Comparative Analysis of Rhizosphere and Endosphere Fungal Communities in Healthy and Diseased Faba Bean Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection
2.3. DNA Extraction, Amplification, and Illumina MiSeq Sequencing
2.4. Bioinformatics and Statistical Analysis
3. Results
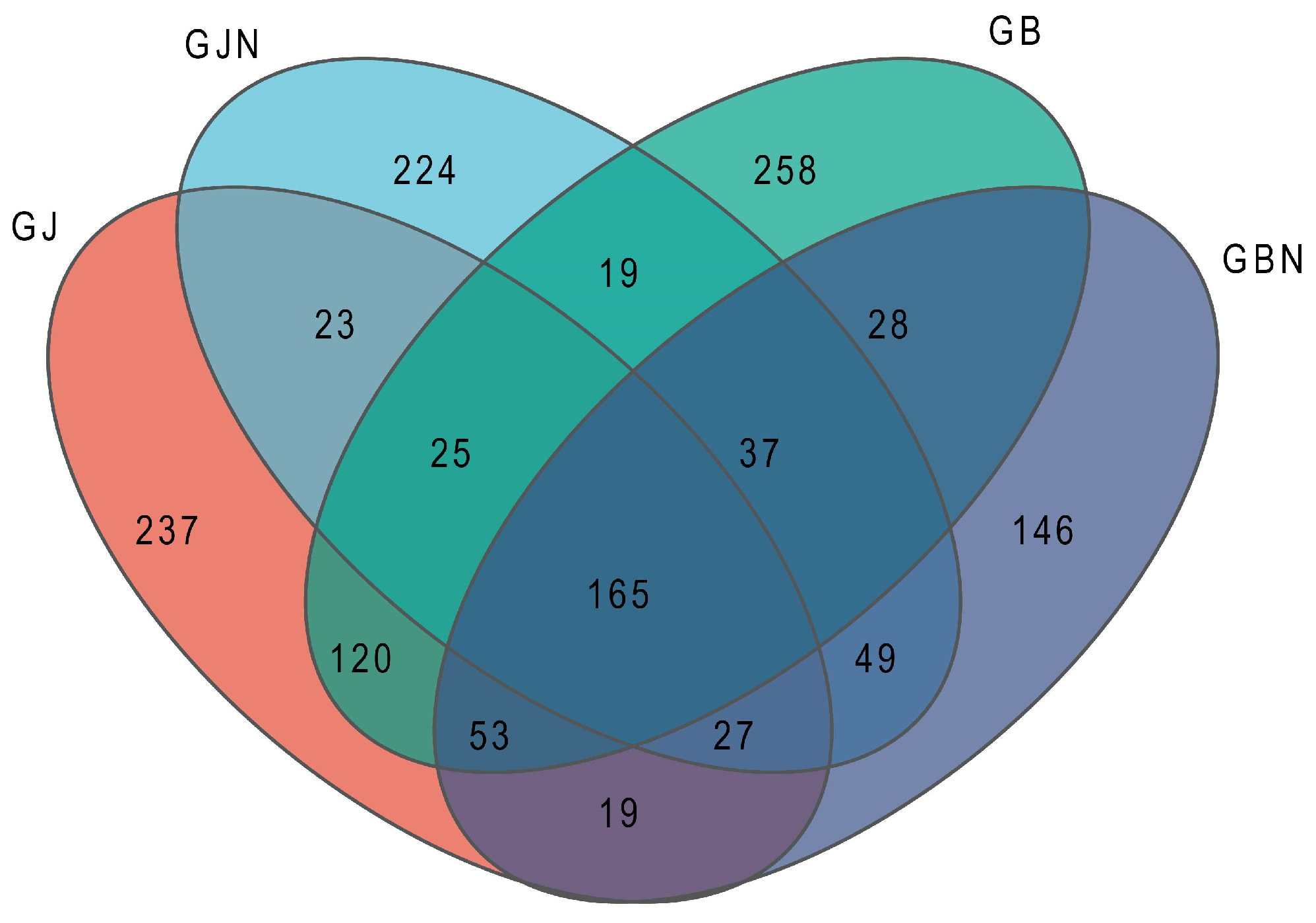
3.1. Analysis of Out Clusters
3.2. Alpha Diversity of Fungal Communities
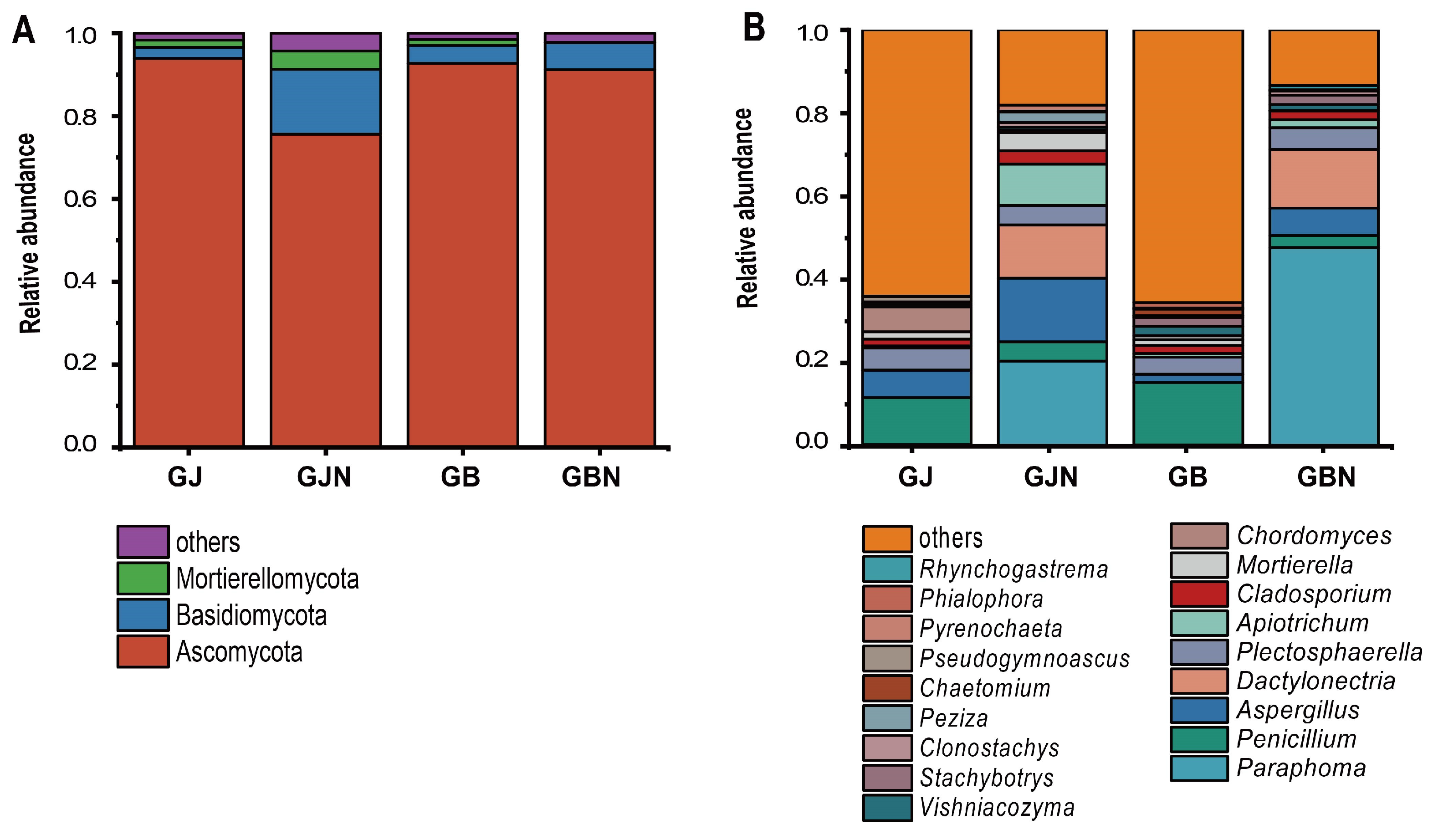
3.3. Variation in Fungal Community Composition
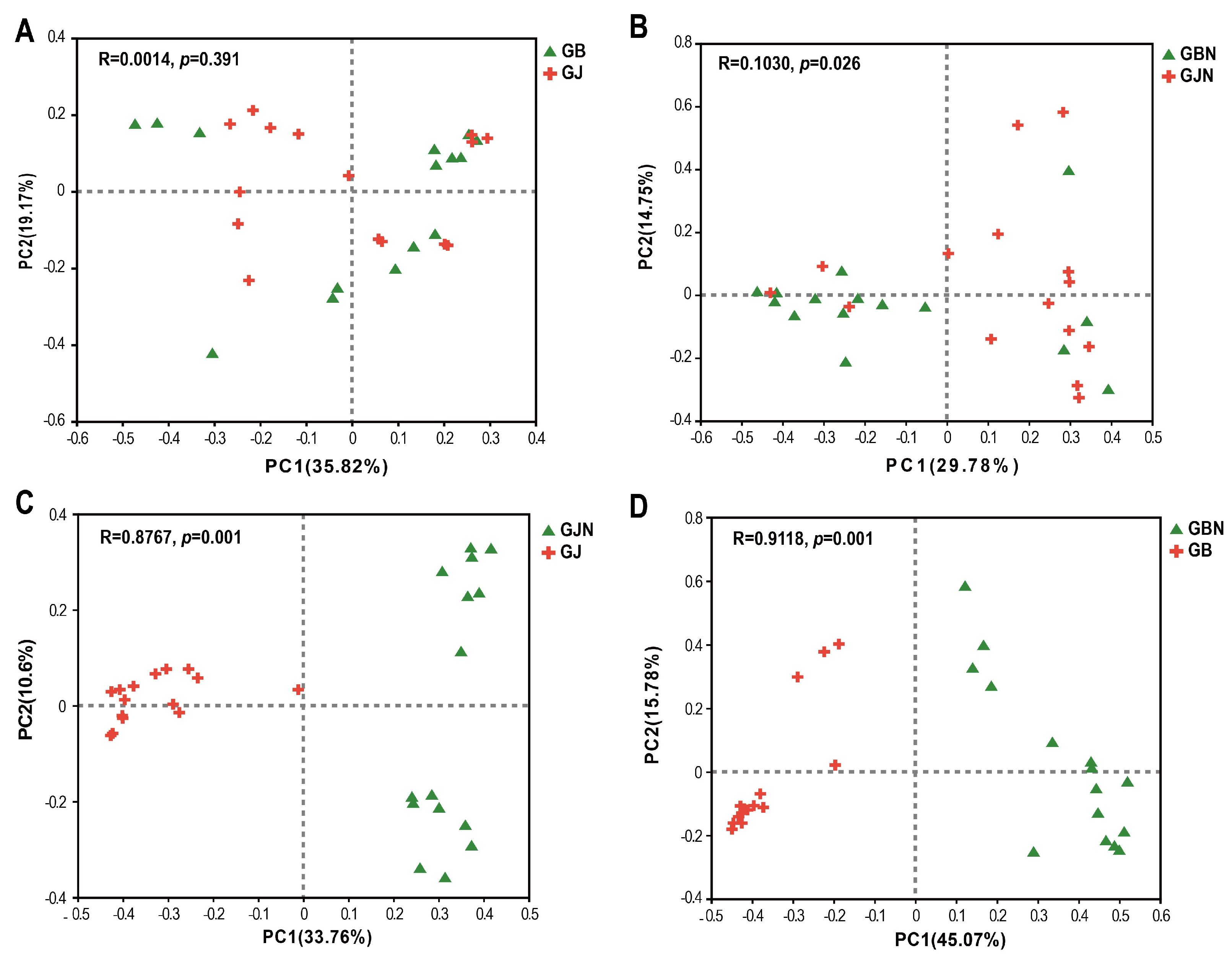
3.4. β-Diversity Analysis of Fungal Communities
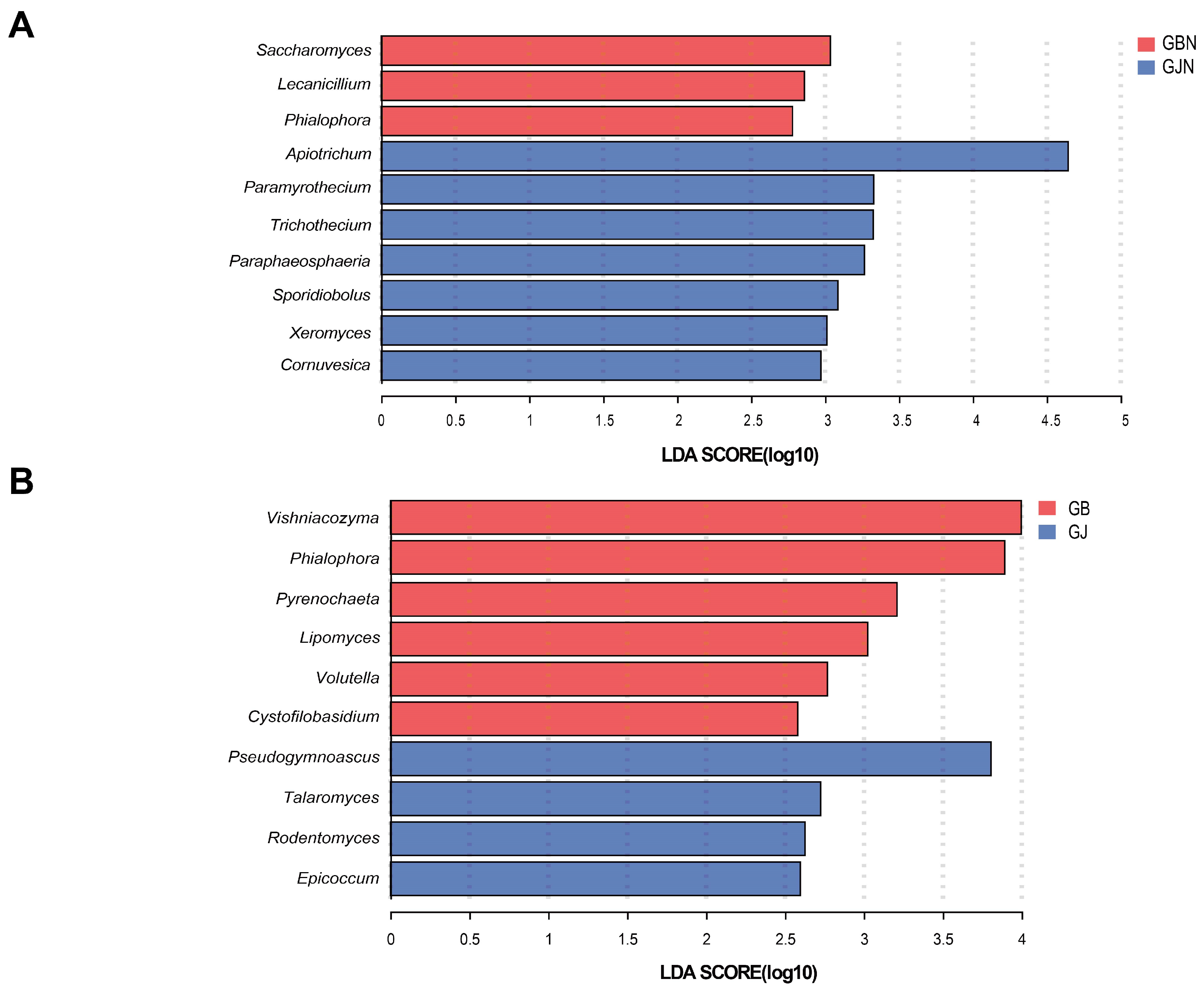
3.5. LEfSe Analysis of Dominant Fungal Taxa
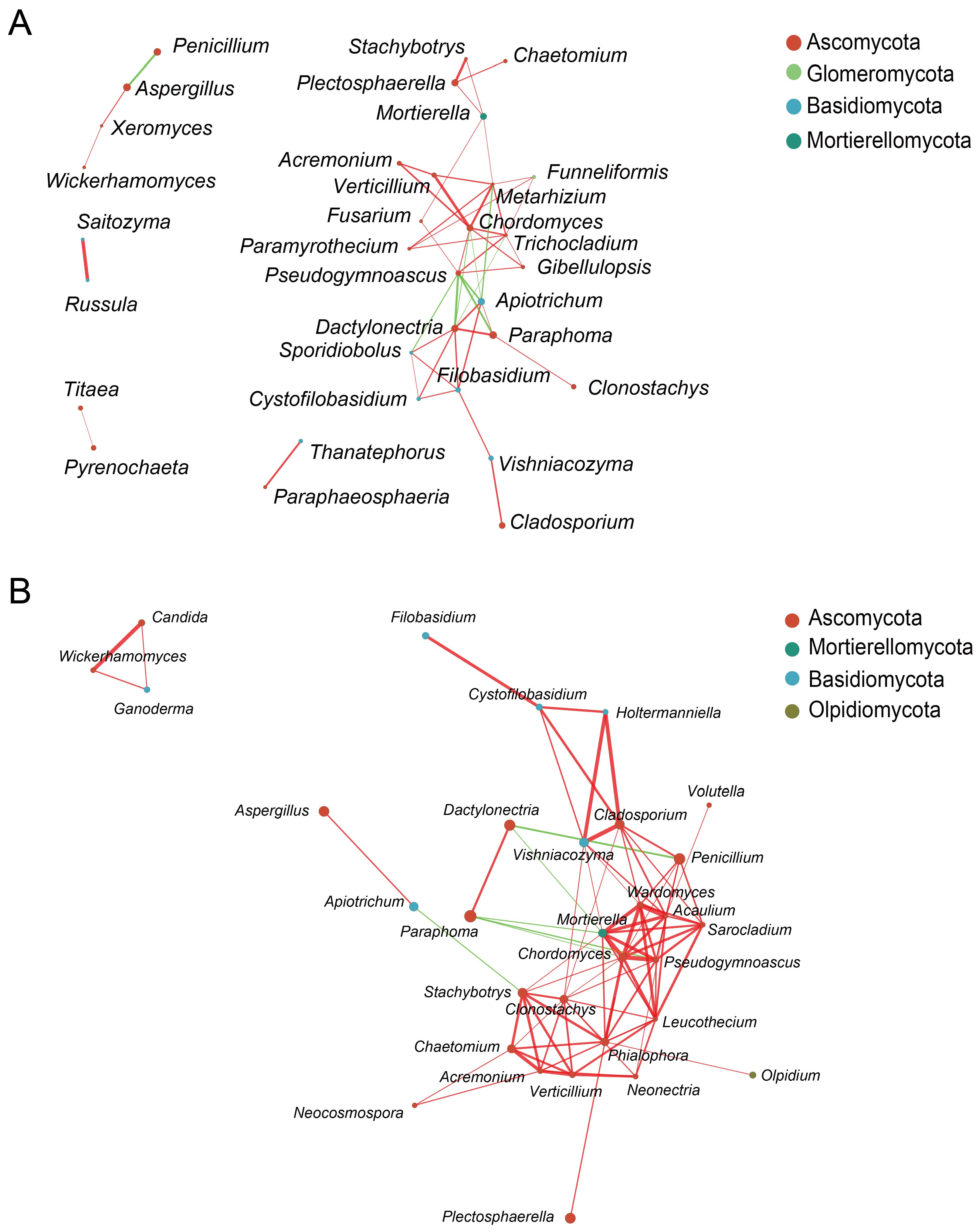
3.6. Network Analysis of Fungal Communities in Rhizosphere and Endosphere
3.7. Functional Prediction Analysis of Fungi in Different Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maalouf, F.; Hu, J.; O’Sullivan, D.M.; Zong, X.; Hamwieh, A.; Kumar, S.; Baum, M.; Ojiewo, C. Breeding and genomics status in faba bean (Vicia faba). Plant Breed. 2019, 138, 465–473. [Google Scholar] [CrossRef]
- Mahmoud, N.M.A. Studies on Chocolate Spot Disease of Broad Bean and Loss Occurrence. Ph.D. Thesis, Menoufia University, Shebin El Kom, Egypt, 1996. [Google Scholar]
- Stoddard, F.L.; Nicholas, A.H.; Rubiales, D.; Thomas, J.; Villegas-Fernández, A.M. Integrated pest management in faba bean. Field Crops Res. 2010, 115, 308–318. [Google Scholar] [CrossRef]
