Dominant Tree Species and Litter Quality Govern Fungal Community Dynamics during Litter Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Litter Bag Embedding, and Sample Collection
2.2. DNA Extraction, Amplification of ITS2 Region, and Sequencing
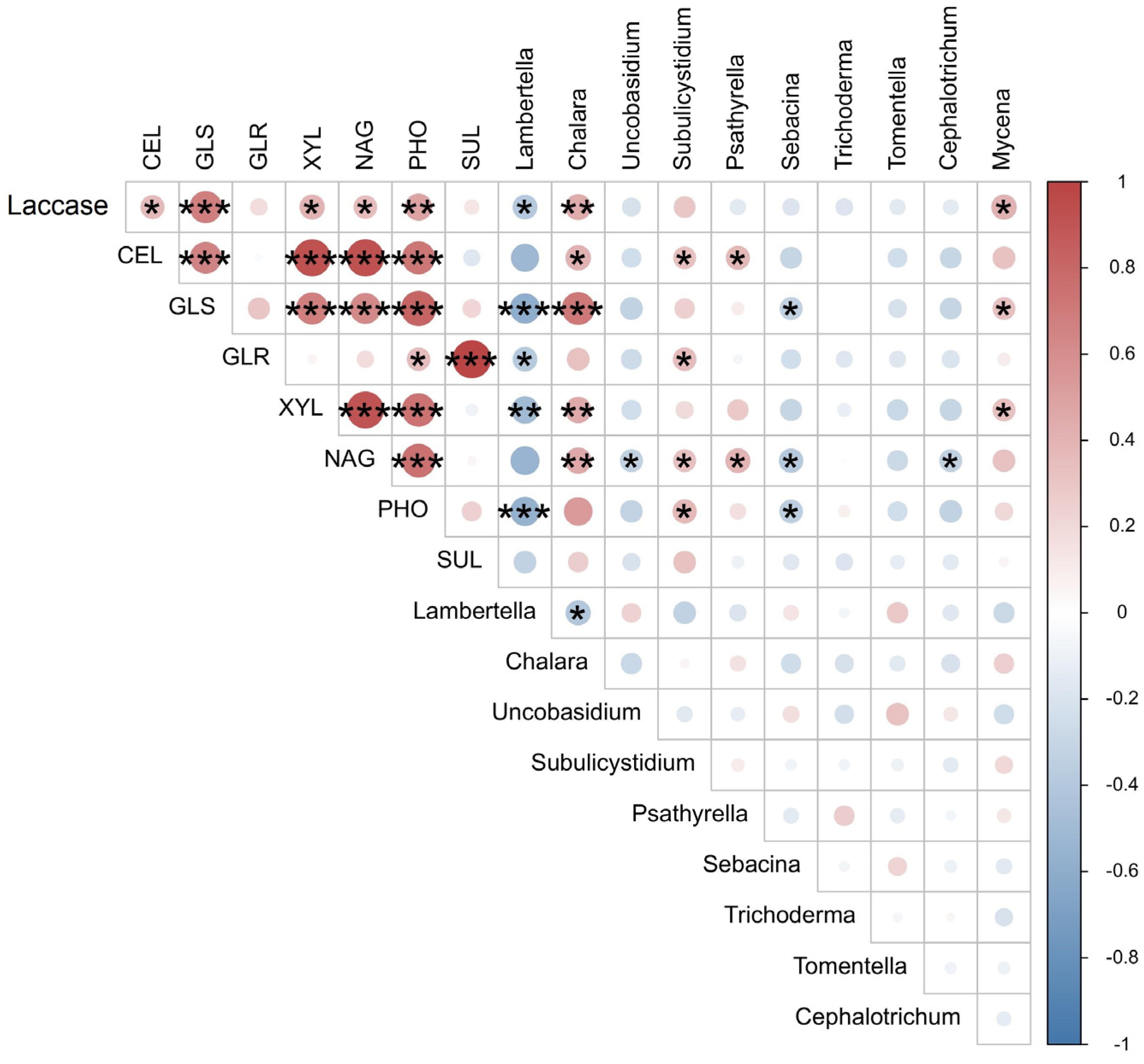
2.3. Enzyme Activity Measurements
2.4. Quantitative Microbial Element Cycling
2.5. Sequence Data Processing and Statistical Analysis
3. Results
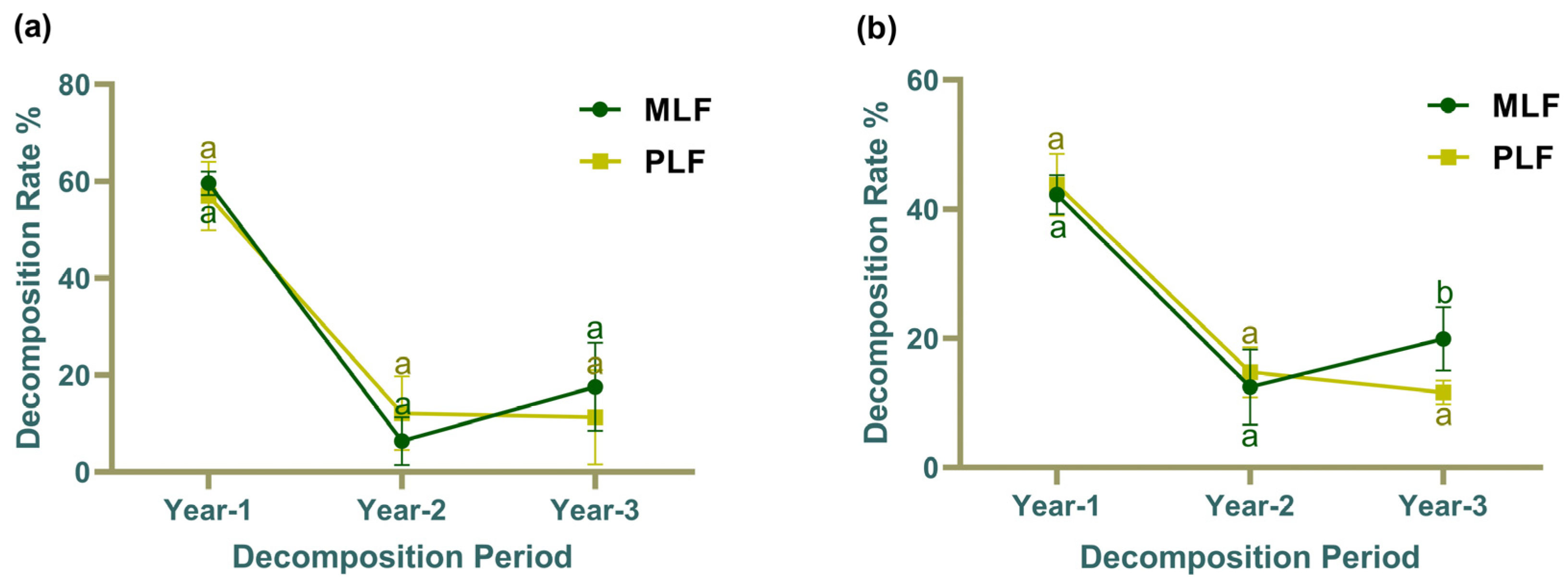
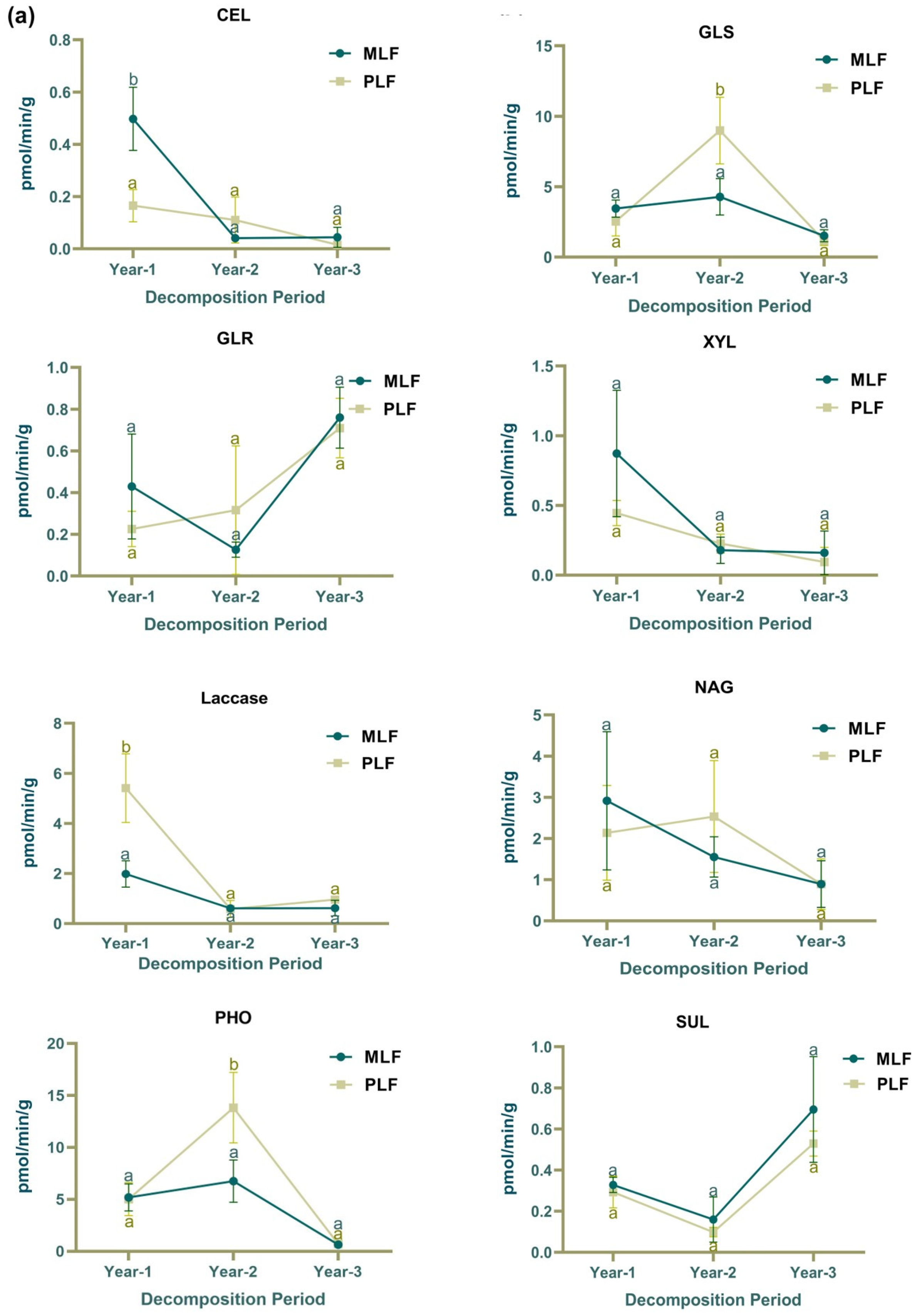
3.1. Decomposition Rate and Enzyme Activities during Litter Decomposition
