010) | 28–30 days [47] |
| Report and “Name of Embryo” | Number of Somite Pairs | Greatest Length (mm) | Proposed Post-Fertil. Age (Days) | Gest. Age (Days) | Heart Activity |
|---|---|---|---|---|---|
| [49] “SR” | 6–7 | 2.2 | ~14 RH | - | No info. |
| [89] von Spee | 7 | 2.69 | 14 RH | 42 | No info. |
| [17,18] Eternod “Du Ga” | 7 | 2.11 | 13–14 RH | - | No info. |
| [90] “Klb” | 5–6 | 1.8 | 12–14 RH | - | No info. |
| [67] Dandy | 7 | 2 | 13–14 RH 24 IM | 43 | No info. |
| [84] “H 98“ | 8 | 1.27 | 38 | No info. | |
| [68] “H87” | 7–8 | 2 | 21 IM | - | No info. |
| [91] Veit & Esch | 8 | 2.5 | - | 40 | No info. |
| [92] Payne | 7 | 2.17 | - | - | No info. |
| [93] “H279” | 4 | 2.5 | - | 28 or 35 | No info. |
| [93] “H197” | 12 | 2.08 | - | 44 | No info. |
| [93] “H392” | 11 | 3.6 | - | 45 | No info. |
| [94] Sternberg | 4 | 2.3 | - | - | No info. |
| [95] “Da2” | 10 | - | - | - | No info. |
| [85] “H10” | 10 | 3 | - | 42 | No info. |
| [96]; “Bi II” | 4–5 | - | - | - | No info. |
| [96]; “Bi III” | 4–5 | - | - | - | No info. |
| [96,97] “Bi XI” | 10 | 2.2 | - | - | No info. |
| [98] Politzer | 7 | ~3 | - | - | No info. |
| [99] West | 8 | - | - | - | No info. |
| [100] “H.Schm.2.” | 10 | 2.4 | 20 C | 29 | No info. |
| [101,102] Litzenberg | 12 | - | - | 47 | No info. |
| [103] Orts Llorca | 4 | - | - | - | No info. |
| [86] Baxter & Boyd | 10 | - | 28 MC | 42 | No info. |
| [104] Arey & Henderson | 6 | - | - | - | No info. |
| [87] Holmdahl | 11 | 1.7 | - | 47 | No info. |
| [105] Streiter | 7 | 1.93 | 21–22 MC | - | No info. |
| [106] Schenk | 5 | - | No info. | ||
| [14] “2795” | 4–5 | 2 | 22 ± 1 St | - | No info. |
| [14] “H1404” | 7–8 | 2.83 | 22 ± 1 St | - | No info. |
| [14] “8244” | 6 | 1.55 | 22 ± 1 St | - | No info. |
| [14] “H637” | 12 | - | 22 ± 1 St | - | No info. |
| [107] “No. 103” | 5 | - | - | - | No info. |
| [107] “No. 101” | 8 | - | - | - | No info. |
| [88] “No. 5074” | 12 | - | - | - | No info. |
| Name of Collection, Report | Number of Embryos | * Greatest Length or CS of Embryos | Number of CS-10 Embryos | Greatest Length of CS-10 Embryos (mm) | Proposed Post-Fertil. Age of CS-10 Embryos (Days) | Gestational Age of CS-10 Embryos (Days) | Number of Embryos with HA | * Length or CS of Embryos with HA |
|---|---|---|---|---|---|---|---|---|
| Carnegie-Coll., [108] | 533 | * 1–220 mm | ? | - | - | - | 22 | * 11–96 mm |
| Carnegie-Coll., [109] | 2500 | - | ? | - | - | - | 1 | * 21 mm |
| Carnegie-Coll., [115] | 483 | 6–23 | 11 | 2.16 (mean) | - | - | 0 | - |
| Kyoto-Coll. [64] | 1213 | 12–23 | 0 | - | - | - | 0 | - |
| Kyoto-Coll. [116] | 90 | 7–13 | 8 | 1.5–2.7 | 27 (mean) | 41 (mean) | 0 | - |
| Kyoto-Coll. [117] | 37 | 6–11 | 10 | - | 22–23 | - | 0 | - |
| Edinburgh-Coll., [118] | 310 | 7–23 | 13 | 2.9 ± 0.12 | - | 45.2 ± 1.2 | 0 | - |
| Newcastle-Coll., [119] | 60 | 10–22 | 1 | - | - | - | 0 | - |
| Carnegie-Coll., [120] | 494 | 2–23 | 20 | 2 (mean) | 27 (mean) | - | 0 | - |
| Carnegie-Coll., [47] | 407 | 1–23 | 13 | 1.5–3.6 | 28–30 | - | 0 | - |
| Study/Source of Specimens | Number of Specimens | Range of CS | Number of CS-10 Embryos | Greatest Length of CS-10 Embryos (mm) | Proposed Post-Fert. Age at CS-10 (Days) | Number of Embryos with HA | CS of Embryos with HA |
|---|---|---|---|---|---|---|---|
| [121]/Carnegie-Coll. | 58 | 10–23, + 4 fetuses | 3 | 2–2.5 | - | 0 | - |
