Poly-Unsaturated Fatty Acids (PUFAs) from Cunninghamella elegans Grown on Glycerol Induce Cell Death and Increase Intracellular Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material, Microbial Culture Media, and Coditions and Analyses Related to Microbial Growth
2.2. Preparation of C. elegans Fatty Acid Lithium Salts (FALS)
2.3. Optimization of the Saponification Reaction of C. elegans Lipids
2.4. Determination of Fatty Acid Lithium Salts Content in Fatty Acids
2.5. Cell Culture
2.6. Proliferation Assays
2.7. Migration Assays
2.8. Fluorescence and Confocal Microscopy
2.9. Flow Cytometry
3. Results
3.1. Growth of C. elegans on Glycerol—Comparisons with the Growth on Glucose
3.2. Fatty Acid (FA) Composition of the Microbial Lipids Produced by C. elegans
3.3. Optimization of the Saponification Reaction of C. elegans Lipids
3.4. C. elegans Fatty Acid Lithium Salts Inhibit the Growth of Normal and Cancerous Cell Lines
3.5. C. elegans Fatty Acid Lithium Salts Decrease the Migration and Wound Healing Ability of Cancer Cells
3.6. Fatty Acids from C. elegans Fatty Acid Lithium Salts Are Stored Inside the Endoplasmic Reticulum
3.7. C. elegans Fatty Acid Lithium Salts Can Induce Cell Death
3.8. Administration of C. elegans Fatty Acid Lithium Salts Increases the Levels of the Intracellular Reactive Oxygen Species (ROS)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Response | |||
|---|---|---|---|---|
| Run | A: Time (min) | B: Hexane (mL) | C: Salt (g) | Weight of FFAs (g) |
| 1 | 82.5 | 18.0 | 2.0 | 0.6824 |
| 2 | 60.0 | 21.0 | 1.0 | 0.7693 |
| 3 | 82.5 | 13.0 | 2.0 | 0.5876 |
| 4 | 82.5 | 18.0 | 2.0 | 0.6948 |
| 5 | 105.0 | 21.0 | 3.0 | 0.7414 |
| 6 | 82.5 | 18.0 | 2.0 | 0.6976 |
| 7 | 120.3 | 18.0 | 2.0 | 0.7906 |
| 8 | 82.5 | 23.0 | 2.0 | 0.6926 |
| 9 | 105.0 | 15.0 | 1.0 | 0.7518 |
| 10 | 82.5 | 18.0 | 2.0 | 0.7583 |
| 11 | 44.7 | 18.0 | 2.0 | 0.8732 |
| 12 | 82.5 | 18.0 | 0.3 | 0.7632 |
| 13 | 82.5 | 18.0 | 2.0 | 0.7364 |
| 14 | 60.0 | 15.0 | 3.0 | 0.6810 |
| 15 | 60.0 | 15.0 | 1.0 | 0.7475 |
| 16 | 105.0 | 21.0 | 1.0 | 0.7290 |
| 17 | 82.5 | 18.0 | 3.7 | 0.6722 |
| 18 | 82.5 | 18.0 | 2.0 | 0.6739 |
| 19 | 105.0 | 15.0 | 3.0 | 0.7558 |
| 20 | 60.0 | 21.0 | 3.0 | 0.7429 |
Appendix B




Appendix C

Appendix D

Appendix E


References
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2002; Volume 51, pp. 1–52. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef]