- Devi, S.I.; Talukdar, N.C.; Sharma, K.C.; Jeyaram, K.; Rohinikumar, M. Screening of rhizobacteria for their plant growth promotion ability and antagonism against damping off and root rot diseases of Broad Bean (Vicia faba L.). Indian J. Microbiol. 2011, 51, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sangle, U.; Kumar, B.; Tripathi, H.; Singh, K.; Gupta, A. Integrated disease management of Faba bean (Vicia faba L.). In Faba Bean (Vicia faba L.): A Potential Leguminous Crop of India; Singh, A.K., Bhatt, B.P., Eds.; Indian Council of Agricultural Research: Patna, India, 2012; pp. 279–301. [Google Scholar]
- Infantino, A.; Kharrat, M.; Riccioni, L.; Coyne, C.J.; McPhee, K.E.; Grünwald, N.J. Screening techniques and sources of resistance to root diseases in cool season food legumes. Euphytica 2006, 147, 201–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Guo, Y.P.; Shao, Y. Impact of different planting densities on root rot and crop growth of faba bean in intercropping systems. Agric. Sci. Bullet. 2018, 24, 60–61. [Google Scholar]
- Dong, Y.; Dong, K.; Zheng, Y.; Tang, L.; Yang, Z.X. Faba bean fusarium wilt (Fusarium oxysporum) control and its mechanismin different wheat varieties and faba bean intercropping system. Chin. J. Appl. Ecol. 2014, 25, 1979–1987. [Google Scholar]
- Rubiales, D.; Khazaei, H. Advances in disease and pest resistance in faba bean. Theor. Appl. Genet. 2022, 135, 3735–3756. [Google Scholar] [CrossRef]
- Prior, R.; Mittelbach, M.; Begerow, D. Impact of three different fungicides on fungal epi- and endophytic communities of common bean (Phaseolus vulgaris) and broad bean (Vicia faba). J. Environ. Sci. Health B 2017, 52, 376–386. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kamilova, F.; Validov, S.; Gafurova, L.; Kucharova, Z.; Lugtenberg, B. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ. Microbiol. 2008, 10, 1–9. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Hamel, C.; Gan, Y. Edaphic properties override the influence of crops on the composition of the soil bacterial community in a semiarid agroecosystem. Appl. Soil Ecol. 2016, 105, 160–168. [Google Scholar] [CrossRef]
- Aghdam, S.A.; Brown, A.M.V. Deep learning approaches for natural product discovery from plant endophytic microbiomes. Environ. Microbiome 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, H.; Chu, M.; Niu, X.; Wang, N.; Lin, Q.; Lou, K.; Zuo, C.; Wang, J.; Zou, Q.; et al. Differentiation and variability in the rhizosphere and endosphere microbiomes of healthy and diseased cotton (Gossypium sp.). Front. Microbiol. 2021, 12, 765269. [Google Scholar] [CrossRef]
- Kim, M.J.; Do, H.; Cho, G.; Jeong, R.D.; Kwak, Y.S. Comparison of microbial community of rhizosphere and endosphere in Kiwifruit. Plant Pathol. 2019, 35, 705–711. [Google Scholar] [CrossRef]
- Chen, J.; Xu, D.; Chao, L.; Liu, H.; Bao, Y. Microbial assemblages associated with the rhizosphere and endosphere of an herbage, Leymus chinensis. Microb. Biotechnol. 2020, 13, 1390–1402. [Google Scholar] [CrossRef]
- Firdu, Z.; Maia, L.; Teodoro, J.; Alemu, T.; Assefa, F. Characterization of faba bean (Vicia faba L.) rhizosphere associating rhizobacteria against Botrytis fabae AAUBF-12 and their plant growth-promoting properties. Heliyon 2022, 8, e08861. [Google Scholar] [CrossRef]
- Xin, Y.; Chen, C.; Zhong, Y.; Bu, X.; Huang, S.; Tahir, M.; Du, Z.; Liu, W.; Yang, W.; Li, J.; et al. Effect of storage time on the silage quality and microbial community of mixed maize and faba bean in the Qinghai-Tibet Plateau. Front. Microbiol. 2023, 13, 1090401. [Google Scholar] [CrossRef]