3.2. Fungal Community α-Diversity during Litter Decomposition
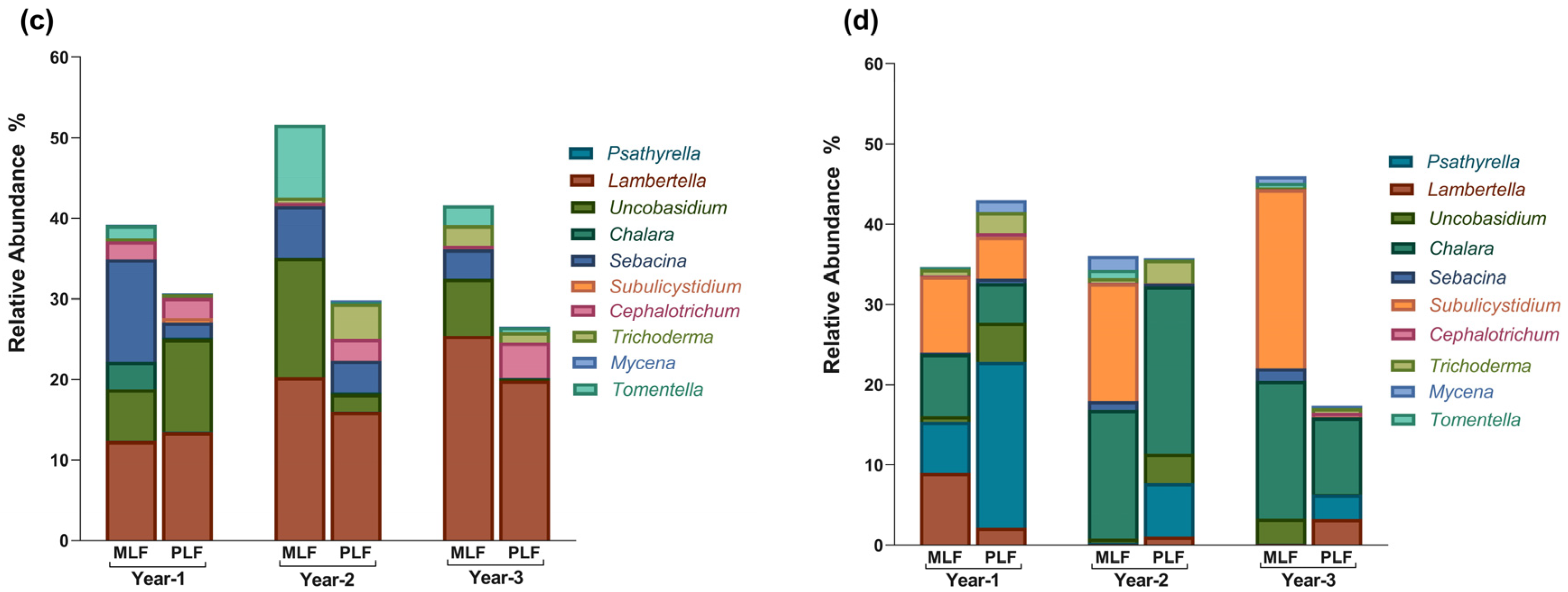
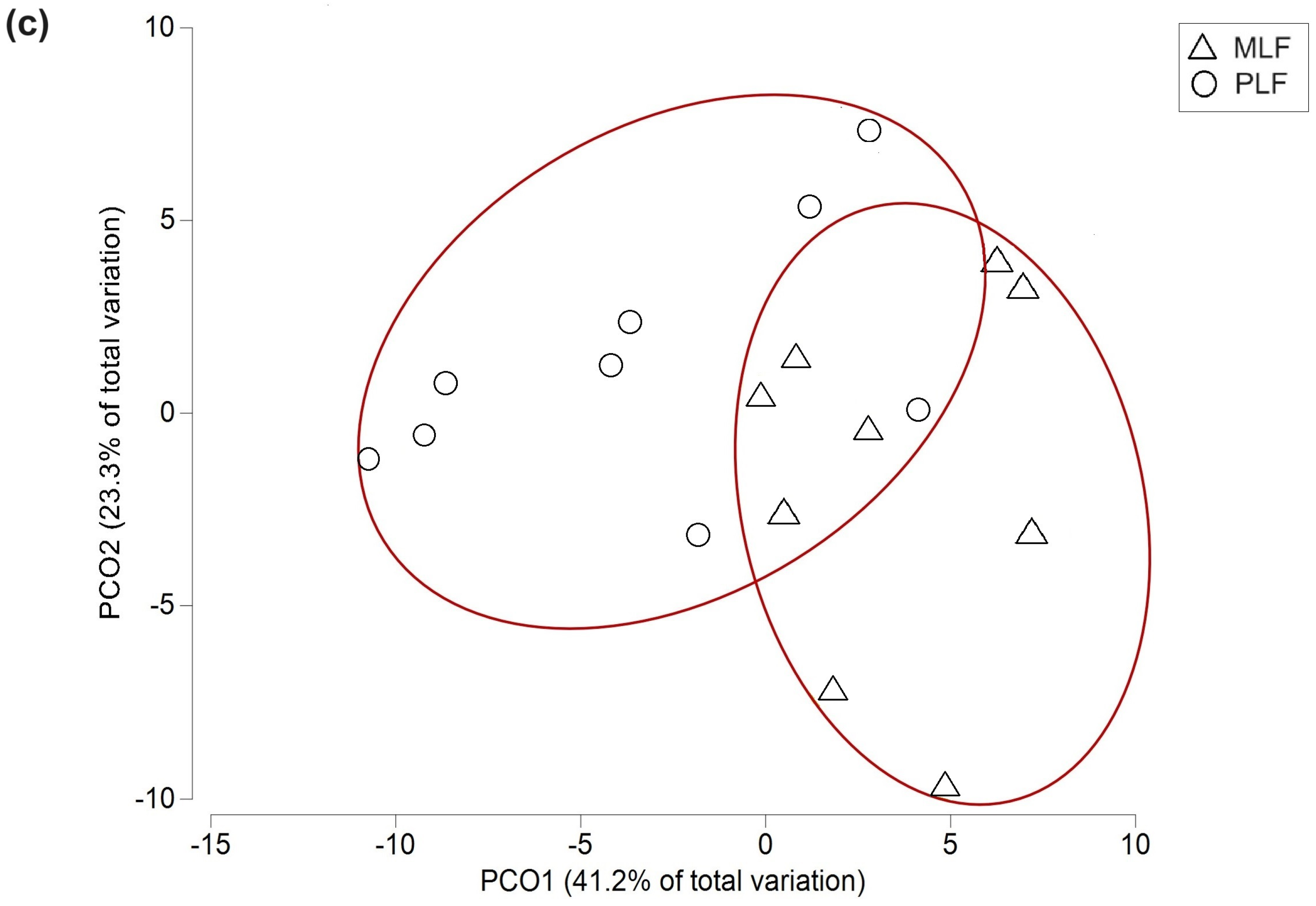
3.3. Fungal Composition at Taxonomic Level during Litter Decomposition
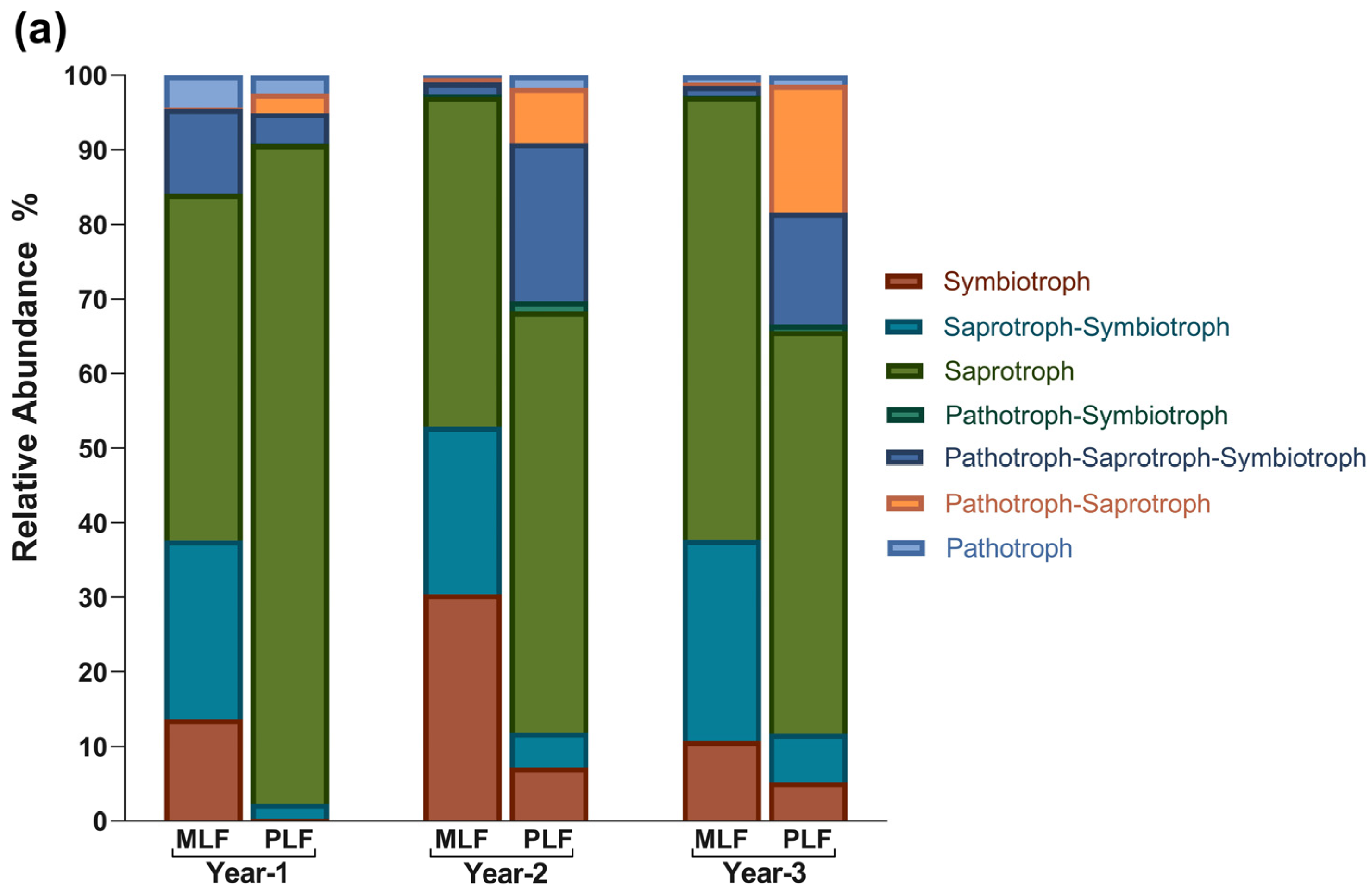
3.4. Fungal Functional Structure during Litter Decomposition
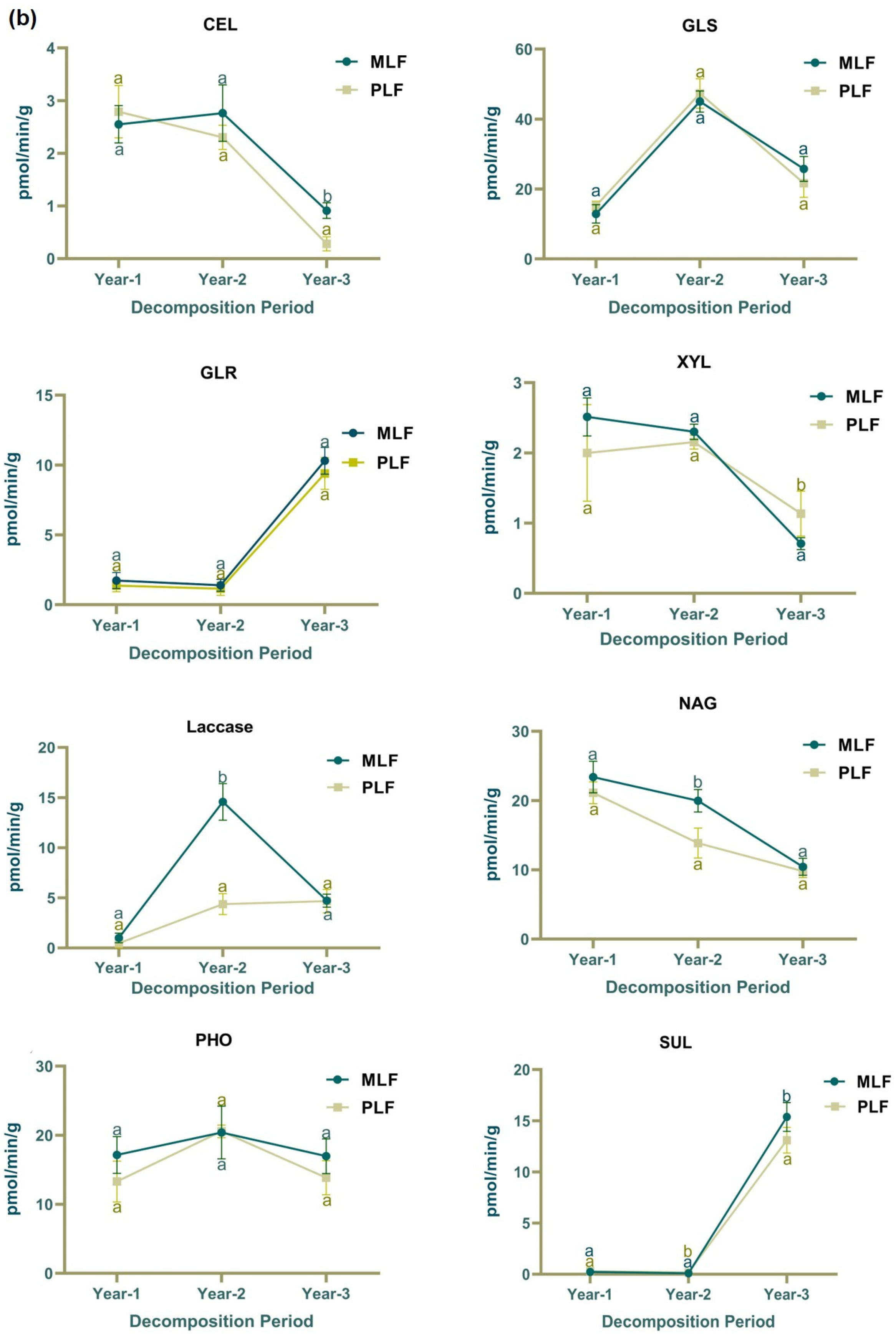
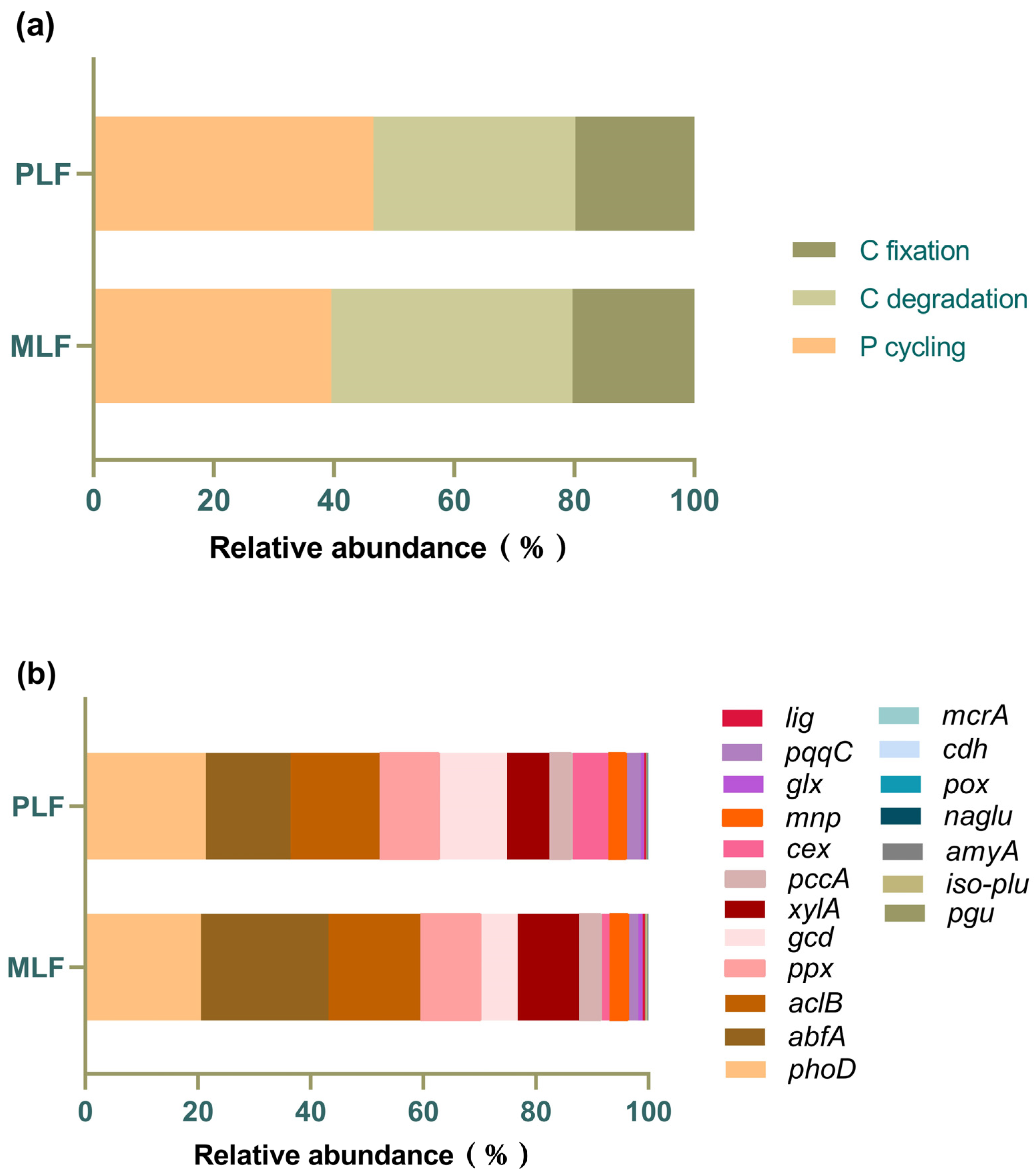
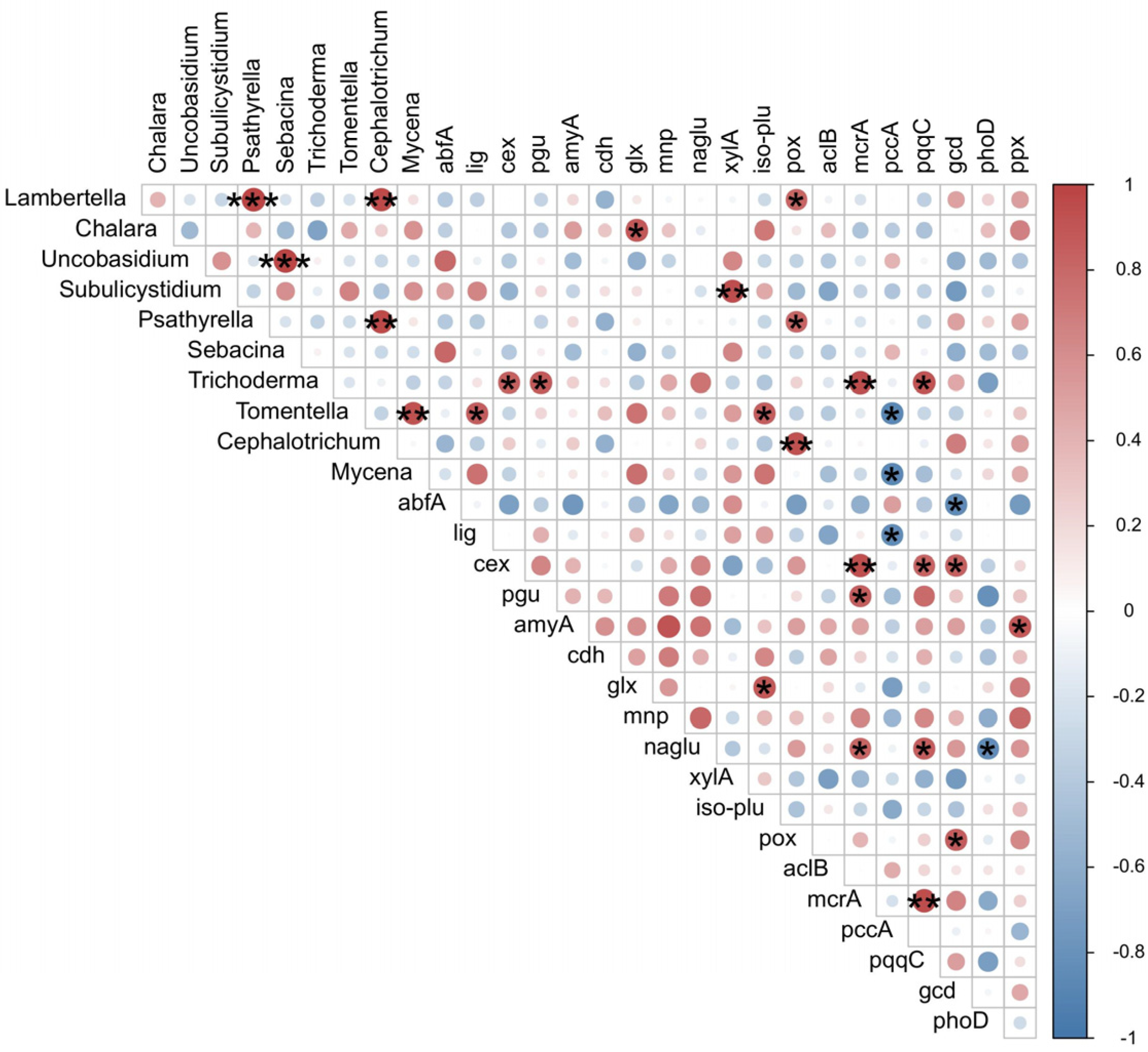
3.5. Fungal Function Gene Structure in Twig Litter in the Third-Year Decomposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prescott, C.E.; Vesterdal, L. Decomposition and Transformations along the Continuum from Litter to Soil Organic Matter in Forest Soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Latterini, F.; Dyderski, M.K.; Horodecki, P.; Picchio, R.; Venanzi, R.; Lapin, K.; Jagodziński, A.M. The Effects of Forest Operations and Silvicultural Treatments on Litter Decomposition Rate: A Meta-Analysis. Curr. For. Rep. 2023, 9, 276–290. [Google Scholar] [CrossRef]
- Robertson, G.P.; Paul, E.A. Decomposition and Soil Organic Matter Dynamics. In Methods in Ecosystem Science; Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W., Eds.; Springer: New York, NY, USA, 2000; pp. 104–116. ISBN 978-1-4612-1224-9. [Google Scholar]
- Krishna, M.P.; Mohan, M. Litter Decomposition in Forest Ecosystems: A Review. Energ. Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A Thready Affair: Linking Fungal Diversity and Community Dynamics to Terrestrial Decomposition Processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Chen, G.; Lin, P.; Xie, J. A Review on Litter Decomposition in Forest Ecosystem. Available online: http://html.rhhz.net/linyekexue/html/20060417.htm (accessed on 5 October 2023).