- Fazili, A.B.A.; Shah, A.M.; Zan, X.; Naz, T.; Nosheen, S.; Nazir, Y.; Ullah, S.; Zhang, H.; Song, Y. Mucor circinelloides: A model organism for oleaginous fungi and its potential applications in bioactive lipid production. Microb. Cell Fact. 2022, 21, 29. [Google Scholar] [CrossRef]
- Robles-Iglesias, R.; Naveira-Pazos, C.; Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. Factors affecting the optimisation and scale-up of lipid accumulation in oleaginous yeasts for sustainable biofuels production. Renew. Sustain. Energy Rev. 2023, 171, 113043. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Awad, M.F.; Shah, A.M.; Nazir, Y.; Naz, T.; Hassane, A.; Nosheen, S.; Song, Y. Evaluation of Different Standard Amino Acids to Enhance the Biomass, Lipid, Fatty Acid, and γ-Linolenic Acid Production in Rhizomucor pusillus and Mucor circinelloides. Front. Nutr. 2022, 9, 876817. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Yeasts, moulds, algae and bacteria as sources of lipids. In Technological Advances in Improved and Alternative Sources of Lipids; Kamel, B.S., Kakuda, Y., Eds.; Springer: Boston, MA, USA, 1994; pp. 235–291. [Google Scholar]
- González-Fernández, M.J.; Ortea, I.; Guil-Guerrero, J.L. α-Linolenic and γ-linolenic acids exercise differential antitumor effects on HT-29 human colorectal cancer cells. Toxicol. Res. 2020, 9, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Huerta-Yepez, S. The Involvement of Polyunsaturated Fatty Acids in Apoptosis Mechanisms and Their Implications in Cancer. Int. J. Mol. Sci. 2023, 24, 11691. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, S. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014, 37, 112–119. [Google Scholar] [CrossRef]
- Colquhoun, A. Gamma-linolenic acid alters the composition of mitochondrial membrane subfractions, decreases outer mitochondrial membrane binding of hexokinase and alters carnitine palmitoyltransferase I properties in the Walker 256 rat tumour. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2002, 1583, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Oono, K.; Ohtake, K.; Watanabe, C.; Shiba, S.; Sekiya, T.; Kasono, K. Contribution of Pyk2 pathway and reactive oxygen species (ROS) to the anti-cancer effects of eicosapentaenoic acid (EPA) in PC3 prostate cancer cells. Lipids Health Dis. 2020, 19, 15. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, H.; Shen, Y.; Ni, X.; Shen, S.; Das, U.N. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch. Med. Sci. 2015, 11, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Hofmanová, J.; Vaculová, A.; Kozubík, A. Polyunsaturated fatty acids sensitize human colon adenocarcinoma HT-29 cells to death receptor-mediated apoptosis. Cancer Lett. 2005, 218, 33–41. [Google Scholar] [CrossRef]
