Genetic Analysis of Candida albicans Filamentation by the Iron Chelator BPS Reveals a Role for a Conserved Kinase—WD40 Protein Pair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media and Chemicals
2.2. Plasmids and Strains
2.3. Growth Assays
2.4. Enrichment and Screening for Filamentation-Defective Mutants
2.5. Identification of Transposon Insertion Sites
2.6. Microscopy
2.7. Protein Analysis
3. Results
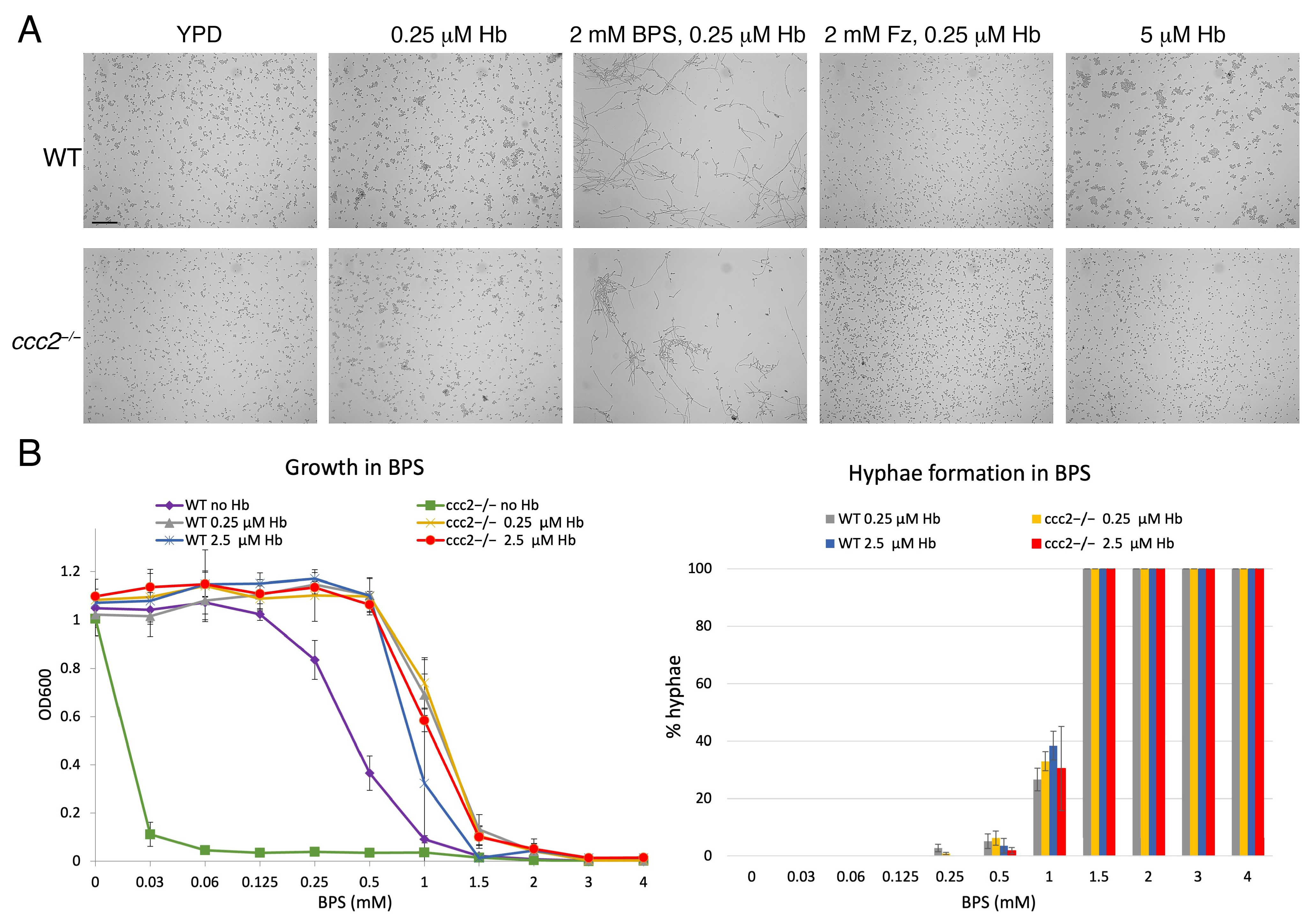
3.1. BPS Induces Filamentous Growth
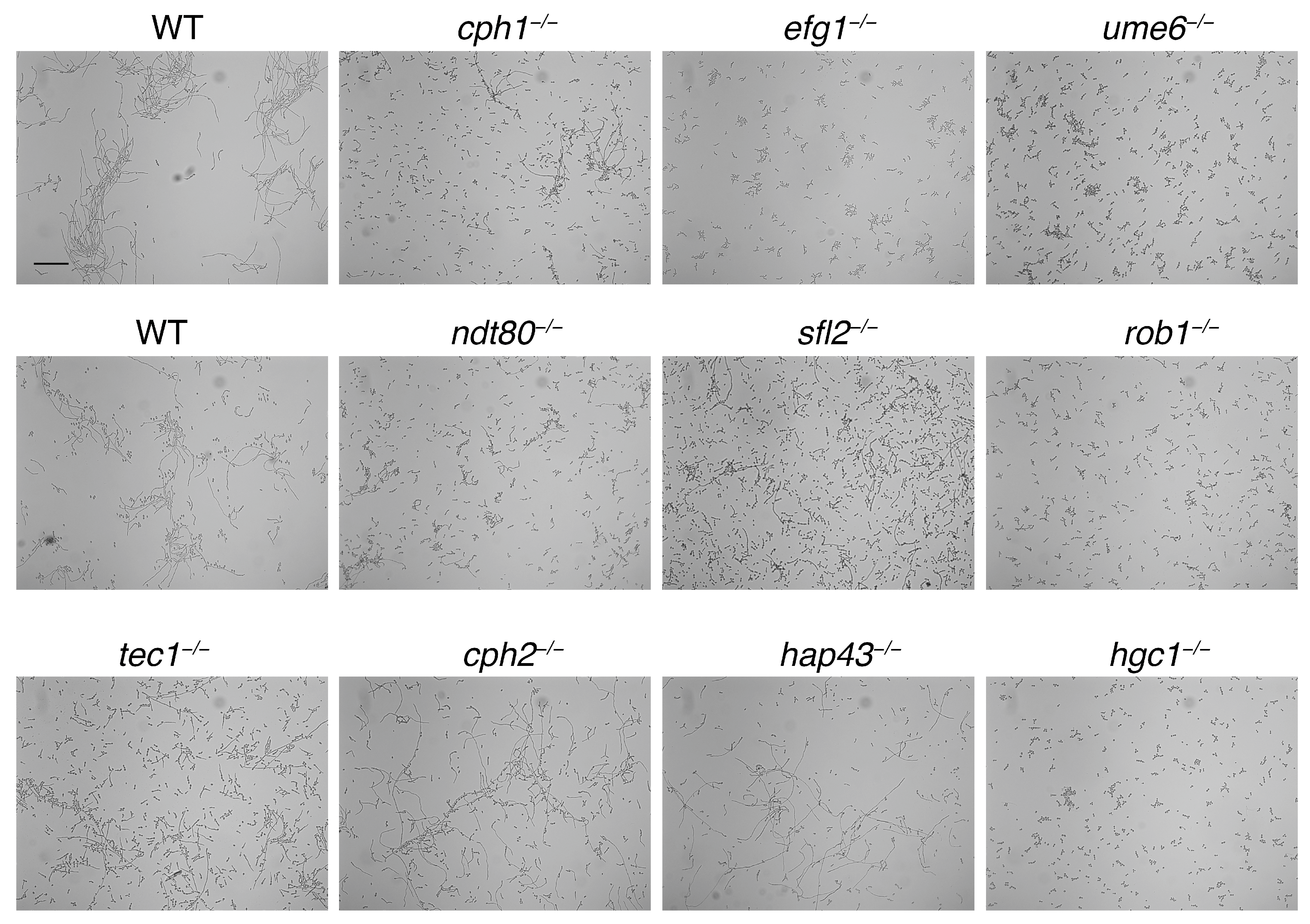
3.2. Identification of Factors Required for BPS-Induced Hyphal Morphogenesis
3.2.1. Screening of Transcription Factor Mutants
3.2.2. Unbiased Selection for Non-Filamenting Mutants
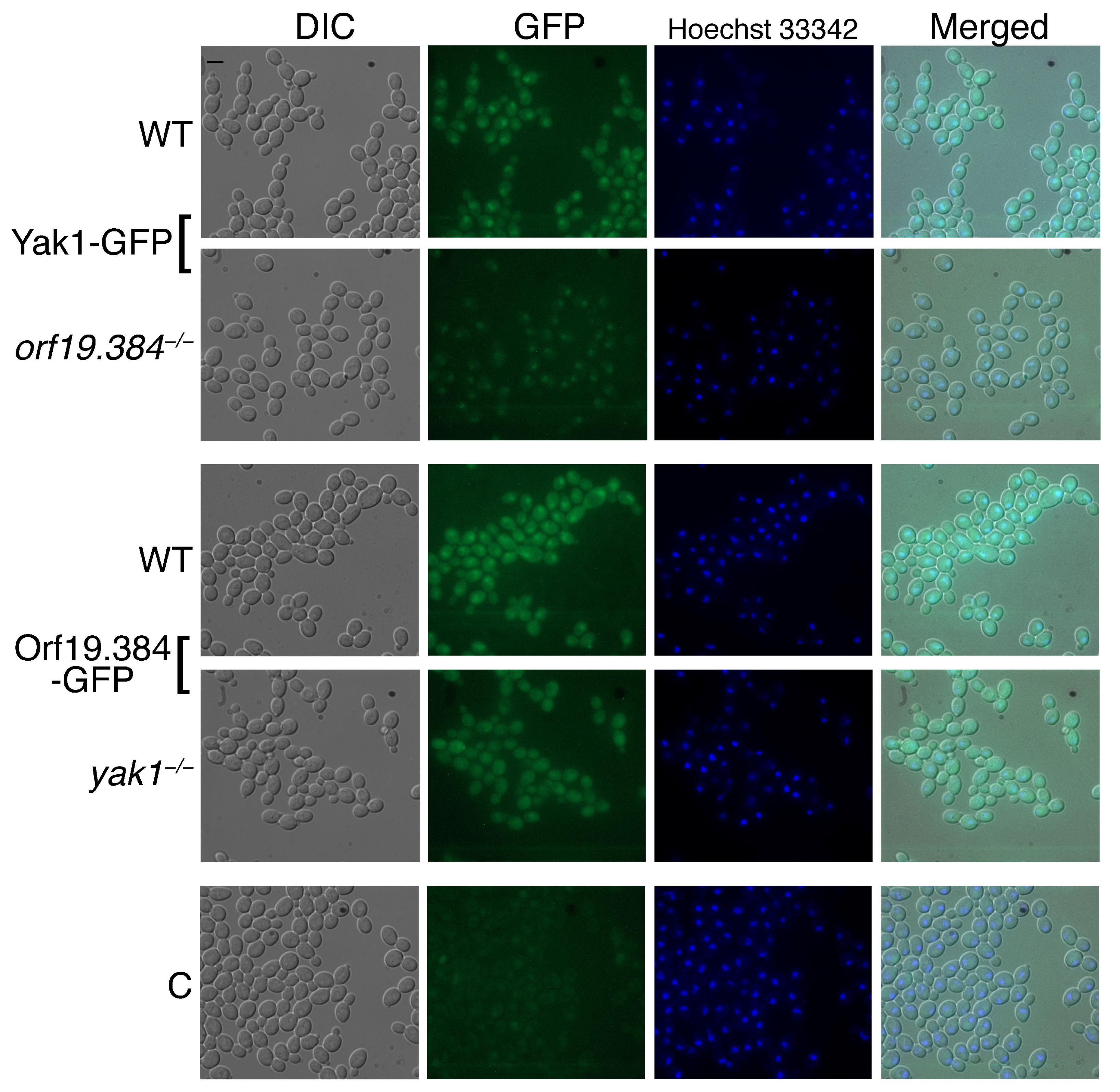
3.3. Yak1 and Orf19.384
3.3.1. Confirmation of the Phenotypes in the Standard Diploid Background
3.3.2. Subcellular Localization
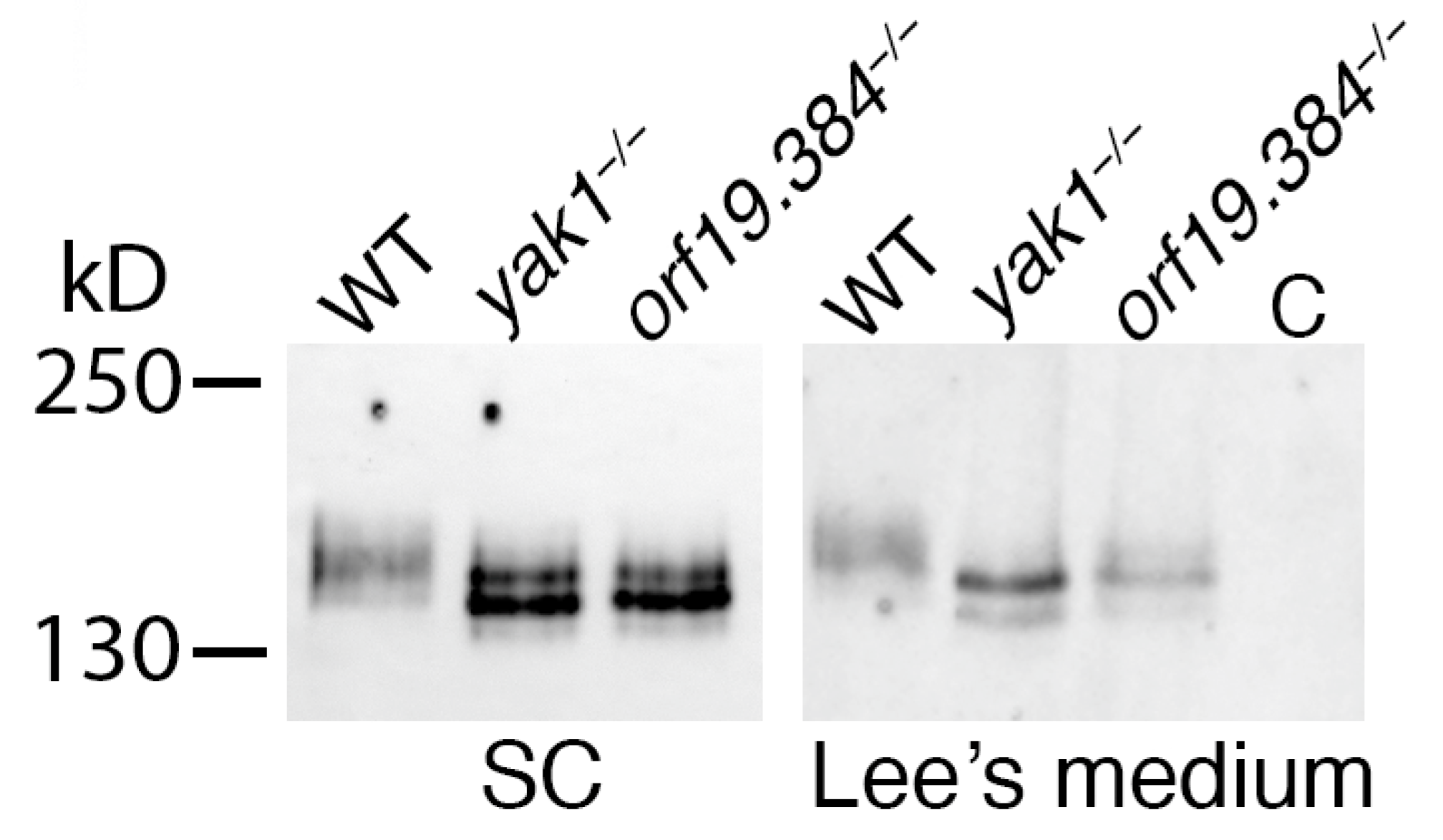
3.3.3. Sfl1 Is a Candidate Substrate of Yak1/Orf19.384
4. Discussion
4.1. BPS and Filamentation
4.2. Mutants Defective in BPS-Dependent Filamentation
4.3. Yak1 and Orf19.384
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderone, R.A.; Clancy, C.J. Candida and Candidiasis, 2nd ed.; Calderone, R.A., Clancy, C.J., Eds.; ASM press: Washington, DC, USA, 2012; ISBN 9781555815394. [Google Scholar]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Veri, A.O.; Ketela, T.; Jiang, B.; Roemer, T.; Cowen, L.E. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat. Commun. 2015, 6, 6741. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wachtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti-Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Romo, J.A.; Pierce, C.G.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Targeting Candida albicans filamentation for antifungal drug development. Virulence 2017, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Kornitzer, D. Regulation of candida albicans hyphal morphogenesis by endogenous signals. J. Fungi 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; D’Enfert, C.; Znaidi, S.; Bachellier-Bassi, S. From genes to networks: The regulatory circuitry controlling candida albicans morphogenesis. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 422, pp. 61–99. [Google Scholar]