- Keller, A.B.; Phillips, R.P. Leaf Litter Decay Rates Differ between Mycorrhizal Groups in Temperate, but Not Tropical, Forests. New Phytol. 2019, 222, 556–564. [Google Scholar] [CrossRef]
- Jing, H.; Wang, G. Temporal Dynamics of Pinus Tabulaeformis Litter Decomposition under Nitrogen Addition on the Loess Plateau of China. For. Ecol. Manag. 2020, 476, 118465. [Google Scholar] [CrossRef]
- Dechaine, J.; Ruan, H.; Sanchez-de Leon, Y.; Zou, X. Correlation between Earthworms and Plant Litter Decomposition in a Tropical Wet Forest of Puerto Rico. Pedobiologia 2005, 49, 601–607. [Google Scholar] [CrossRef]
- Farooq, T.H.; Li, Z.; Yan, W.; Shakoor, A.; Kumar, U.; Shabbir, R.; Peng, Y.; Gayathiri, E.; Alotaibi, S.S.; Wróbel, J.; et al. Variations in Litterfall Dynamics, C:N:P Stoichiometry and Associated Nutrient Return in Pure and Mixed Stands of Camphor Tree and Masson Pine Forests. Front. Environ. Sci. 2022, 10, 903039. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Chen, X.; An, Z.; Zhang, X.; Du, J.; Chang, S.X. Tree Species Composition Alters the Decomposition of Mixed Litter and the Associated Microbial Community Composition and Function in Subtropical Plantations in China. For. Ecol. Manag. 2023, 529, 120743. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of Broadleaf and Needle Litter in Forests of British Columbia: Influences of Litter Type, Forest Type, and Litter Mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Baldrian, P. Forest Microbiome: Diversity, Complexity and Dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The Role of Microbial Community in the Decomposition of Leaf Litter and Deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Baldrian, P.; Valásková, V. Degradation of Cellulose by Basidiomycetous Fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef]
- Chávez-Vergara, B.; Rosales-Castillo, A.; Merino, A.; Vázquez-Marrufo, G.; Oyama, K.; García-Oliva, F. Quercus Species Control Nutrients Dynamics by Determining the Composition and Activity of the Forest Floor Fungal Community. Soil Biol. Biochem. 2016, 98, 186–195. [Google Scholar] [CrossRef]
- Eichlerová, I.; Homolka, L.; Žifčáková, L.; Lisá, L.; Dobiášová, P.; Baldrian, P. Enzymatic Systems Involved in Decomposition Reflects the Ecology and Taxonomy of Saprotrophic Fungi. Fungal Ecol. 2015, 13, 10–22. [Google Scholar] [CrossRef]
- Tian, L.; Dell, E.; Shi, W. Chemical Composition of Dissolved Organic Matter in Agroecosystems: Correlations with Soil Enzyme Activity and Carbon and Nitrogen Mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; Ge, J.; He, N.; Wang, Q.; Wang, D. The Variations in Soil Microbial Communities, Enzyme Activities and Their Relationships with Soil Organic Matter Decomposition along the Northern Slope of Changbai Mountain. Appl. Soil Ecol. 2015, 86, 19–29. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Extracellular Enzyme Activities and Carbon Chemistry as Drivers of Tropical Plant Litter Decomposition. Biotropica 2004, 36, 285–296. [Google Scholar] [CrossRef]
- Waring, B.G. Exploring Relationships between Enzyme Activities and Leaf Litter Decomposition in a Wet Tropical Forest. Soil Biol. Biochem. 2013, 64, 89–95. [Google Scholar] [CrossRef]
- Osono, T.; To-Anun, C.; Hagiwara, Y.; Hirose, D. Decomposition of Wood, Petiole and Leaf Litter by Xylaria Species from Northern Thailand. Fungal Ecol. 2011, 4, 210–218. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The Plant Cell Wall-Decomposing Machinery Underlies the Functional Diversity of Forest Fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, Y.; Xu, G.; Guo, Q.; Tan, J.; Ding, G. Fungal Diversity within the Phyllosphere of Pinus Massoniana and the Possible Involvement of Phyllospheric Fungi in Litter Decomposition. Fungal Biol. 2021, 125, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Osono, T. Ecology of Ligninolytic Fungi Associated with Leaf Litter Decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Frankland, J.C. Fungal Succession—Unravelling the Unpredictable. Mycol. Res. 1998, 102, 1–15. [Google Scholar] [CrossRef]
- Dilly, O.; Bartsch, S.; Rosenbrock, P.; Buscot, F.; Munch, J.C. Shifts in Physiological Capabilities of the Microbiota during the Decomposition of Leaf Litter in a Black Alder (Alnus glutinosa (Gaertn.) L.) Forest. Soil Biol. Biochem. 2001, 33, 921–930. [Google Scholar] [CrossRef]
- Osono, T. Role of Phyllosphere Fungi of Forest Trees in the Development of Decomposer Fungal Communities and Decomposition Processes of Leaf Litter. Can. J. Microbiol. 2006, 52, 701–716. [Google Scholar] [CrossRef]
- Rajala, T.; Peltoniemi, M.; Hantula, J.; Mäkipää, R.; Pennanen, T. RNA Reveals a Succession of Active Fungi during the Decay of Norway Spruce Logs. Fungal Ecol. 2011, 4, 437–448. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in Leaf Litter: Novel Insights into Community Dynamics of Bacteria and Fungi during Litter Decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal Community on Decomposing Leaf Litter Undergoes Rapid Successional Changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of Fungal and Bacterial Communities in Forest Litter and Soil Is Largely Determined by Dominant Trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Sun, H.; Terhonen, E.; Kovalchuk, A.; Tuovila, H.; Chen, H.; Oghenekaro, A.O.; Heinonsalo, J.; Kohler, A.; Kasanen, R.; Vasander, H.; et al. Dominant Tree Species and Soil Type Affect the Fungal Community Structure in a Boreal Peatland Forest. Appl. Environ. Microbiol. 2016, 82, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of Local Forest Composition on Soil Fungal Communities in a Mixed Boreal Forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Treseder, K.K.; Bent, E.; Borneman, J.; McGuire, K.L. Shifts in Fungal Communities during Decomposition of Boreal Forest Litter. Fungal Ecol. 2014, 10, 58–69. [Google Scholar] [CrossRef]
- Foudyl-Bey, S.; Brais, S.; Drouin, P. Litter Heterogeneity Modulates Fungal Activity, C Mineralization and N Retention in the Boreal Forest Floor. Soil Biol. Biochem. 2016, 100, 264–275. [Google Scholar] [CrossRef]
- Qu, Z.-L.; Liu, B.; Ma, Y.; Xu, J.; Sun, H. The Response of the Soil Bacterial Community and Function to Forest Succession Caused by Forest Disease. Funct. Ecol. 2020, 34, 2548–2559. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, Z.; Jin, X.; Zhu, J.; Xu, H.; Zhao, C.; Chen, B.; Guan, Q. Spatial Distribution Pattern and Interspecific Association of Dominant Tree Species in a Broad-Leaved Mixed Forest on Zijin Mountain. J. Nanjing For. Univ. 2018, 61, 84. [Google Scholar] [CrossRef]