- Serini, S.; Piccioni, E.; Merendino, N.; Calviello, G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis 2009, 14, 135–152. [Google Scholar] [CrossRef]
- Zajdel, A.; Wilczok, A.; Tarkowski, M. Toxic effects of n-3 polyunsaturated fatty acids in human lung A549 cells. Toxicol. Vitr. 2015, 30, 486–491. [Google Scholar] [CrossRef]
- Colquhoun, A.; Schumacher, R.I. Modifications in mitochondrial metabolism and ultrastructure and their relationship to tumour growth inhibition by gamma-linolenic acid. Mol. Cell. Biochem. 2001, 218, 13–20. [Google Scholar] [CrossRef]
- Ge, H.; Kong, X.; Shi, L.; Hou, L.; Liu, Z.; Li, P. Gamma-linolenic acid induces apoptosis and lipid peroxidation in human chronic myelogenous leukemia K562 cells. Cell Biol. Int. 2009, 33, 402–410. [Google Scholar] [CrossRef]
- Leaver, H.A.; Wharton, S.B.; Bell, H.S.; Leaver-Yap, I.M.M.; Whittle, I.R. Highly unsaturated fatty acid induced tumour regression in glioma pharmacodynamics and bioavailability of gamma linolenic acid in an implantation glioma model: Effects on tumour biomass, apoptosis and neuronal tissue histology. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 283–292. [Google Scholar] [CrossRef]
- Kairemo, K.J.A.; Jekunen, A.P.; Korppi-Tommola, E.T.; Pyrhonen, S.O. The effect of lithium γ-linolenate therapy of pancreatic cancer on perfusion in liver and pancreatic tissues. Pancreas 1998, 16, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Ilc, K.; Ferrero, J.M.; Fischel, J.L.; Formento, P.; Bryce, R.; Etienne, M.C.; Milano, G. Cytotoxic effects of two gamma linoleic salts (lithium gammalinolenate or meglumine gammalinolenate) alone or associated with a nitrosourea: An experimental study on human glioblastoma cell lines. Anticancer. Drugs 1999, 10, 413–417. [Google Scholar] [CrossRef]
- Alakhras, R.; Bellou, S.; Fotaki, G.; Stephanou, G.; Demopoulos, N.A.; Papanikolaou, S.; Aggelis, G. Fatty acid lithium salts from Cunninghamella echinulata have cytotoxic and genotoxic effects on HL-60 human leukemia cells. Eng. Life Sci. 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Sayegh, F.; Elazzazy, A.; Bellou, S.; Moustogianni, A.; Elkady, A.I.; Baeshen, M.N.; Aggelis, G. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann. Microbiol. 2016, 66, 937–948. [Google Scholar] [CrossRef]
