Genome Sequencing and Analysis of Nigrospora oryzae, a Rice Leaf Disease Fungus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Strain
2.2. Identification of Strain
2.3. Whole-Genome Sequencing
2.4. Genomic Component Analysis
2.5. Protein-Encoding Gene Prediction
2.6. Gene Function Annotation
2.7. Data Availability
2.8. Collinearity Analysis
3. Results
3.1. Strain Identification
3.2. Genome Assembly and Evaluation
3.3. Repeat Sequence Prediction
3.4. Protein-Coding Gene, Non-Coding RNA, and Pseudogene Prediction
3.5. Transcription Factor Prediction
3.6. Functional Annotation of the Genome
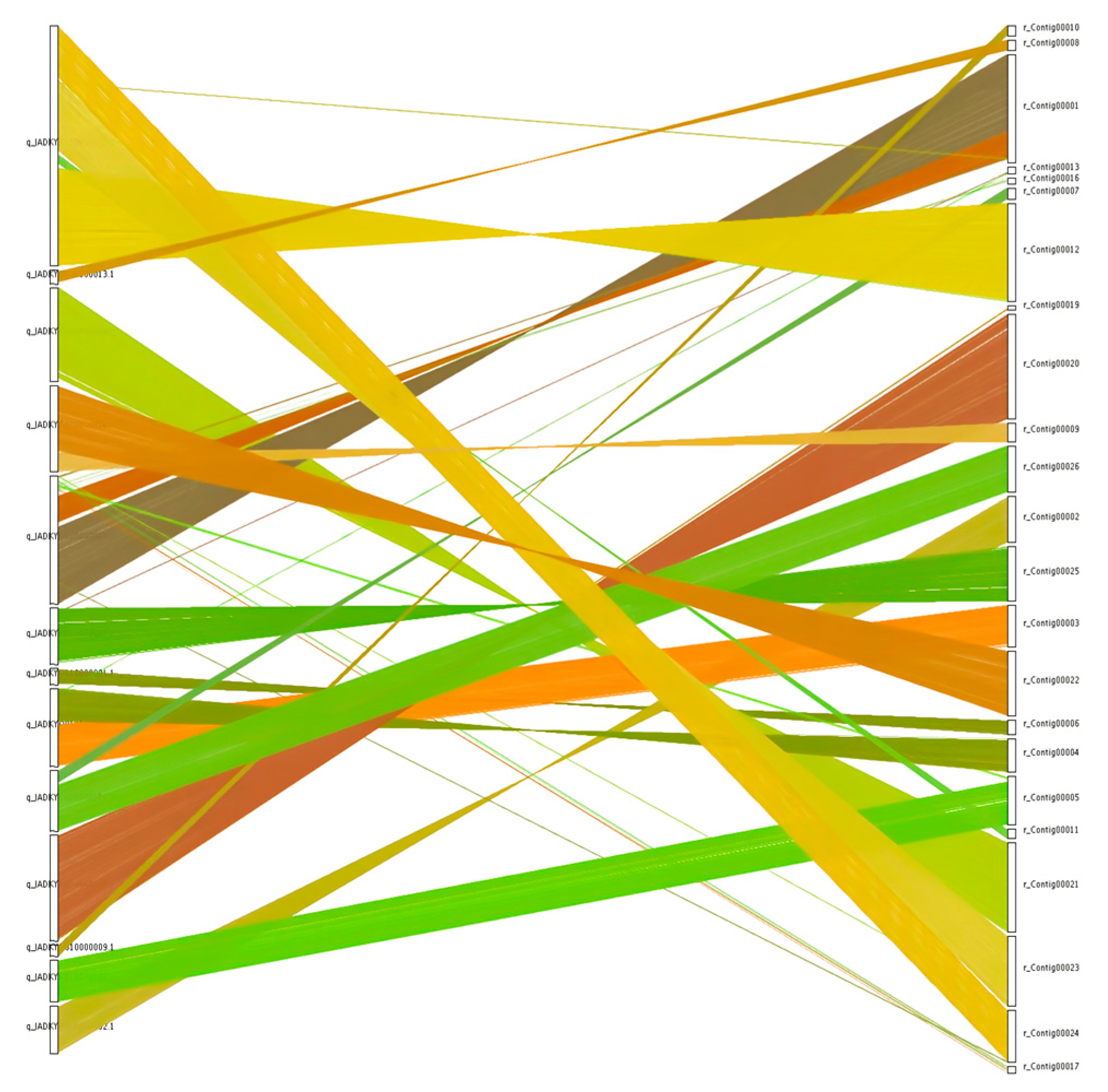
3.7. Collinearity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.X.; Li, S.S.; Tan, G.J.; Shen, J.T.; He, T. First report of Nigrospora oryzae causing leaf spot of cotton in China. Plant Dis. 2012, 96, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shi, F.; Kelly, D.; Hsiang, T. First report of leaf spot of Kentucky Bluegrass (Poa pratensis) caused by Nigrospora oryzae in Ontario. Plant Dis. 2012, 96, 909. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukushima, T.; Tsujino, Y.; Fujimori, T. Nigrosporins A and B, new phytotoxic and antibacterial metabolites produced by a Fungus Nigrospora oryzae. Biosci. Biotechnol. Biochem. 1997, 61, 1848–1852. [Google Scholar] [CrossRef]
- Laemmlen, F.F. Interdependence of a mite, siteroptes reniformis, and a fungus, Nigrospora oryzae, in the nigrospora lint rot of cotton. Phytopathology 1973, 63, 308. [Google Scholar] [CrossRef]
- Palmateer, A.J.; McLean, K.S.; van Santen, E.; Morgan-Jones, G. Occurrence of nigrospora lint rot caused by Nigrospora oryzae on cotton in Alabama. Plant Dis. 2003, 87, 873. [Google Scholar] [CrossRef]
- van den Brink, J.; de Vries, R.P. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef] [PubMed]
- Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Standen, J.H. Nigrospora oryzae (B. and Br.) fetch on maize. Phytopathology 1945, 35, 552–564. [Google Scholar]
- Liu, L.M.; Zhao, Y.; Zhang, Y.L.; Fu, Q.; Huang, S.W. Nigrospora oryzae causing Panicle Branch Rot Disease on Oryza sativa (rice). Plant Dis. 2021, 105, 2724. [Google Scholar] [CrossRef]
- Sha, H.; Liu, X.; Xiao, X.; Zhang, H.; Gu, X.; Chen, W.; Mao, B. Nigrospora oryzae causing Leaf Spot Disease on Chrysanthemum × morifolium Ramat and Screening of its potential antagonistic bacteria. Microorganisms 2023, 11, 2224. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Zhao, C.; Li, L.; Wu, Z. Potential Fungicide Candidates: A Dual Action Mode Study of Novel Pyrazole-4-carboxamides against Gibberella zeae. J. Agric. Food Chem. 2023, 71, 1862–1872. [Google Scholar] [CrossRef]
- Yun, Y.; Song, A.; Bao, J.; Chen, S.; Lu, S.; Cheng, C.; Zheng, W.; Wang, Z.; Zhang, L. Genome data of Fusarium oxysporum f.sp. cubense race 1 and tropical race 4 isolates using long-read sequencing. Mol. Plant Microbe Interact. 2019, 32, 1270–1272. [Google Scholar] [PubMed]