- Arkowitz, R.A.; Bassilana, M. Recent advances in understanding Candida albicans hyphal growth. F1000Research 2019, 8, 700. [Google Scholar] [CrossRef]
- Trinder, P. The improved determination of iron in serum. J. Clin. Pathol. 1956, 9, 170–172. [Google Scholar] [CrossRef]
- Berman, J.; Sudbery, P.E. Candida Albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002, 3, 918–930. [Google Scholar] [CrossRef]
- Homann, O.R.; Dea, J.; Noble, S.M.; Johnson, A.D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009, 5, e1000783. [Google Scholar] [CrossRef]
- Roemer, T.; Jiang, B.; Davison, J.; Ketela, T.; Veillette, K.; Breton, A.; Tandia, F.; Linteau, A.; Sillaots, S.; Marta, C.; et al. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 2003, 50, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Hickman, M.A.; Zeng, G.; Forche, A.; Hirakawa, M.P.; Abbey, D.; Harrison, B.D.; Wang, Y.M.; Su, C.H.; Bennett, R.J.; Wang, Y.; et al. The “obligate diploid” Candida albicans forms mating-competent haploids. Nature 2013, 494, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chow, E.W.L.; Wang, H.; Xu, X.; Cai, C.; Song, Y.; Wang, J.; Wang, Y. LncRNA DINOR is a virulence factor and global regulator of stress responses in Candida auris. Nat. Microbiol. 2021, 6, 842–851. [Google Scholar] [CrossRef]
- Segal, E.S.; Gritsenko, V.; Levitan, A.; Yadav, B.; Dror, N.; Steenwyk, J.L.; Silberberg, Y.; Mielich, K.; Rokas, A.; Gow, N.A.R.; et al. Gene essentiality analyzed by in vivo transposon mutagenesis and machine learning in a stable haploid isolate of candida albicans. MBio 2018, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mielich, K.; Shtifman-Segal, E.; Golz, J.C.; Zeng, G.; Wang, Y.; Berman, J.; Kunze, R. Maize transposable elements Ac/Ds as insertion mutagenesis tools in Candida albicans. G3 Genes Genomes Genet. 2018, 8, 1139–1145. [Google Scholar] [CrossRef]
- Atir-Lande, A.; Gildor, T.; Kornitzer, D. Role for the SCFCDC4 ubiquitin ligase in Candida albicans morphogenesis. Mol. Biol. Cell 2005, 16, 2772–2785. [Google Scholar] [CrossRef]
- Avitan, D. A Novel Inducer of Hyphal Morphogenesis in Candida Albicans; Technion-Israel Institute of Technology: Haifa, Israel, 2021. [Google Scholar]