- Filippousi, R.; Tsouko, E.; Mordini, K.; Ladakis, D.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Sustainable arabitol production by a newly isolated Debaryomyces prosopidis strain cultivated on biodiesel-derived glycerol. Carbon Resour. Convers. 2022, 5, 92–99. [Google Scholar] [CrossRef]
- Dritsas, P.; Aggelis, G. Studies on the co-metabolism of glucose and glycerol in the fungus Umbelopsis isabellina. Carbon Resour. Convers. 2023, 6, 326–333. [Google Scholar] [CrossRef]
- Sarantou, S.; Stoforos, N.G.; Kalantzi, O.; Papanikolaou, S. Biotechnological valorization of biodiesel-derived glycerol: Trials with the non-conventional yeasts Yarrowia lipolytica and Rhodosporidium sp. Carbon Resour. Convers. 2021, 4, 61–75. [Google Scholar] [CrossRef]
- André, A.; Diamantopoulou, P.; Philippoussis, A.; Sarris, D.; Komaitis, M.; Papanikolaou, S. Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: Production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 2010, 31, 407–416. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; pp. 1–428. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C. Boyden chamber assay. Methods Mol. Biol. 2005, 294, 15–22. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Moens, S.J.B.; Tiessens, F.; Bakker, G.J.; Dallinga-Thie, G.M.; Groen, A.K.; Nieuwdorp, M.; Stroes, E.S.G.; Kroon, J. Nile Red Quantifier: A novel and quantitative tool to study lipid accumulation in patient-derived circulating monocytes using confocal microscopy. J. Lipid Res. 2017, 58, 2210–2219. [Google Scholar] [CrossRef]
- Cooksey, K.E.; Guckert, J.B.; Williams, S.A.; Callis, P.R. Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J. Microbiol. Methods 1987, 6, 333–345. [Google Scholar] [CrossRef]
- Kimura, K.; Yamaoka, M.; Kamisaka, Y. Rapid estimation of lipids in oleaginous fungi and yeasts using Nile red fluorescence. J. Microbiol. Methods 2004, 56, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar] [CrossRef]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids containing polyunsaturated fatty acids synthesized by zygomycetes grown on glycerol. Appl. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Philippoussis, A.; Mallouchos, A.; Diamantopoulou, P. Valorization of low-cost, carbon-rich substrates by edible ascomycetes and basidiomycetes grown on liquid cultures. FEMS Microbiol. Lett. 2020, 367, fnaa168. [Google Scholar] [CrossRef]