- Li, H.; Zeng, T.; Du, Z.; Dong, X.; Xin, Y.; Wu, Y.; Huang, L.; Liu, L.; Kang, B.; Jiang, D.; et al. Assessment on the fermentation quality and bacterial community of mixed silage of faba bean with forage wheat or oat. Front. Microbiol. 2022, 13, 875819. [Google Scholar] [CrossRef]
- Zhao, S.; Niu, C.; Wang, Y.; Zan, Y.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Revealing the succession of spatial heterogeneity of the microbial community during broad bean paste fermentation. Appl. Environ. Microbiol. 2023, 89, e0062123. [Google Scholar] [CrossRef]
- Granzow, S.; Kaiser, K.; Wemheuer, B.; Pfeiffer, B.; Daniel, R.; Vidal, S.; Wemheuer, F. The effects of cropping regimes on fungal and bacterial communities of wheat and faba bean in a greenhouse pot experiment differ between plant species and compartment. Front. Microbiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, C.; Bei, S.; Wang, G.; Geisen, S.; Bedoussac, L.; Christie, P.; Zhang, J. High bacterial diversity and siderophore-producing bacteria collectively suppress Fusarium oxysporum in maize/faba bean intercropping. Front. Microbiol. 2022, 13, 972587. [Google Scholar] [CrossRef]
- Luo, Y.T.; Tang, L.; Zheng, Y.; Dong, Y. Effects of wheat-faba bean intercropping on the yield and rhizosphere pathogen in different n application rates. Chin. J. Soil Sci. 2012, 43, 826–831. [Google Scholar]
- Alfauomy, G.A.; Atwa, M.A.M. Influences of biological control on damping off diseases of faba beans as well as physico-chemical and technological properties. Middle East J. Agric. Res. 2020, 9, 812–827. [Google Scholar]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Core