- Zhang, C.; Konopka, J.B. A photostable green fluorescent protein variant for analysis of protein localization in Candida albicans. Eukaryot. Cell 2010, 9, 224–226. [Google Scholar] [CrossRef]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [CrossRef]
- Liu, H.; Kohler, J.; Fink, G.R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 1994, 266, 1723–1726. [Google Scholar] [CrossRef]
- Lo, H.-J.; Kohler, J.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–950. [Google Scholar] [CrossRef]
- Noble, S.M.; Johnson, A.D. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 2005, 4, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, S.; Pinsky, M.; Weissman, Z.; Kornitzer, D. Regulation of the Candida albicans Hypha-Inducing Transcription Factor Ume6 by the CDK1 Cyclins Cln3 and Hgc1. mSphere 2017, 2, e00248-16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004, 23, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformaion of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, S.; Li, J.; Zheng, B.; Bao, M.; Ning, G. Fusion primer and nested integrated PCR (FPNI-PCR): A new high-efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotechnol. 2011, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Weissman, Z.; Kornitzer, D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol. Microbiol. 2004, 53, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Weissman, Z.; Shemer, R.; Conibear, E.; Kornitzer, D. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans. Mol. Microbiol. 2008, 69, 201–217. [Google Scholar] [CrossRef]
- Casanova, M.; Cervera, A.M.; Gozalbo, D.; Martinez, J.P. Hemin induces germ tube formation in Candida albicans. Infect. Immun. 1997, 65, 4360–4364. [Google Scholar] [CrossRef]
- Santos, R.; Buisson, N.; Knight, S.; Dancis, A.; Camadro, J.-M.; Lesuisse, E. Haemin uptake and use as an iron source by Candida albicans: Role of CaHMX1-encoded haem oxygenase. Microbiology 2003, 149, 579–588. [Google Scholar] [CrossRef]