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Liu, T.M. Inoculum effects on the production of γ-linolenic acid by the shake culture of Cunninghamella echinulata CCRC 31840. Enzyme Microb. Technol. 1997, 21, 137–142. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G. Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J. Appl. Microbiol. 2008, 105, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Zikou, E.; Chatzifragkou, A.; Koutinas, A.A.; Papanikolaou, S. Evaluating glucose and xylose as cosubstrates for lipid accumulation and γ-linolenic acid biosynthesis of Thamnidium elegans. J. Appl. Microbiol. 2013, 114, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged cultivation of selected macro-fungi to produce mycelia rich in β-glucans and other bioactive compounds, valorizing side streams of the food industry. Carbon Resour. Convers. 2024, 7, 100198. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G.; Papanikolaou, S. Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur. J. Lipid Sci. Technol. 2010, 112, 1048–1057. [Google Scholar] [CrossRef]
- Chen, H.C.; Chang, C.C. Production of γ-Linolenic Acid by the Fungus Cunninghamella echinulata CCRC 31840. Biotechnol. Prog. 1996, 12, 338–341. [Google Scholar] [CrossRef]
- Dierge, E.; Feron, O. Dealing with saturated and unsaturated fatty acid metabolism for anticancer therapy. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Seegers, J.C.; De Kock, M.; Lottering, M.L.; Grobler, C.J.S.; Van Papendorp, D.H.; Shou, Y.; Habbersett, R.; Lehnert, B.E. Effects of gamma linolenic acid and arachidonic acid on cell cycle progression and apoptosis induction in normal and transformed cells. Prostaglandins Leukot. Essent. Fat. Acids 1997, 56, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Andreoli Miyake, J.; Nascimento Gomes, R.; Colquhoun, A. Gamma-Linolenic acid alters migration, proliferation and apoptosis in human and rat glioblastoma cells. Prostaglandins Other Lipid Mediat. 2020, 150, 106452. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wu, C.H.; Wu, K.C.; Shiao, L.R.; Chuang, C.M.; Leung, Y.M.; Chow, L. A basal level of γ-linolenic acid depletes Ca2+ stores and induces endoplasmic reticulum and oxidative stresses to cause death of breast cancer BT-474 cells. Chin. J. Physiol. 2021, 64, 202–209. [Google Scholar] [CrossRef]