| [10]/Carnegie-Coll. + published cases | 11 | 9–11 | 7 | 1.8–3.09 | - | 0 | - |
| [70]/Carnegie-Coll. + own specimens | 24 | 9–15 | 9 | - | - | 0 | - |
| [122]/Carnegie-Coll. | 54 | 9–23 | 4 | - | - | 0 | - |
| [111]/Carnegie-Coll. | 351 | 9–23 | 12 | 2.2 ± 0.8 | - | 10 | 17–22 |
| [123]/own specimens | 11 | 10–16 | 2 | - | 22–23 | 0 | - |
| [124]/own specimens | 29 | 10–16 | 3 | - | 22 ± 1 | 0 | - |
| [125]/own specimens | 300 | 10–16 | ? | - | - | 0 | - |
| [126]/own specimens | ? | 10–16 | ? | - | - | 0 | - |
| Report | Number of Specimens | Greatest Length of Embryos/ Fetuses (mm) | CS | Proposed Post-Fert. Age | Gest. Age (Weeks) | Kind of Specimens | Kind of Study | Spontaneous Heart Rate (bpm] |
|---|---|---|---|---|---|---|---|---|
| [138] | 10 | - | - | - | 4.5–10 | myocardial cells | Electro-physiol. | 60–150 37 °C |
| [147] | 165 | - | - | - | 5–15 | hearts | Heart rate recordings | 157–180 37 °C |
| [131,132] | 1 | 2.5 | 11 | 14 days RH | 6 | embryo | Case report | 90 RT |
| [127] | 1 | - | 11 | 18–20 days | - | embryo | Case report | Occasional RT |
| [133] | 1 | 4 | 13 or 14 | 47–50 days | - | embryo | Case report | 70 RT |
| [135] | 5 | 6–60 | - | 2 weeks–2.5 months | - | 2 embryos + 3 fetuses | ECG | Occasional RT |
| [139] | 37 | - | - | - | 7–12 | hearts | Electro-physiol. | 50–132 37 °C |
| [136] | 1 | 23 | - | 7.5 weeks | 9.5 | embryo | ECG | 40–80 |
| [145] | 2 | 30 + 30 | - | - | 9 + 10 | hearts | Experiments | 140 37 °C |
| [134] | 11 | - | - | - | 9.5–25 | fetuses | ECG | 48–100 RT |
| [137] | 11 | 55–170 | - | 10–18 | fetuses | ECG | No info. | |
| [129] | 1 | 75 | - | 13 | fetus | Case report | No info. | |
| [141] | 5 | 100–165 | - | - | 12–16 | hearts | Electro-physiol. | No info. |
| [140] | 14 | - | - | - | 12–22 | hearts | Electro-physiol. | 142 37 °C |
| [146] | 17 | - | - | - | 12–22 | hearts | Experiments | 142 37 °C |
| [143] | 1 | 100 | - | 13 weeks | - | heart | Experiments | 157 37 °C |
| [128] | 1 | 100 | - | - | 16 | fetus | Case report | No info. |
| [130] | 1 | - | - | - | 15 | fetus | Case report | 30 RT |
| [144] | 9 | - | - | - | 16–24 | hearts | Experiments | No info. |
| [142] | 2 | - | - | - | 24 | hearts | Experiments | 40 + 100 37 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Männer, J. When Does the Human Embryonic Heart Start Beating? A Review of Contemporary and Historical Sources of Knowledge about the Onset of Blood Circulation in Man. J. Cardiovasc. Dev. Dis. 2022, 9, 187. https://doi.org/10.3390/jcdd9060187
Männer J. When Does the Human Embryonic Heart Start Beating? A Review of Contemporary and Historical Sources of Knowledge about the Onset of Blood Circulation in Man. Journal of Cardiovascular Development and Disease. 2022; 9(6):187. https://doi.org/10.3390/jcdd9060187
Chicago/Turabian StyleMänner, Jörg. 2022. "When Does the Human Embryonic Heart Start Beating? A Review of Contemporary and Historical Sources of Knowledge about the Onset of Blood Circulation in Man" Journal of Cardiovascular Development and Disease 9, no. 6: 187. https://doi.org/10.3390/jcdd9060187
APA StyleMänner, J. (2022). When Does the Human Embryonic Heart Start Beating? A Review of Contemporary and Historical Sources of Knowledge about the Onset of Blood Circulation in Man. Journal of Cardiovascular Development and Disease, 9(6), 187. https://doi.org/10.3390/jcdd9060187