- Pendrak, M.L.; Roberts, D.D. Hemoglobin is an effective inducer of hyphal differentiation in Candida albicans. Med. Mycol. 2007, 45, 61–71. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine-A New Spectrophotometric Reagent for Iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Weissman, Z.; Shemer, R.; Kornitzer, D. Deletion of the copper transporter CaCCC2 reveals two distinct pathways for iron acquisition in Candida albicans. Mol. Microbiol. 2002, 44, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Ardon, O.; Bussey, H.; Philpott, C.; Ward, D.M.; Davis-Kaplan, S.; Verroneau, S.; Jiang, B.; Kaplan, J. Identification of a Candida albicans ferrichrome transporter and its characterization by expression in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 43049–43055. [Google Scholar] [CrossRef]
- Villa, S.; Hamideh, M.; Weinstock, A.; Qasim, M.N.; Hazbun, T.R.; Sellam, A.; Hernday, A.D.; Thangamani, S. Transcriptional control of hyphal morphogenesis in Candida albicans. FEMS Yeast Res. 2020, 20, 5. [Google Scholar] [CrossRef]
- Cao, F.; Lane, S.; Raniga, P.P.; Lu, Y.; Zhou, Z.; Ramon, K.; Chen, J.; Liu, H. The Flo8 Transcription Factor Is Essential for Hyphal Development and Virulence in Candida albicans. Mol. Biol. Cell 2006, 17, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Joost, H.G. Structural and Functional Characteristics of Dyrk, a Novel Subfamily of Protein Kinases with Dual Specificity. Prog. Nucleic Acid Res. Mol. Biol. 1998, 62, 1–17. [Google Scholar] [CrossRef]
- Aranda, S.; Laguna, A.; de la Luna, S. DYRK family of protein kinases: Evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011, 25, 449–462. [Google Scholar] [CrossRef]
- Goyard, S.; Knechtle, P.; Chauvel, M.; Mallet, A.; Prévost, M.-C.; Proux, C.; Coppée, J.-Y.; Schwarz, P.; Dromer, F.; Park, H.; et al. The Yak1 Kinase Is Involved in the Initiation and Maintenance of Hyphal Growth in Candida albicans. Mol. Biol. Cell 2008, 19, 2251–2266. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- De Vetten, N.; Quattrocchio, F.; Mol, J.; Koes, R. The an11 locus controlling flower pigmentation in petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Genes Dev. 1997, 11, 1422–1434. [Google Scholar] [CrossRef]