- Kong, X.; Ge, H.; Hou, L.; Shi, L.; Liu, Z. Induction of apoptosis in K562/ADM cells by gamma-linolenic acid involves lipid peroxidation and activation of caspase-3. Chem. Biol. Interact. 2006, 162, 140–148. [Google Scholar] [CrossRef]
- Tanaka, A.; Yamamoto, A.; Murota, K.; Tsujiuchi, T. Biochemical and Biophysical Research Communications Polyunsaturated fatty acids induce ovarian cancer cell death through ROS-dependent MAP kinase activation. Biochem. Biophys. Res. Commun. 2017, 493, 468–473. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Matsushita, M.; Freigang, S.; Schneider, C.; Conrad, M.; Bornkamm, G.W.; Kopf, M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568. [Google Scholar] [CrossRef]
- Sarparast, M.; Pourmand, E.; Hinman, J.; Vonarx, D.; Reason, T.; Zhang, F.; Paithankar, S.; Chen, B.; Borhan, B.; Watts, J.L.; et al. Dihydroxy-Metabolites of Dihomo-γ-linolenic Acid Drive Ferroptosis-Mediated Neurodegeneration. ACS Cent. Sci. 2023, 9, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Ta, N.; Jiang, X.; Zhang, Y.; Wang, H. Ferroptosis as a promising therapeutic strategy for melanoma. Front. Pharmacol. 2023, 14, 1252567. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Lalrinzuali, S.; Khushboo, M.; Dinata, R.; Bhanushree, B.; Nisa, N. Long-term consumption of fermented pork fat-based diets differing in calorie, fat content, and fatty acid levels mediates oxidative stress, inflammation, redox imbalance, germ cell apoptosis, disruption of steroidogenesis, and testicular dysfun. Environ. Sci. Pollut. Res. 2023, 30, 52446–52471. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Vázquez, E.Y.; Quintas-Granados, L.I.; Cortés, H.; González-Del Carmen, M.; Leyva-Gómez, G.; Rodríguez-Morales, M.; Bustamante-Montes, L.P.; Silva-Adaya, D.; Perez-Plasencia, C.; Jacobo-Herrera, N.; et al. Lithium: A Promising Anticancer Agent. Life 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Hossein, G.; Janzamin, E.; Azimian-Zavareh, V. Effect of lithium chloride and antineoplastic drugs on survival and cell cycle of androgen-dependent prostate cancer LNCap cells. Indian J. Pharmacol. 2012, 44, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Hossein, G.; Zavareh, V.A.; Fard, P.S. Combined Treatment of Androgen-Independent Prostate Cancer Cell Line DU145 with Chemotherapeutic Agents and Lithium Chloride: Effect on Growth Arrest and/or Apoptosis. Avicenna J. Med. Biotechnol. 2012, 4, 75–87. [Google Scholar] [PubMed]