- Zhu, B.; Wang, S.; Mi, C.Y.; Yang, R.H.; Zen, G.H.; Hu, X.F. Genome sequence resource for Ilyonectria mors-panacis, causing rusty root rot of Panax notoginseng. Mol. Plant-Microbe Interact. 2019, 32, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, K.; Coetzee, M.P.A.; Hoffmeister, D. Secrets of the subterranean pathosystem of Armillaria. Mol. Plant Pathol. 2011, 12, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.A.; Yoon, G.M.; Aryal, U.K.; Lail, K.; Amirebrahimi, M.; LaButti, K.; Lipzen, A.; Riley, R.; Barry, K.; Henrissat, B.; et al. Symbiotic nitrogen fixation in the reproductive structures of a basidiomycete fungus. Curr. Biol. 2021, 31, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Sipos, G.; Prasanna, A.N.; Walter, M.C.; O’Connor, E.; Bálint, B.; Krizsán, K.; Kiss, B.; Hess, J.; Varga, T.; Slot, J.; et al. Genome expansion and lineage-specific genetic innovations in the forest pathogenic fungi Armillaria. Nat. Ecol. Evol. 2017, 1, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Mahuku, G.S. A simple extraction method suitable for PCR-based analysis of plant, fungal, and bacterial DNA. Plant Mol. Biol. Report. 2012, 22, 71–81. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, J.Z.; Zhang, L.Y.; Xu, L.Z.; Yan, C.; Gong, Z.P. Identification and characterization of Cucurbita gummy stem blight fungi in Northeast China. J. Phytopathol. 2018, 166, 305–313. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. bioRxiv 2016, 071282. [Google Scholar] [CrossRef]
- Ruan, J.; Li, H. Fast and accurate long-read assembly with wtdbg2. bioRxiv 2019. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H. LTR_FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007, 35, W265–W268. [Google Scholar] [CrossRef]
- Han, Y.; Wessler, S.R. MITE-Hunter: A program for discovering miniature inverted-repeat transposable elements from genomic sequences. Nucleic Acids Res. 2010, 38, e199. [Google Scholar] [CrossRef]
- Price, A.L.; Jones, N.C.; Pevzner, P.A. De novo identification of repeat families in large genomes. Bioinformatics 2005, 21, i351–i358. [Google Scholar] [CrossRef]
- Edgar, R.C.; Myers, E.W. PILER: Identification and classification of genomic repeats. Bioinformatics 2015, 21, i152–i158. [Google Scholar] [CrossRef]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Jurka, J.; Kapitonov, V.V.; Pavlicek, A.; Klonowski, P.; Kohany, O.; Walichiewicz, J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 2005, 110, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Tarailo-Graovac, M.; Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinform. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.; Karlin, S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997, 268, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Waack, S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 2003, 19, ii215–ii225. [Google Scholar] [CrossRef] [PubMed]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Parra, G.; Guigó, R. Using geneid to identify genes. Curr. Protoc. Bioinform. 2007, 43, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Korf, I. Gene finding in novel genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef]