- Nissen, R.M.; Amsterdam, A.; Hopkins, N. A zebrafish screen for craniofacial mutants identifies wdr68 as a highly conserved gene required for endothelin-1 expression. BMC Dev. Biol. 2006, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Arias, E.E.; Chen, J.; Harper, J.W.; Walter, J.C. A Family of Diverse Cul4-Ddb1-Interacting Proteins Includes Cdt2, which Is Required for S Phase Destruction of the Replication Factor Cdt1. Mol. Cell 2006, 23, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nishida, E. DYRK1A binds to an evolutionarily conserved WD40-repeat protein WDR68 and induces its nuclear translocation. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Yousefelahiyeh, M.; Xu, J.; Alvarado, E.; Yu, Y.; Salven, D.; Nissen, R.M. DCAF7/WDR68 is required for normal levels of DYRK1A and DYRK1B. PLoS ONE 2018, 13, e0207779. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, G.; Kovshilovsky, M.; Yen, D.; Mohanty, A.; Mohanty, S.; Nee, A.; Nissen, R.M. The zebrafish dyrk1b gene is important for endoderm formation. Genesis 2010, 48, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Himpel, S.; Tegge, W.; Frank, R.; Leder, S.; Joost, H.G.; Becker, W. Specificity determinants of substrate recognition by the protein kinase DYRK1A. J. Biol. Chem. 2000, 275, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, C.; Mao, X.; Cao, F.; Chen, J. Roles of Candida albicans Sfl1 in hyphal development. Eukaryot. Cell 2007, 6, 2112–2121. [Google Scholar] [CrossRef]
- Znaidi, S.; Nesseir, A.; Chauvel, M.; Rossignol, T.; d’Enfert, C. A Comprehensive Functional Portrait of Two Heat Shock Factor-Type Transcriptional Regulators Involved in Candida albicans Morphogenesis and Virulence. PLOS Pathog. 2013, 9, e1003519. [Google Scholar] [CrossRef]
- Moors, M.A.; Stull, T.L.; Blank, K.J.; Buckley, H.R.; Mosser, D.M. A role for complement receptor-like molecules in iron acquisition by Candida albicans. J. Exp. Med. 1992, 175, 1643–1651. [Google Scholar] [CrossRef]
- Hameed, S.; Prasad, T.; Banerjee, D.; Chandra, A.; Mukhopadhyay, C.K.; Goswami, S.K.; Lattif, A.A.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A.; et al. Iron deprivation induces EFG1-mediated hyphal development in Candida albicans without affecting biofilm formation. FEMS Yeast Res. 2008, 8, 744–755. [Google Scholar] [CrossRef]
- van Wijlick, L.; Znaidi, S.; Hernández-Cervantes, A.; Basso, V.; Bachellier-Bassi, S.; d’Enfert, C. Functional Portrait of Irf1 (Orf19.217), a Regulator of Morphogenesis and Iron Homeostasis in Candida albicans. Front. Cell. Infect. Microbiol. 2022, 12, 960884. [Google Scholar] [CrossRef] [PubMed]