- Bgatova, N.P.; Gavrilova, Y.S.; Lykov, A.P.; Solovieva, A.O.; Makarova, V.; Borodin, Y.I.; Konenkov, V.I. Apoptosis and autophagy in hepatocarcinoma cells induced by different forms of lithium salts. Cell Tissue Biol. 2017, 11, 261–267. [Google Scholar] [CrossRef]
- Taskaeva, Y.S.; Bgatova, N.P.; Dossymbekova, R.S.; Solovieva, A.O.; Miroshnichenko, S.M.; Sharipov, K.O.; Tungushbaeva, Z.B. In vitro effects of lithium carbonate on cell cycle, apoptosis, and autophagy in hepatocellular carcinoma-29 cells. Bull. Exp. Biol. Med. 2020, 170, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, S.; Xie, H.; Wang, C.; Gao, X.; Rong, Y.; Liu, Z.; Lu, Y. KIF18B promotes hepatocellular carcinoma progression through activating Wnt/β-catenin-signaling pathway. J. Cell. Physiol. 2020, 235, 6507–6514. [Google Scholar] [CrossRef] [PubMed]
- Vijay, G.V.; Zhao, N.; Hollander, P.D.; Toneff, M.J.; Joseph, R.; Pietila, M.; Taube, J.H.; Sarkar, T.R.; Ramirez-Pena, E.; Werden, S.J.; et al. GSK3β regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Ramshini, S.; Omidi, M. The Psychiatric Drug Lithium Increases DNA Damage and Decreases Cell Survival in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines Expos ed to Ionizing Radiation. Curr. Mol. Pharmacol. 2019, 12, 301–310. [Google Scholar]
- Cohen-Harazi, R.; Hofmann, S.; Kogan, V.; Fulman-Levy, H.; Abaev, K.; Shovman, O.; Brider, T.; Koman, I. Cytotoxicity of Exogenous Acetoacetate in Lithium Salt Form Is Mediated by Lithium and Not Acetoacetate. Anticancer Res. 2020, 40, 3831–3837. [Google Scholar] [CrossRef]
- Kim, H.; Li, S.; Lee, D.; Park, J.H.; Muramatsu, T.; Harada, H.; Jung, Y.; Jung, H. Activation of Wnt signalling reduces the population of cancer stem cells in ameloblastoma. Cell Prolif. 2021, 54, e13073. [Google Scholar] [CrossRef]
- Varela-lópez, A.; Battino, M.; Navarro-hortal, M.D.; Giampieri, F.; Forbes-hernández, T.Y.; Romero-márquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef]
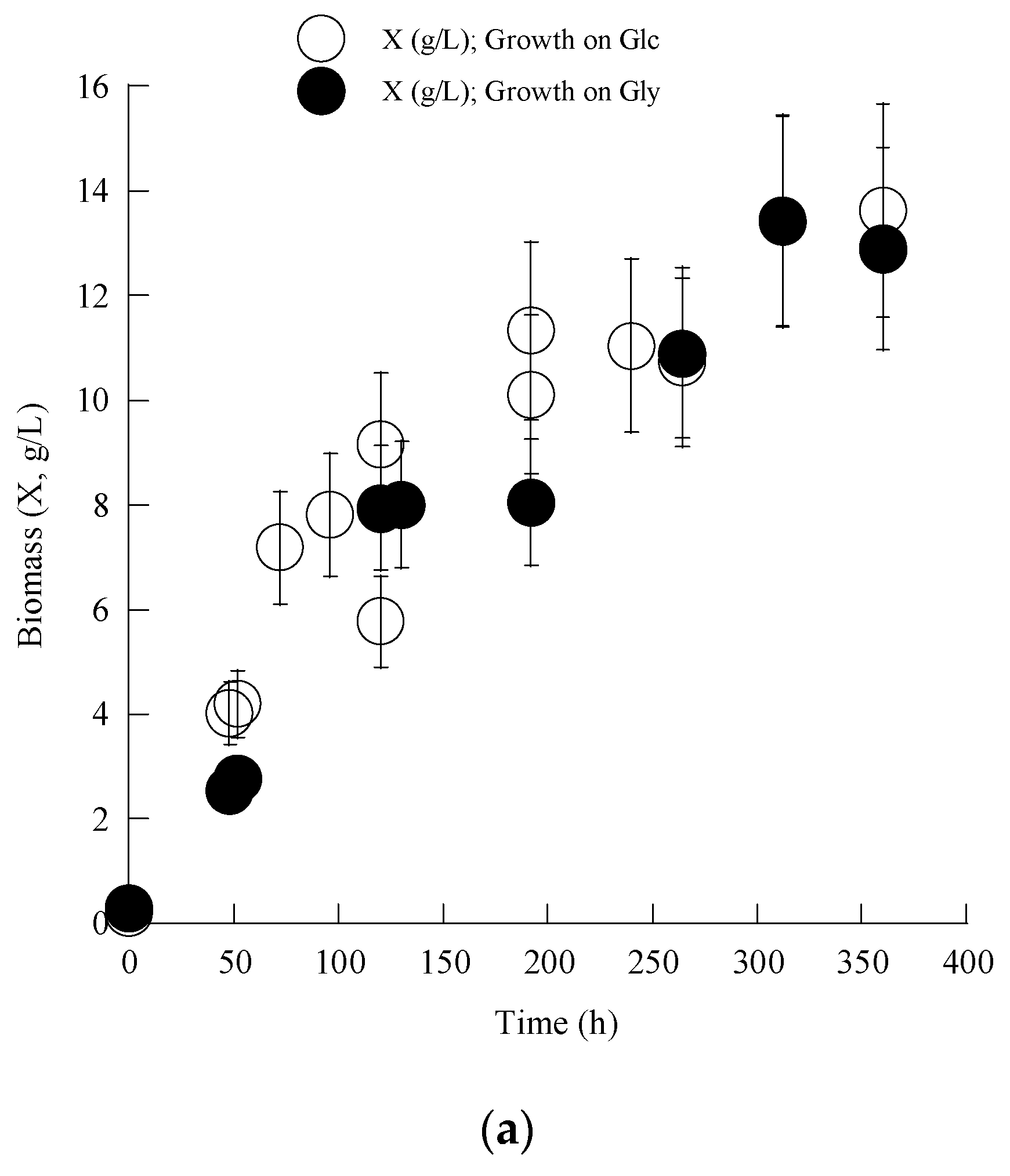






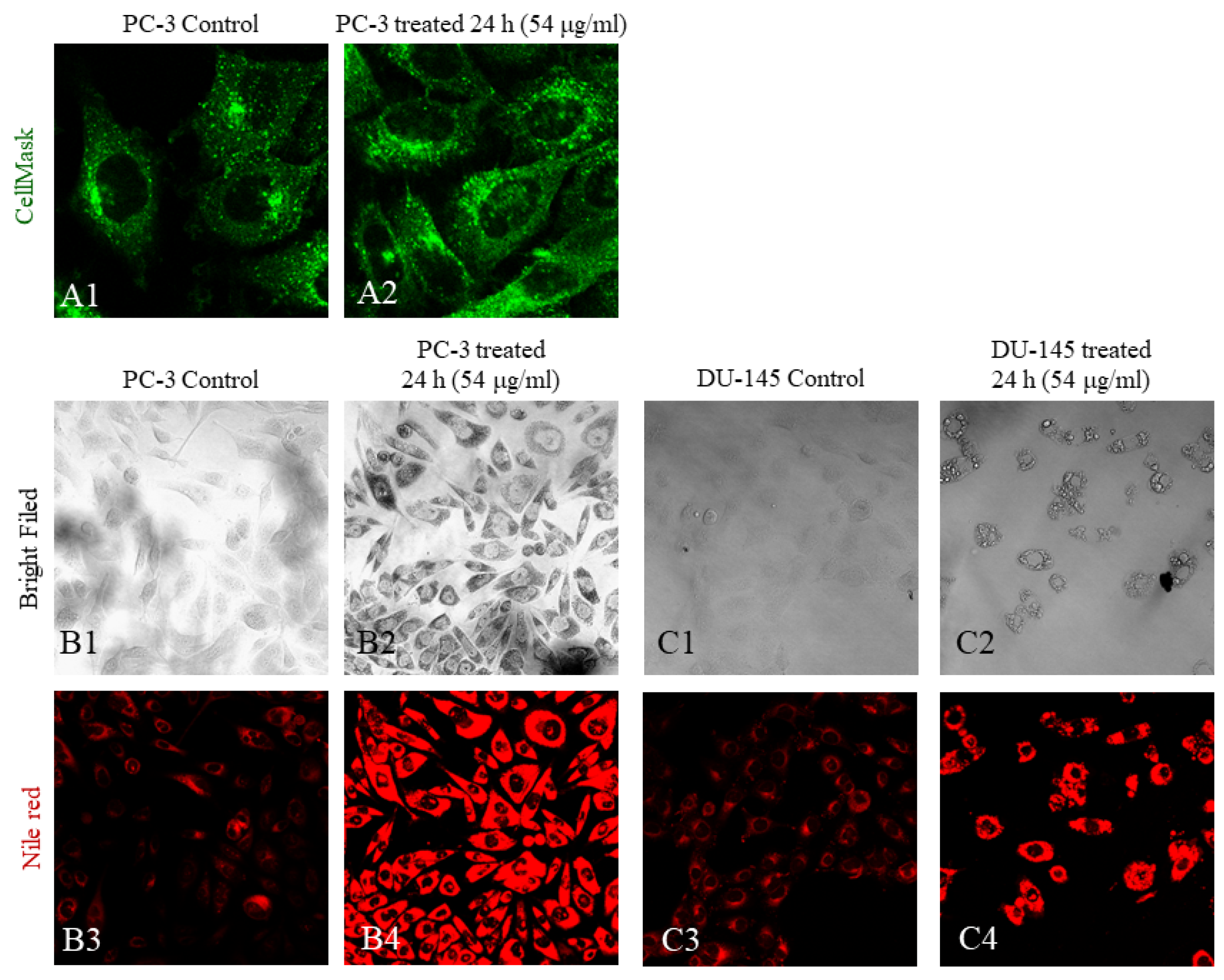


| Cellular Fatty Acids | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ6,9,12C18:3 |
|---|---|---|---|---|---|
| C. elegans on glycerol | |||||
| 50 h | 19.8 ± 3.1 | 10.5 ± 2.8 | 47.7 ± 4.2 | 12.2 ± 2.4 | 9.2 ± 1.8 |
| 192 h | 21.4 ± 3.9 | 7.4 ± 1.7 | 48.8 ± 4.4 | 11.9 ± 2.1 | 9.5 ± 1.7 |