microbiomes for sustainable agroecosystems. Nat. Plants 2018, 4, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Lynch, R.C.; Kane, N.C.; Fierer, N. Plant domestication and the assembly of bacterial and fungal communities associated with strains of the common sunflower, Helianthus annuus. New Phytol. 2017, 214, 412–423. [Google Scholar] [CrossRef]
- Woźniak, M.; Grządziel, J.; Gałązka, A.; Frąc, M. Metagenomic analysis of bacterial and fungal community composition associated with Paulownia elongata × Paulownia fortunei. BioResearch 2019, 14, 8511–8529. [Google Scholar] [CrossRef]
- Yan, K.; Abbas, M.; Meng, L.; Cai, H.; Peng, Z.; Li, Q.; El-Sappah, A.H.; Yan, L.; Zhao, X. Analysis of the fungal diversity and community structure in sichuan dark tea during pile-fermentation. Front. Microbiol. 2021, 12, 706714. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Shi, W.; Zhao, J.; Hou, Q.; Zhang, H.; Jia, L.; Sun, K. Analysis of endophyte diversity of Rheum palmatum among different tissues and ages. Arch. Microbiol. 2023, 205, 14. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, W.; Shao, Y.; Li, Y.J.; Lin, L.A.; Zhang, Y.J.; Han, H.; Chen, Z.J. Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch. Microbiol. 2020, 202, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.; Chen, C.; Xu, Z.; Ghadiri, H. Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical Australia. J. Soil Sediment 2010, 10, 1267–1277. [Google Scholar] [CrossRef]
- Hao, Z.P.; Christie, P.; Zheng, F.; Li, J.L.; Chen, Q.; Wang, J.G.; Li, X.L. Excessive nitrogen inputs in intensive greenhouse cultivation may influence soil microbial biomass and community composition. Commun. Soil Sci. Plant Anal. 2009, 40, 2323–2337. [Google Scholar] [CrossRef][Green Version]