- Stoldt, V.R.; Sonneborn, A.; Leuker, C.E.; Ernst, J.F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997, 16, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Sellam, A.; Askew, C.; Epp, E.; Tebbji, F.; Mullick, A.; Whiteway, M.; Nantel, A. Role of transcription factor CaNdt80p in cell separation, hyphal growth, and virulence in Candida albicans. Eukaryot. Cell 2010, 9, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A Recently Evolved Transcriptional Network Controls Biofilm Development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Mancera, E.; Nocedal, I.; Hammel, S.; Gulati, M.; Mitchell, K.F.; Andes, D.R.; Nobile, C.J.; Butler, G.; Johnson, A.D. Evolution of the complex transcription network controlling biofilm formation in candida species. Elife 2021, 10, e64682. [Google Scholar] [CrossRef] [PubMed]
- Spiering, M.J.; Moran, G.P.; Chauvel, M.; MacCallum, D.M.; Higgins, J.; Hokamp, K.; Yeomans, T.; d’Enfert, C.; Coleman, D.C.; Sullivan, D.J. Comparative transcript profiling of Candida albicans and Candida dubliniensis identifies SFL2, a C. albicans gene required for virulence in a reconstituted epithelial infection model. Eukaryot. Cell 2010, 9, 251–265. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Chen, J. Candida albicans Sfl2, a temperature-induced transcriptional regulator, is required for virulence in a murine gastrointestinal infection model. FEMS Yeast Res. 2011, 11, 209–222. [Google Scholar] [CrossRef]
- Fox, E.P.; Bui, C.K.; Nett, J.E.; Hartooni, N.; Mui, M.C.; Andes, D.R.; Nobile, C.J.; Johnson, A.D. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015, 96, 1226–1239. [Google Scholar] [CrossRef]
- Lee, P.; Cho, B.R.; Joo, H.S.; Hahn, J.S. Yeast Yak1 kinase, a bridge between PKA and stress-responsive transcription factors, Hsf1 and Msn2/Msn4. Mol. Microbiol. 2008, 70, 882–895. [Google Scholar] [CrossRef]
- MacAlpine, J.; Liu, Z.; Hossain, S.; Whitesell, L.; Robbins, N.; Cowen, L.E. DYRK-family kinases regulate Candida albicans morphogenesis and virulence through the Ras1/PKA pathway. MBio 2023, 14, e02183-23. [Google Scholar] [CrossRef]
