Inhibitory Effect of L-Methionine on Alternaria alternata Based on Metabolomics Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Chemicals
2.2. Preparation of Spore Suspension
2.3. A. alternata Microbial Index Determination
2.3.1. Determination of the Mycelial Growth Inhibition Rate of A. alternata
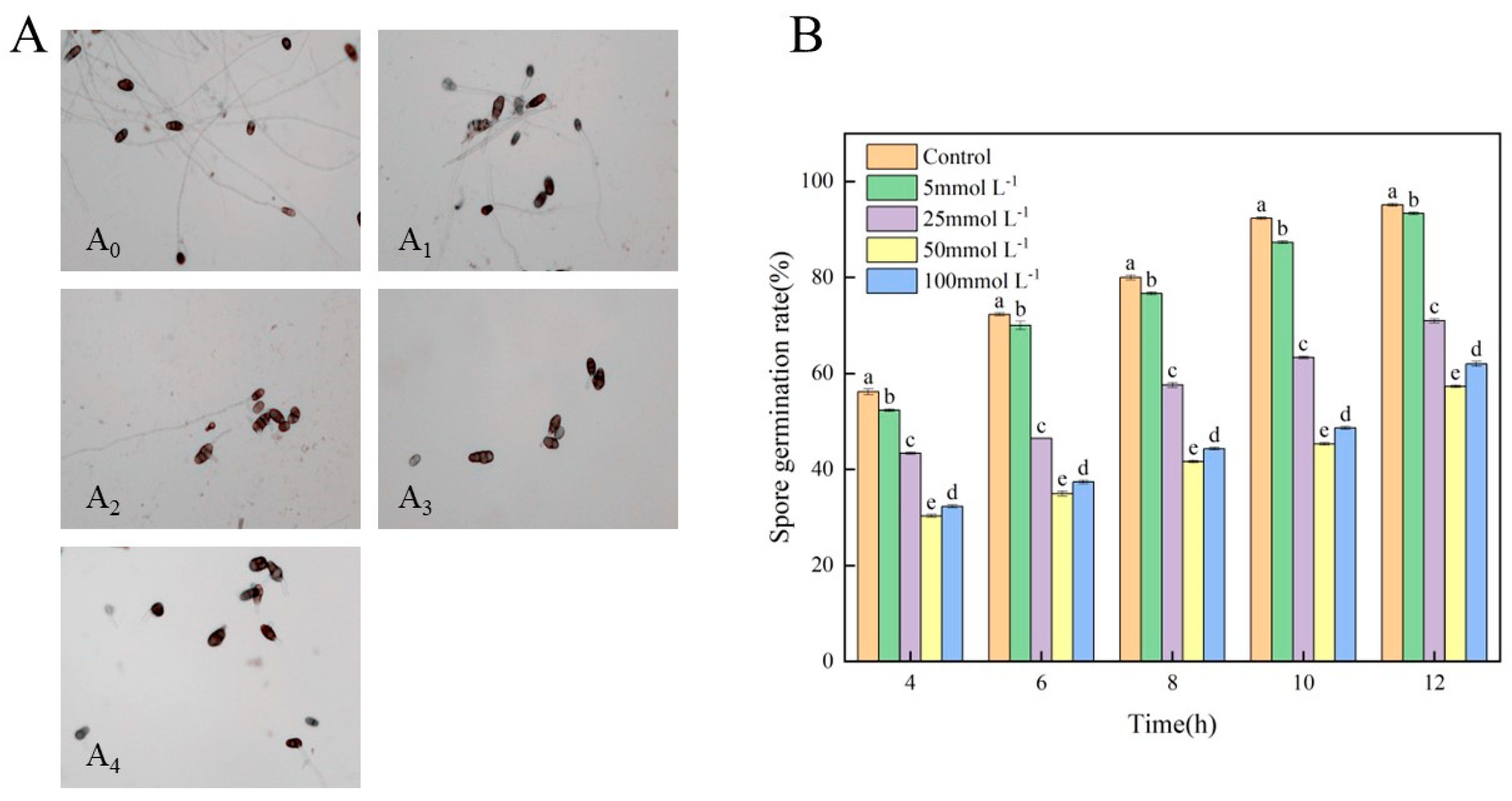
2.3.2. Assay for the Spore Germination of A. alternata