- Deng, J.; Yin, Y.; Luo, J.; Zhu, W.; Zhou, Y. Different Revegetation Types Alter Soil Physical-Chemical Characteristics and Fungal Community in the Baishilazi Nature Reserve. PeerJ 2019, 6, e6251. [Google Scholar] [CrossRef]
- Liu, K.; Meng, W.; Qu, Z.; Zhang, Y.; Liu, B.; Ma, Y.; Chang, L.; Sun, H. Changes in Bacterial Communities and Functions Associated with Litter Degradation during Forest Succession Caused by Forest Disease. Phytobiomes J. 2023, 7, 491–499. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, Z.; Jin, X.; Guan, Q.; Zhu, J.; Dai, K.; Zhao, C. Characteristics of Coniferous and Broad-Leaved Mixed Forest Community on Zijin Mountain. J. Cent. South Univ. For. Technol. 2020, 40, 113–119. [Google Scholar]
- Jiang, A.; Wan, F.; Hu, F. Study on Soil Anti-Erodibility in Different Forests in Spirit Valley of Mount Zijin in Nanjing. Available online: http://stbcyj.paperonce.org/oa/DArticle.aspx?id=20180103 (accessed on 7 November 2023).
- Anderson, J.M. The Breakdown and Decomposition of Sweet Chestnut (Castanea sativa Mill.) and Beech (Fagus sylvatica L.) Leaf Litter in Two Deciduous Woodland Soils: I. Breakdown, Leaching and Decomposition. Oecologia 1973, 12, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qu, Z.-L.; Liu, B.; Tan, J.-J.; Asiegbu, F.O.; Sun, H. Bacterial Community Structure of Pinus Thunbergii Naturally Infected by the Nematode Bursaphelenchus xylophilus. Microorganisms 2020, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.P.; Peay, K.G. Sequence Depth, Not PCR Replication, Improves Ecological Inference from next Generation DNA Sequencing. PLoS ONE 2014, 9, e90234. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Saiya-Cork, K.; Long, T.; Osgood, M.P.; Neher, D.A.; Zak, D.R.; Norby, R.J. Soil Microbial Activity in a Liquidambar Plantation Unresponsive to CO2-Driven Increases in Primary Production. Appl. Soil Ecol. 2003, 24, 263–271. [Google Scholar] [CrossRef]
- Xu, J.; Liu, B.; Qu, Z.-L.; Ma, Y.; Sun, H. Age and Species of Eucalyptus Plantations Affect Soil Microbial Biomass and Enzymatic Activities. Microorganisms 2020, 8, 811. [Google Scholar] [CrossRef]
- Zheng, B.; Zhu, Y.; Sardans, J.; Peñuelas, J.; Su, J. QMEC: A Tool for High-Throughput Quantitative Assessment of Microbial Functional Potential in C, N, P, and S Biogeochemical Cycling. Sci. China Life Sci. 2018, 61, 1451–1462. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Tedersoo, L.; Nilsson, R.H.; Abarenkov, K.; Jairus, T.; Sadam, A.; Saar, I.; Bahram, M.; Bechem, E.; Chuyong, G.; Kõljalg, U. 454 Pyrosequencing and Sanger Sequencing of Tropical Mycorrhizal Fungi Provide Similar Results but Reveal Substantial Methodological Biases. New Phytol. 2010, 188, 291–301. [Google Scholar] [CrossRef]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE Database for Molecular Identification of Fungi--Recent Updates and Future Perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Anderson, M. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E Limited: Plymouth, UK, 2008. [Google Scholar]
- Jackrel, S.L.; Gilbert, J.A.; Wootton, J.T. The Origin, Succession, and Predicted Metabolism of Bacterial Communities Associated with Leaf Decomposition. mBio 2019, 10, e01703-19. [Google Scholar] [CrossRef] [PubMed]
- Laganière, J.; Paré, D.; Bradley, R.L. How Does a Tree Species Influence Litter Decomposition? Separating the Relative Contribution of Litter Quality, Litter Mixing, and Forest Floor Conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar] [CrossRef]
- Kamau, S.; Barrios, E.; Karanja, N.K.; Ayuke, F.O.; Lehmann, J. Dominant Tree Species and Earthworms Affect Soil Aggregation and Carbon Content along a Soil Degradation Gradient in an Agricultural Landscape. Geoderma 2020, 359, 113983. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, S.; Yang, X.; Liu, Z.; Zhou, X.; Du, G.; Zhang, L.; Guo, A.; Chen, S.; Nielsen, U.N. Dominant Plant Species Influence Nematode Richness by Moderating Understory Diversity and Microbial Assemblages. Soil Biol. Biochem. 2019, 137, 107566. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Xiao, S.; Liu, Z.; Zhou, X.; Du, G.; Liu, K.; Wang, Y.; Chen, S.; Nielsen, U.N. Dominant Plants Affect Litter Decomposition Mainly through Modifications of the Soil Microbial Community. Soil Biol. Biochem. 2021, 161, 108399. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.; Peng, C.; Peng, Y.; Zhang, C.; Huang, C.; Tan, Y.; Wu, F. Foliar Litter Decomposition in an Alpine Forest Meta-Ecosystem on the Eastern Tibetan Plateau. Sci. Total Environ. 2016, 566–567, 279–287. [Google Scholar] [CrossRef]
- Bray, S.R.; Kitajima, K.; Mack, M.C. Temporal Dynamics of Microbial Communities on Decomposing Leaf Litter of 10 Plant Species in Relation to Decomposition Rate. Soil Biol. Biochem. 2012, 49, 30–37. [Google Scholar] [CrossRef]
- Gołębiewski, M.; Tarasek, A.; Sikora, M.; Deja-Sikora, E.; Tretyn, A.; Niklińska, M. Rapid Microbial Community Changes During Initial Stages of Pine Litter Decomposition. Microb. Ecol. 2019, 77, 56–75. [Google Scholar] [CrossRef]
- Cheng, F.; Peng, X.; Zhao, P.; Yuan, J.; Zhong, C.; Cheng, Y.; Cui, C.; Zhang, S. Soil Microbial Biomass, Basal Respiration and Enzyme Activity of Main Forest Types in the Qinling Mountains. PLoS ONE 2013, 8, e67353. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Berg, S.; Wallenstein, M.D.; Simmons, B.L.; Wall, D.H. Tree Species Traits Influence Soil Physical, Chemical, and Biological Properties in High Elevation Forests. PLoS ONE 2009, 4, e5964. [Google Scholar] [CrossRef]
- Bauhus, J.; Paré, D.; Côtéc, L. Effects of Tree Species, Stand Age and Soil Type on Soil Microbial Biomass and Its Activity in a Southern Boreal Forest. Soil Biol. Biochem. 1998, 30, 1077–1089. [Google Scholar] [CrossRef]
- Aciego Pietri, J.C.; Brookes, P.C. Substrate Inputs and pH as Factors Controlling Microbial Biomass, Activity and Community Structure in an Arable Soil. Soil Biol. Biochem. 2009, 41, 1396–1405. [Google Scholar] [CrossRef]
- Šnajdr, J.; Cajthaml, T.; Valášková, V.; Merhautová, V.; Petránková, M.; Spetz, P.; Leppänen, K.; Baldrian, P. Transformation of Quercus Petraea Litter: Successive Changes in Litter Chemistry Are Reflected in Differential Enzyme Activity and Changes in the Microbial Community Composition. FEMS Microbiol. Ecol. 2011, 75, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.T.; Cajthaml, T.; Šnajdr, J.; Baldrian, P. Differential Degradation of Oak (Quercus petraea) Leaf Litter by Litter-Decomposing Basidiomycetes. Res. Microbiol. 2007, 158, 447–455. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Decomposition as a Process—Some Main Features. In Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Berg, B., McClaugherty, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 13–43. ISBN 978-3-030-59631-6. [Google Scholar]