- MacAlpine, J.; Daniel-Ivad, M.; Liu, Z.; Yano, J.; Revie, N.M.; Todd, R.T.; Stogios, P.J.; Sanchez, H.; O’Meara, T.R.; Tompkins, T.A.; et al. A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 2021, 12, 6151. [Google Scholar] [CrossRef] [PubMed]
- Guedj, F.; Pereira, P.L.; Najas, S.; Barallobre, M.J.; Chabert, C.; Souchet, B.; Sebrie, C.; Verney, C.; Herault, Y.; Arbones, M.; et al. DYRK1A: A master regulatory protein controlling brain growth. Neurobiol. Dis. 2012, 46, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, F.; Zhu, X.R.; Kaltenbach, E.; Ackermann, A.; Baumann, A.; Canal, I.; Heisenberg, M.; Fischbach, K.F.; Pongs, O. minibrain: A new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron 1995, 14, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Glenewinkel, F.; Cohen, M.J.; King, C.R.; Kaspar, S.; Bamberg-Lemper, S.; Mymryk, J.S.; Becker, W. The adaptor protein DCAF7 mediates the interaction of the adenovirus E1A oncoprotein with the protein kinases DYRK1A and HIPK2. Sci. Rep. 2016, 6, 28241. [Google Scholar] [CrossRef]
- Ho, Y.; Gruhler, A.; Heilbut, A.; Bader, G.D.; Moore, L.; Adams, S.L.; Millar, A.; Taylor, P.; Bennett, K.; Boutilier, K.; et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 2002, 415, 180–183. [Google Scholar] [CrossRef]
- Breitkreutz, A.; Choi, H.; Sharom, J.R.; Boucher, L.; Neduva, V.; Larsen, B.; Lin, Z.Y.; Breitkreutz, B.J.; Stark, C.; Liu, G.; et al. A global protein kinase and phosphatase interaction network in yeast. Science 2010, 328, 1043–1046. [Google Scholar] [CrossRef]
- Bauer, J.; Wendland, J. Candida albicans Sfl1 suppresses flocculation and filamentation. Eukaryot. Cell 2007, 6, 1736–1744. [Google Scholar] [CrossRef]
- McCall, A.D.; Kumar, R.; Edgerton, M. Candida albicans Sfl1/Sfl2 regulatory network drives the formation of pathogenic microcolonies. PLoS Pathog. 2018, 14, e1007316. [Google Scholar] [CrossRef]
- Unoje, O.; Yang, M.; Lu, Y.; Su, C.; Liu, H. Linking Sfl1 Regulation of Hyphal Development to Stress Response Kinases in Candida albicans. mSphere 2020, 5, e00672-19. [Google Scholar] [CrossRef]

| Name | Genotype/Strain Number | Origin |
|---|---|---|
| Diploid strains | ||
| KC2 = CAI4 | ura3Δ::imm434/ura3Δ::imm434 | [21] |
| KC148 = JKC18 | ura3Δ::imm434/ura3Δ::imm434 cph1Δ/cph1Δ | [22] |