| 312 h | 19.5 ± 2.8 | 5.2 ± 1.7 | 52.5 ± 4.9 | 14.8 ± 2.7 | 5.8 |
| C. elegans on glucose | |||||
| 50 h | 23.2 ± 3.9 | 8.1 ± 1.8 | 47.4 | 11.8 | 8.4 |
| 192 h | 23.3 ± 3.1 | 7.6 ± 2.8 | 48.1 | 11.0 | 8.8 |
| 312 h | 18.6 ± 2.5 | 6.8 ± 1.3 | 50.9 | 15.1 | 7.5 |
| Number | Time | Hexane | Salt | Weight of FFAs (g) | Desirability |
|---|---|---|---|---|---|
| 1 | 60.00 | 15.000 | 1.00 | 0.749 | 0.849 selected |
| 2 | 60.00 | 15.022 | 1.00 | 0.749 | 0.848 |
| 3 | 60.00 | 15.035 | 1.00 | 0.750 | 0.848 |
| 4 | 60.20 | 15.000 | 1.00 | 0.748 | 0.847 |
| Cell Line | Nthy-Ori 3-1 | TPC-1 | K1 | PC-3 | DU-145 |
|---|---|---|---|---|---|
| Mean IC50 (μg/mL) | 58.81 | 58.60 | 57.89 | 59.21 | 60.69 |
| Std. Deviation | 3.063 | 5.358 | 4.468 | 6.855 | 2.862 |
| Std. Error | 1.768 | 3.093 | 2.580 | 3.957 | 1.652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalampounias, G.; Gardeli, C.; Alexis, S.; Anagnostopoulou, E.; Androutsopoulou, T.; Dritsas, P.; Aggelis, G.; Papanikolaou, S.; Katsoris, P. Poly-Unsaturated Fatty Acids (PUFAs) from Cunninghamella elegans Grown on Glycerol Induce Cell Death and Increase Intracellular Reactive Oxygen Species. J. Fungi 2024, 10, 130. https://doi.org/10.3390/jof10020130
Kalampounias G, Gardeli C, Alexis S, Anagnostopoulou E, Androutsopoulou T, Dritsas P, Aggelis G, Papanikolaou S, Katsoris P. Poly-Unsaturated Fatty Acids (PUFAs) from Cunninghamella elegans Grown on Glycerol Induce Cell Death and Increase Intracellular Reactive Oxygen Species. Journal of Fungi. 2024; 10(2):130. https://doi.org/10.3390/jof10020130
Chicago/Turabian StyleKalampounias, Georgios, Chrysavgi Gardeli, Spyridon Alexis, Elena Anagnostopoulou, Theodosia Androutsopoulou, Panagiotis Dritsas, George Aggelis, Seraphim Papanikolaou, and Panagiotis Katsoris. 2024. "Poly-Unsaturated Fatty Acids (PUFAs) from Cunninghamella elegans Grown on Glycerol Induce Cell Death and Increase Intracellular Reactive Oxygen Species" Journal of Fungi 10, no. 2: 130. https://doi.org/10.3390/jof10020130
APA StyleKalampounias, G., Gardeli, C., Alexis, S., Anagnostopoulou, E., Androutsopoulou, T., Dritsas, P., Aggelis, G., Papanikolaou, S., & Katsoris, P. (2024). Poly-Unsaturated Fatty Acids (PUFAs) from Cunninghamella elegans Grown on Glycerol Induce Cell Death and Increase Intracellular Reactive Oxygen Species. Journal of Fungi, 10(2), 130. https://doi.org/10.3390/jof10020130