- Liao, L.B.; Chen, X.X.; Xiang, J.; Zhang, N.N.; Wang, E.T.; Shi, F.S. Zanthoxylum bungeanum root-rot associated shifts in microbiomes of root endosphere, rhizosphere, and soil. PeerJ 2022, 10, e13808. [Google Scholar] [CrossRef]
- Daniel, R. The metagenomics of soil. Nat. Rev. Microbiol. 2005, 3, 470–478. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Kemmelmeier, K. The Glomeromycota in the neotropics. Front. Microbiol. 2021, 11, 553679. [Google Scholar] [CrossRef]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Gianinazzi-Pearson, V.; Cleyet-Marel, J.C.; Baudoin, E.; van Tuinen, D. Glomeromycota communities survive extreme levels of metal toxicity in an orphan mining site. Sci. Total Environ. 2017, 598, 121–128. [Google Scholar] [CrossRef]
- Jie, W.; Lin, J.; Guo, N.; Cai, B.; Yan, X. Community composition of rhizosphere fungi as affected by Funneliformis mosseae in soybean continuous cropping soil during seedling period. Chil. J. Agric. Res. 2019, 79, 356–365. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, T.; Zhang, H.; Zhao, M.; Penton, C.R.; Thomashow, L.S.; Shen, Q. Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. ISME J. 2020, 14, 2936–2950. [Google Scholar] [CrossRef]
- Parka, M.S.; Leea, J.W.; Kima, S.H.; Parka, J.-H.; Youb, Y.-H.; Lim, Y.W. Penicillium from Rhizosphere Soil in Terrestrial and Coastal Environments. Mycobiology 2020, 48, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Zachow, C.; Lottmann, J.; Götz, M.; Costa, R.; Smalla, K. Impact of plant species and site on rhizosphere-associated fungi antagonistic to Verticillium dahliae Kleb. Appl. Environ. Microbiol. 2005, 71, 4203–4213. [Google Scholar] [CrossRef] [PubMed]