- Lehmann, J.; Schroth, G.; Zech, W. Decomposition and Nutrient Release from Leaves, Twigs and Roots of Three Alley-Cropped Tree Legumes in Central Togo. Agroforest Syst. 1995, 29, 21–36. [Google Scholar] [CrossRef]
- Cai, Y.J.; Chapman, S.J.; Buswell, J.A.; Chang, S. Production and Distribution of Endoglucanase, Cellobiohydrolase, and β-Glucosidase Components of the Cellulolytic System of Volvariella Volvacea, the Edible Straw Mushroom. Appl. Environ. Microbiol. 1999, 65, 553–559. [Google Scholar] [CrossRef]
- Kähkönen, M.A.; Lankinen, P.; Hatakka, A. Hydrolytic and Ligninolytic Enzyme Activities in the Pb Contaminated Soil Inoculated with Litter-Decomposing Fungi. Chemosphere 2008, 72, 708–714. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.; Wu, H.; Ouyang, S.; Zhou, B.; Zeng, Y.; Chen, Y.; Kuzyakov, Y. Tree Species Identity Surpasses Richness in Affecting Soil Microbial Richness and Community Composition in Subtropical Forests. Soil Biol. Biochem. 2019, 130, 113–121. [Google Scholar] [CrossRef]
- Loreau, M. Microbial Diversity, Producer–Decomposer Interactions and Ecosystem Processes: A Theoretical Model. Proc. R. Soc. London Ser. B Biol. Sci. 2001, 268, 303–309. [Google Scholar] [CrossRef]
- Loreau, M.; Hector, A. Partitioning Selection and Complementarity in Biodiversity Experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef]
- LeBauer, D.S. Litter Degradation Rate and β-Glucosidase Activity Increase with Fungal Diversity. Can. J. For. Res. 2010, 40, 1076–1085. [Google Scholar] [CrossRef]
- Novotný, Č.; Svobodová, K.; Erbanová, P.; Cajthaml, T.; Kasinath, A.; Lang, E.; Šašek, V. Ligninolytic Fungi in Bioremediation: Extracellular Enzyme Production and Degradation Rate. Soil Biol. Biochem. 2004, 36, 1545–1551. [Google Scholar] [CrossRef]
- Baldrian, P. Chapter 2 Enzymes of Saprotrophic Basidiomycetes. In British Mycological Society Symposia Series; Boddy, L., Frankland, J.C., van West, P., Eds.; Ecology of Saprotrophic Basidiomycetes; Academic Press: Cambridge, MA, USA, 2008; Volume 28, pp. 19–41. [Google Scholar]
- Geethanjali, P.A.; Jayashankar, M. A Review on Litter Decomposition by Soil Fungal Community. IOSR J. Pharm. Biol. Sci. 2016, 11, 01–03. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; de Vries, R.P.; Hildén, K.S. Chapter Eleven—Genomics, Lifestyles and Future Prospects of Wood-Decay and Litter-Decomposing Basidiomycota. In Advances in Botanical Research; Martin, F.M., Ed.; Fungi; Academic Press: Cambridge, MA, USA, 2014; Volume 70, pp. 329–370. [Google Scholar]
- Hirose, A.; Kudo, S.; Murakami, T.; Tanaka, K.; Harada, Y.; Hashimoto, M. Lambertellin System, the Mechanism for Fungal Replacement of Monilinia fructigena with Lambertella corni-maris without Competitive Inhibition on Agar Media. Bioorganic Med. Chem. 2014, 22, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Becarelli, S.; Chicca, I.; Siracusa, G.; La China, S.; Gentini, A.; Lorenzi, R.; Munz, G.; Petroni, G.; Levin, D.B.; Di Gregorio, S. Hydrocarbonoclastic Ascomycetes to Enhance Co-Composting of Total Petroleum Hydrocarbon (TPH) Contaminated Dredged Sediments and Lignocellulosic Matrices. N. Biotechnol. 2019, 50, 27–36. [Google Scholar] [CrossRef]
- Koukol, O. New Species of Chalara Occupying Coniferous Needles. Fungal Divers. 2011, 49, 75–91. [Google Scholar] [CrossRef]
- Vanden Wymelenberg, A.; Sabat, G.; Mozuch, M.; Kersten, P.J.; Cullen, D.; Blanchette, R.A. Structure, Organization, and Transcriptional Regulation of a Family of Copper Radical Oxidase Genes in the Lignin-Degrading Basidiomycete Phanerochaete Chrysosporium. Appl. Environ. Microbiol. 2006, 72, 4871–4877. [Google Scholar] [CrossRef]
- Barbi, F.; Kohler, A.; Barry, K.; Baskaran, P.; Daum, C.; Fauchery, L.; Ihrmark, K.; Kuo, A.; LaButti, K.; Lipzen, A.; et al. Fungal Ecological Strategies Reflected in Gene Transcription—A Case Study of Two Litter Decomposers. Environ. Microbiol. 2020, 22, 1089–1103. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Toots, M.; Diédhiou, A.G.; Henkel, T.W.; Kjøller, R.; Morris, M.H.; Nara, K.; Nouhra, E.; Peay, K.G.; et al. Towards Global Patterns in the Diversity and Community Structure of Ectomycorrhizal Fungi. Mol. Ecol. 2012, 21, 4160–4170. [Google Scholar] [CrossRef]
- Fernandez, C.W.; See, C.R.; Kennedy, P.G. Decelerated Carbon Cycling by Ectomycorrhizal Fungi Is Controlled by Substrate Quality and Community Composition. New Phytol. 2020, 226, 569–582. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Ryberg, M.; Otsing, E.; Kõljalg, U.; Abarenkov, K. Global Biogeography of the Ectomycorrhizal /Sebacina Lineage (Fungi, Sebacinales) as Revealed from Comparative Phylogenetic Analyses. Mol. Ecol. 2014, 23, 4168–4183. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.C.; Cairney, J.W.G. Ectomycorrhizal Fungi: Exploring the Mycelial Frontier. FEMS Microbiol. Rev. 2007, 31, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Strullu-Derrien, C.; Selosse, M.-A.; Kenrick, P.; Martin, F.M. The Origin and Evolution of Mycorrhizal Symbioses: From Palaeomycology to Phylogenomics. New Phytol. 2018, 220, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.I.; Frum, A.; Dobrea, C.M.; Cristea, R.; Gligor, F.G.; Vicas, L.G.; Ionete, R.E.; Sutan, N.A.; Georgescu, C. Comparative Antioxidant and Antimicrobial Activities of Several Conifer Needles and Bark Extracts. Pharmaceutics 2024, 16, 52. [Google Scholar] [CrossRef]
- Simon, J.; Dörken, V.M.; L.-M.-Arnold, A.; Adamczyk, B. Environmental Conditions and Species Identity Drive Metabolite Levels in Green Leaves and Leaf Litter of 14 Temperate Woody Species. Forests 2018, 9, 775. [Google Scholar] [CrossRef]
- Kusuda, M.; Ueda, M.; Miyatake, K.; Terashita, T. Characterization of the Carbohydrase Productions of an Ectomycorrhizal Fungus, Tricholoma Matsutake. Mycoscience 2008, 49, 291–297. [Google Scholar] [CrossRef]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canbäck, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent Losses of Decay Mechanisms and Rapid Turnover of Symbiosis Genes in Mycorrhizal Mutualists. Nat. Genet. 2015, 47, 410–415. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sánchez-García, M.; Morin, E.; Andreopoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-Scale Genome Sequencing of Mycorrhizal Fungi Provides Insights into the Early Evolution of Symbiotic Traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef]