- Keilwagen, J.; Wenk, M.; Erickson, J.L.; Schattat, M.H.; Jan, G.; Frank, H. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 2016, 44, e89. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650. [Google Scholar] [CrossRef]
- Duarte, G.T.; Volkova, P.Y.; Geras’kin, S.A. A pipeline for non-model organisms for de novo transcriptome assembly, annotation, and gene ontology analysis using open tools: Case study with scots pine. Bio Protoc. 2021, 11, e3912. [Google Scholar] [CrossRef]
- Campbell, M.A.; Haas, B.J.; Hamilton, J.P.; Mount, S.M.; Buell, C.R. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC Genom. 2006, 7, 327. [Google Scholar] [CrossRef]
- Haas, B.J.; Salzberg, S.L.; Zhu, W.; Pertea, M.; Allen, J.E.; Orvis, J.; White, O.; Buell, C.R.; Wortman, J.R. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008, 9, R7. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; González-Calle, V.; Carbonero, P.; Barrero-Sicilia, C. The family of DOF transcription factors in Brachypodium distachyon: Phylogenetic comparison with rice and barley DOFs and expression profiling. BMC Plant Biol. 2012, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.D.; Zhu, W.H.; Niu, T.T.; Liu, Z.Y. Nigrospora oryzae causing leaf spot on Asiatic Dayflower in Chongqing, China. Plant Dis. 2012, 106, 763. [Google Scholar] [CrossRef]



| Features | N. oryzae |
|---|---|
| Assembly size (Mb) | 42.09 |
| Scaffolds | 26 |
| GC (%) | 58.83 |
| Repeated sequences (%) | 2.93 |
| Protein-coding genes | 10,688 |
| Gene density (genes per Mb) | 254 |
| Secreted proteins | 879 |
| tRNA | 272 |
| Pseudogenes | 17 |
| Average gene length (bp) | 2356 |
| Species | Total Gene Number | Cluster Gene Number | Total Gene Family Number | Unique Gene Family Number |
|---|---|---|---|---|
| GZL1 | 10,544 | 10,028 | 9588 | 27 |
| ZQ1 | 10,688 | 10,068 | 9585 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Zhang, L.; Wu, J. Genome Sequencing and Analysis of Nigrospora oryzae, a Rice Leaf Disease Fungus. J. Fungi 2024, 10, 100. https://doi.org/10.3390/jof10020100
Zhao Q, Zhang L, Wu J. Genome Sequencing and Analysis of Nigrospora oryzae, a Rice Leaf Disease Fungus. Journal of Fungi. 2024; 10(2):100. https://doi.org/10.3390/jof10020100
Chicago/Turabian StyleZhao, Qian, Liyan Zhang, and Jianzhong Wu. 2024. "Genome Sequencing and Analysis of Nigrospora oryzae, a Rice Leaf Disease Fungus" Journal of Fungi 10, no. 2: 100. https://doi.org/10.3390/jof10020100
APA StyleZhao, Q., Zhang, L., & Wu, J. (2024). Genome Sequencing and Analysis of Nigrospora oryzae, a Rice Leaf Disease Fungus. Journal of Fungi, 10(2), 100. https://doi.org/10.3390/jof10020100