- Elias, F.; Woyessa, D.; Muleta, D. Phosphate solubilization potential of rhizosphere fungi isolated from plants in Jimma Zone, Southwest Ethiopia. Int. J. Microbiol. 2016, 2016, 5472601. [Google Scholar] [CrossRef] [PubMed]
- Lygin, A.V.; Hill, C.B.; Zernova, O.V.; Crull, L.; Widholm, J.M.; Hartman, G.L.; Lozovaya, V.V. Response of soybean pathogens to glyceollin. Phytopathology 2010, 100, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Bertolini, P.; Pratella, G.C. Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. 2003, 94, 761–766. [Google Scholar] [CrossRef]
- Ortega, H.E.; Torres-Mendoza, D.; Cubilla-Rios, L. Patents on endophytic fungi for agriculture and bio- and phytoremediation applications. Microorganisms 2020, 8, 1237. [Google Scholar] [CrossRef]
- Kumla, J.; Nundaeng, S.; Suwannarach, N.; Lumyong, S. Evaluation of multifarious plant growth promoting trials of yeast isolated from the soil of Assam Tea (Camellia sinensis var. assamica) plantations in northern Thailand. Microorganisms 2020, 8, 1168. [Google Scholar] [CrossRef]
- Li, L.Y.; Sun, B.D.; Zhang, G.S.; Deng, H.; Wang, M.H.; Tan, X.M.; Zhang, X.Y.; Jia, H.M.; Zhang, H.W.; Zhang, T.; et al. Polyketides with different post-modifications from desert endophytic fungus Paraphoma sp. Nat. Prod. Res. 2017, 32, 939–943. [Google Scholar] [CrossRef]