- Looney, B.; Miyauchi, S.; Morin, E.; Drula, E.; Courty, P.-E.; Kohler, A.; Lindquist, E.; Kuo, A.; Labutti, K.; Pangilinan, J.; et al. Evolutionary Priming and Transition to the Ectomycorrhizal Habit in an Iconic Lineage of Mushroom-Forming Fungi: Is Preadaptation a Requirement? bioRxiv 2021. [Google Scholar] [CrossRef]
- Lebreton, A.; Zeng, Q.; Miyauchi, S.; Kohler, A.; Dai, Y.-C.; Martin, F.M. Evolution of the Mode of Nutrition in Symbiotic and Saprotrophic Fungi in Forest Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 385–404. [Google Scholar] [CrossRef]
- Nehls, U.; Plassard, C. Nitrogen and Phosphate Metabolism in Ectomycorrhizas. New Phytol. 2018, 220, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial Separation of Litter Decomposition and Mycorrhizal Nitrogen Uptake in a Boreal Forest. New Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Hug, L.A.; Co, R. It Takes a Village: Microbial Communities Thrive through Interactions and Metabolic Handoffs. mSystems 2018, 3, e00152-17. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.A.; Starke, R.; Martinovic, T.; Parente Fernandes, M.L.; Orgiazzi, A.; Bastida, F. Distribution of Phosphorus Cycling Genes across Land Uses and Microbial Taxonomic Groups Based on Metagenome and Genome Mining. Soil Biol. Biochem. 2022, 174, 108826. [Google Scholar] [CrossRef]
- Matsumura, K.; Obata, H.; Hata, Y.; Kawato, A.; Abe, Y.; Akita, O. Isolation and Characterization of a Novel Gene Encoding α-L-Arabinofuranosidase from Aspergillus Oryzae. J. Biosci. Bioeng. 2004, 98, 77–84. [Google Scholar] [CrossRef]
- Rodionov, D.A.; Rodionova, I.A.; Rodionov, V.A.; Arzamasov, A.A.; Zhang, K.; Rubinstein, G.M.; Tanwee, T.N.N.; Bing, R.G.; Crosby, J.R.; Nookaew, I.; et al. Transcriptional Regulation of Plant Biomass Degradation and Carbohydrate Utilization Genes in the Extreme Thermophile Caldicellulosiruptor Bescii. mSystems 2021, 6, e0134520. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Xiao, M.; Feng, Z.; Yu, Y.; Yao, H. Effects of Microplastics on Soil Carbon Dioxide Emissions and the Microbial Functional Genes Involved in Organic Carbon Decomposition in Agricultural Soil. Sci. Total Environ. 2022, 806, 150714. [Google Scholar] [CrossRef]
- Li, M.; Hao, Y.; Yan, Z.; Kang, E.; Wang, J.; Zhang, K.; Li, Y.; Wu, H.; Kang, X. Long-term Degradation from Marshes into Meadows Shifts Microbial Functional Diversity of Soil Phosphorus Cycling in an Alpine Wetland of the Tibetan Plateau. Land. Degrad. Dev. 2022, 33, 628–637. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Wang, J.; He, L.; Wang, J.; Guo, Y.; Zhao, F. Contrasting Soil Microbial Functional Potential for Phosphorus Cycling in Subtropical and Temperate Forests. Forests 2022, 13, 2002. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Rai, P.; Srivastava, A.K.; Kumar, S. Trichoderma for Climate Resilient Agriculture. World J. Microbiol. Biotechnol. 2017, 33, 155. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma Biofertilizer Links to Altered Soil Chemistry, Altered Microbial Communities, and Improved Grassland Biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.J.; Girguis, P.R.; Preston, C.M.; Richardson, P.M.; DeLong, E.F. Identification of Methyl Coenzyme M Reductase A (mcrA) Genes Associated with Methane-Oxidizing Archaea. Appl. Environ. Microbiol. 2003, 69, 5483–5491. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.S.; Borovkova, A.N.; Shalamitskiy, M.Y.; Naumov, G.I. Natural Polymorphism of Pectinase PGU Genes in the Saccharomyces Yeasts. Microbiology 2021, 90, 349–360. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, T.; Han, P.; Zhou, J.; Zhao, Y.; Huang, L.; Yuan, Y. Isolation, Genomic Characterization, and Mushroom Growth-Promoting Effect of the First Fungus-Derived Rhizobium. Front. Microbiol. 2022, 13, 947687. [Google Scholar] [CrossRef]
- Granja-Travez, R.S.; Persinoti, G.F.; Squina, F.M.; Bugg, T.D.H. Functional Genomic Analysis of Bacterial Lignin Degraders: Diversity in Mechanisms of Lignin Oxidation and Metabolism. Appl. Microbiol. Biotechnol. 2020, 104, 3305–3320. [Google Scholar] [CrossRef]
- Zhang, Y.; He, G.; Yang, L.; Wen, S.; Yan, J.; Min, B.; Peng, T.; Ji, L. Phosphorus Fertilizer Application Shifts the Rhizosphere Bacterial Community and Their Carbon, Nitrogen and Phosphorus-Cycle Genes in a Phoebe bournei Young Plantation. Appl. Soil Ecol. 2024, 198, 105391. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree Species Influence on Microbial Communities in Litter and Soil: Current Knowledge and Research Needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Y.; Zhu, J.; Sun, L.; Ma, X. Impacts of Global Warming on Forest Litters. Available online: http://www.sjlyyj.com/article/doi/10.13348/j.cnki.sjlyyj.2022.0079.y (accessed on 28 December 2023).
- Lamarche, J.; Bradley, R.L.; Hooper, E.; Shipley, B.; Simao Beaunoir, A.-M.; Beaulieu, C. Forest Floor Bacterial Community Composition and Catabolic Profiles in Relation to Landscape Features in Québec’s Southern Boreal Forest. Microb. Ecol. 2007, 54, 10–20. [Google Scholar] [CrossRef]
- Habtewold, J.Z.; Helgason, B.L.; Yanni, S.F.; Janzen, H.H.; Ellert, B.H.; Gregorich, E.G. Litter Composition Has Stronger Influence on the Structure of Soil Fungal than Bacterial Communities. Eur. J. Soil Biol. 2020, 98, 103190. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, W.; Chang, L.; Qu, Z.; Liu, B.; Liu, K.; Zhang, Y.; Huang, L.; Sun, H. Dominant Tree Species and Litter Quality Govern Fungal Community Dynamics during Litter Decomposition. J. Fungi 2024, 10, 690. https://doi.org/10.3390/jof10100690
Meng W, Chang L, Qu Z, Liu B, Liu K, Zhang Y, Huang L, Sun H. Dominant Tree Species and Litter Quality Govern Fungal Community Dynamics during Litter Decomposition. Journal of Fungi. 2024; 10(10):690. https://doi.org/10.3390/jof10100690
Chicago/Turabian StyleMeng, Wenjing, Lin Chang, Zhaolei Qu, Bing Liu, Kang Liu, Yuemei Zhang, Lin Huang, and Hui Sun. 2024. "Dominant Tree Species and Litter Quality Govern Fungal Community Dynamics during Litter Decomposition" Journal of Fungi 10, no. 10: 690. https://doi.org/10.3390/jof10100690
APA StyleMeng, W., Chang, L., Qu, Z., Liu, B., Liu, K., Zhang, Y., Huang, L., & Sun, H. (2024). Dominant Tree Species and Litter Quality Govern Fungal Community Dynamics during Litter Decomposition. Journal of Fungi, 10(10), 690. https://doi.org/10.3390/jof10100690