| KC149 = HLC52 | ura3Δ::imm434/ura3Δ::imm434 efg1Δ/efg1Δ | [23] |
| KC274 = SN148 | ura3Δ::imm434/ura3Δ::imm434, his1Δ/his1Δ, leu2Δ/leu2Δ, arg4Δ/arg4Δ | [24] |
| KC445 | ura3Δ::imm434/ura3Δ::imm434 ume6Δ::hisG/ume6Δ::hisG | [25] |
| KC532 | KC274 hgc1Δ::HIS1/hgc1Δ::ARG4 | [26] |
| KC1175 | KC274 ADE2/ade2::URA3 | This work |
| KC1336 | KC274 YAK1/yak1Δ::hisG-URA3-hisG | [19] |
| KC1337 | KC274 YAK1/yak1Δ::hisG | [19] |
| KC1338 | KC274 yak1Δ::hisG/yak1Δ::hisG-URA3-hisG | [19] |
| KC1339 | KC274 yak1Δ::hisG/yak1Δ::hisG | [19] |
| KC1340 | KC274 ORF19.384/orf19.384Δ::hisG-URA3-hisG | [19] |
| KC1341 | KC274 ORF19.384/orf19.384Δ::hisG | [19] |
| KC1363 | KC274 orf19.384Δ::hisG/orf19.384Δ::hisG | [19] |
| KC1381 | KC274 orf19.384Δ::hisG/orf19.384Δ::hisG | [19] |
| KC1543 | KC274 SFL1-13xMyc URA3 | This work |
| KC1544 | KC1339 SFL1-13xMyc URA3 | This work |
| KC1545 | KC1381 SFL1-13xMyc URA3 | This work |
| KC1669 | KC274 YAK1/YAK1-GFP URA3 | This work |
| KC1670 | KC274 ORF19.384/ORF19.384-GFP URA3 | This work |
| KC1671 | KC1339 ORF19.384/ORF19.384-GFP URA3 | This work |
| KC1672 | KC1381 YAK1/YAK1-GFP URA3 | This work |
| Haploid strains | ||
| KC1139 | ura3Δ ade2::Ds-NAT1 NEU5tl::AcTPase4x URA3 | [16] |
| KC1140 | ura3Δ | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinsky, M.; Kornitzer, D. Genetic Analysis of Candida albicans Filamentation by the Iron Chelator BPS Reveals a Role for a Conserved Kinase—WD40 Protein Pair. J. Fungi 2024, 10, 83. https://doi.org/10.3390/jof10010083
Pinsky M, Kornitzer D. Genetic Analysis of Candida albicans Filamentation by the Iron Chelator BPS Reveals a Role for a Conserved Kinase—WD40 Protein Pair. Journal of Fungi. 2024; 10(1):83. https://doi.org/10.3390/jof10010083
Chicago/Turabian StylePinsky, Mariel, and Daniel Kornitzer. 2024. "Genetic Analysis of Candida albicans Filamentation by the Iron Chelator BPS Reveals a Role for a Conserved Kinase—WD40 Protein Pair" Journal of Fungi 10, no. 1: 83. https://doi.org/10.3390/jof10010083
APA StylePinsky, M., & Kornitzer, D. (2024). Genetic Analysis of Candida albicans Filamentation by the Iron Chelator BPS Reveals a Role for a Conserved Kinase—WD40 Protein Pair. Journal of Fungi, 10(1), 83. https://doi.org/10.3390/jof10010083