- Dang, S.Z.; Li, Y.Z. The characterization and the biological activity of phytotoxin produced by Paraphoma radicina. J. Fungi 2022, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- Osorio, N.W.; Habte, M. Effect of a phosphate-solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in tropical soils with contrasting phosphate sorption capacity. Plant Soil 2014, 389, 375–385. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Suo, M.; Qiu, Z.; Wu, H.; Zhao, M.; Yang, H. Regulating root fungal community using Mortierella alpina for Fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 2022, 13, 850917. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Yang, Q.; Kang, C.; Li, M. Unraveling the characteristics of the microbial community and potential pathogens in the rhizosphere soil of Rehmannia glutinosa with root rot disease. Appl. Soil Ecol. 2018, 130, 271–279. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hou, L.; Zhang, G.; Cheng, L.; Liu, Y. Comparative Analysis of Rhizosphere and Endosphere Fungal Communities in Healthy and Diseased Faba Bean Plants. J. Fungi 2024, 10, 84. https://doi.org/10.3390/jof10010084
Li J, Hou L, Zhang G, Cheng L, Liu Y. Comparative Analysis of Rhizosphere and Endosphere Fungal Communities in Healthy and Diseased Faba Bean Plants. Journal of Fungi. 2024; 10(1):84. https://doi.org/10.3390/jof10010084
Chicago/Turabian StyleLi, Juan, Lu Hou, Gui Zhang, Liang Cheng, and Yujiao Liu. 2024. "Comparative Analysis of Rhizosphere and Endosphere Fungal Communities in Healthy and Diseased Faba Bean Plants" Journal of Fungi 10, no. 1: 84. https://doi.org/10.3390/jof10010084
APA StyleLi, J., Hou, L., Zhang, G., Cheng, L., & Liu, Y. (2024). Comparative Analysis of Rhizosphere and Endosphere Fungal Communities in Healthy and Diseased Faba Bean Plants. Journal of Fungi, 10(1), 84. https://doi.org/10.3390/jof10010084