2.3.3. Determination of the Mycelial Morphology of A. alternata
2.3.4. Assay of Plasma Membrane Integrity
2.3.5. Determination of Electrolyte Leakage and Cellular Content Leakage
2.4. Metabolomics Analysis
2.5. Data Analysis
3. Results
3.1. Effect of Met on the Mycelial Growth of A. alternata
3.2. Effects of Met on the Spore Germination of A. alternata
3.3. Effects of Met on the Mycelial Morphology and Plasma Membrane Integrity of A. alternata
3.4. Effects of Met on the Electrolyte and Cellular Leakage of A. alternata
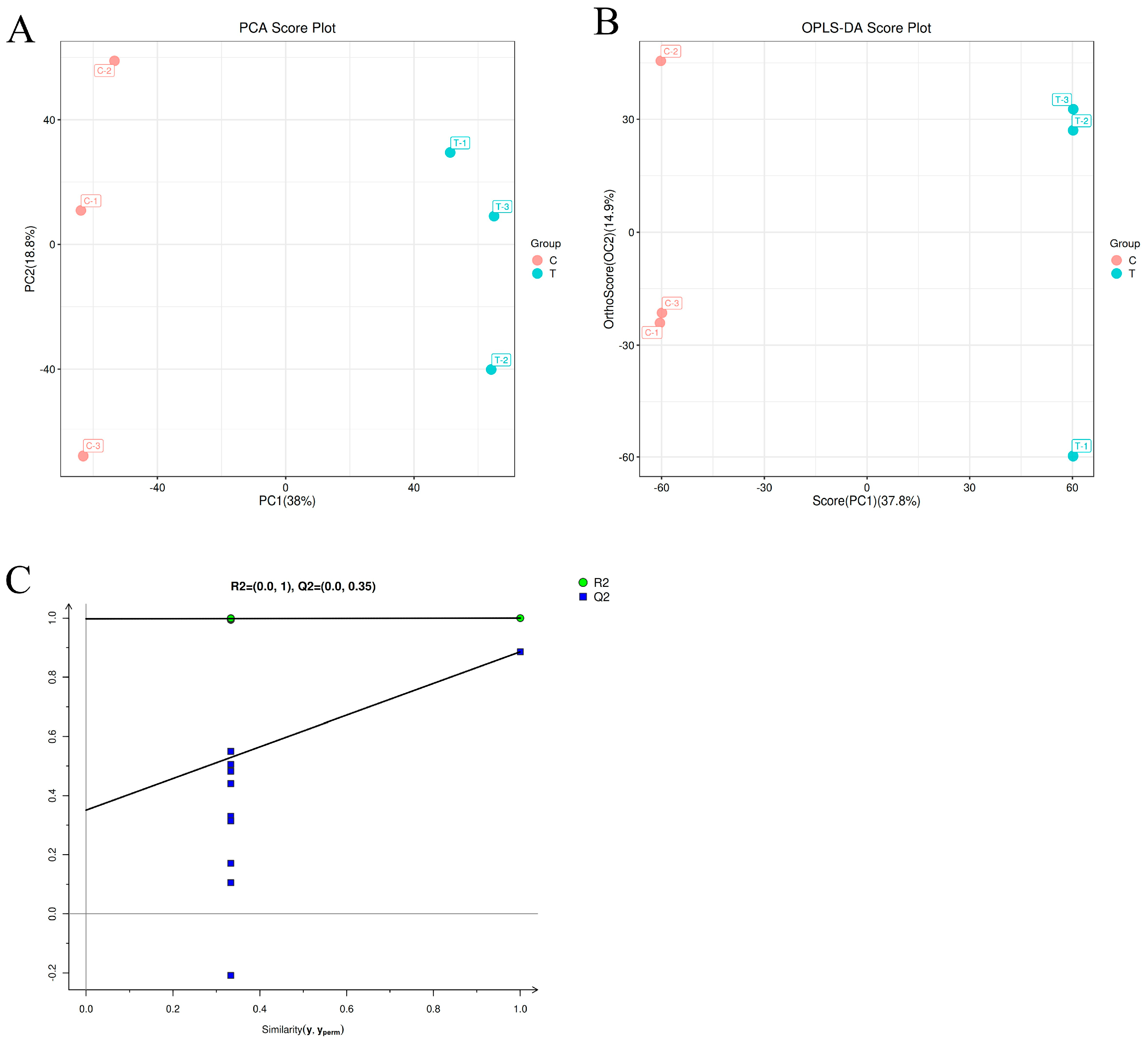
3.5. Metabolomics Data Analysis
3.5.1. PCA Analysis and OPLS-DA Analysis
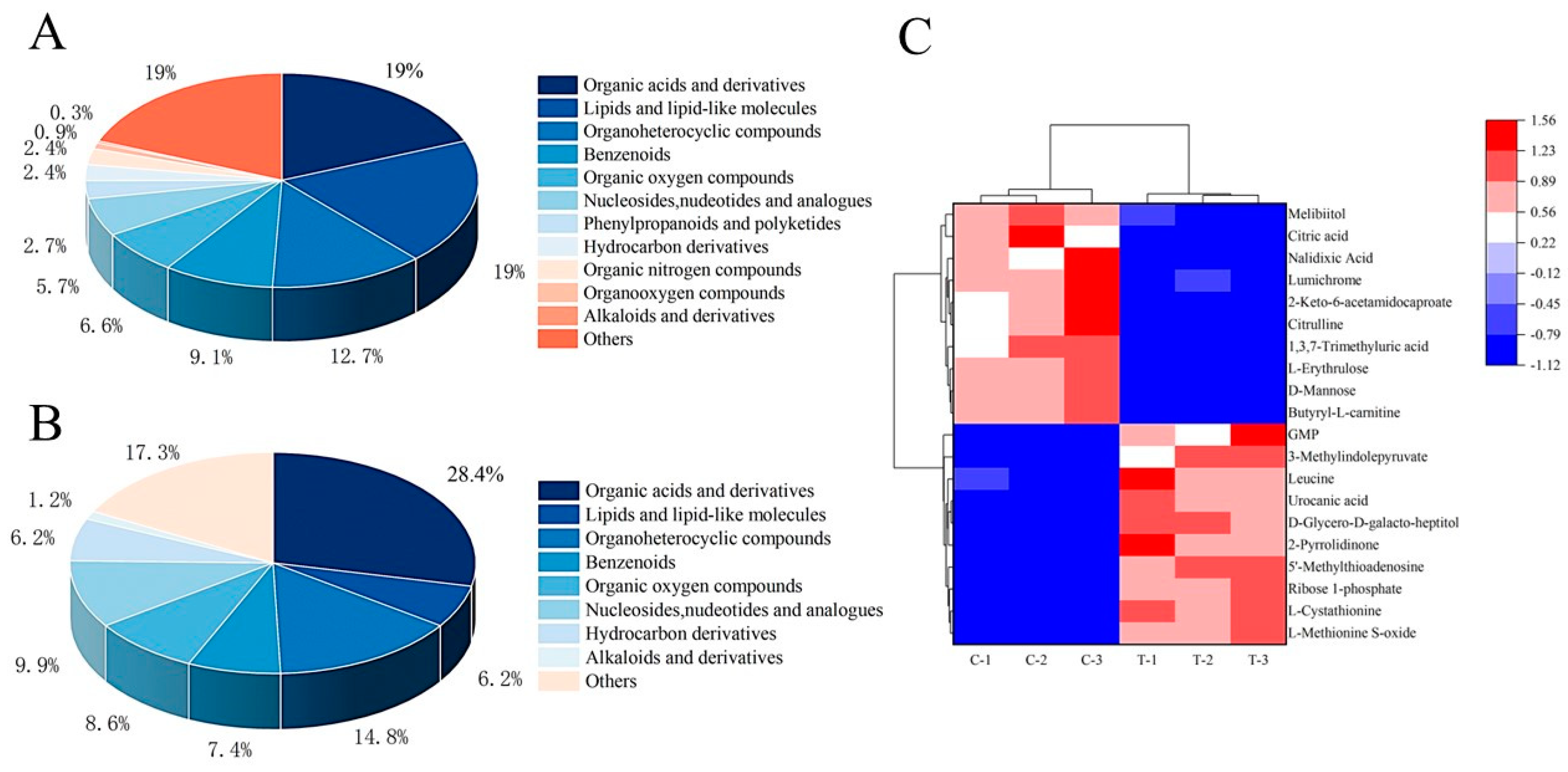
3.5.2. Differential Metabolite Screening and Cluster Analysis
3.5.3. KEGG Enrichment Analysis of Differential Metabolites
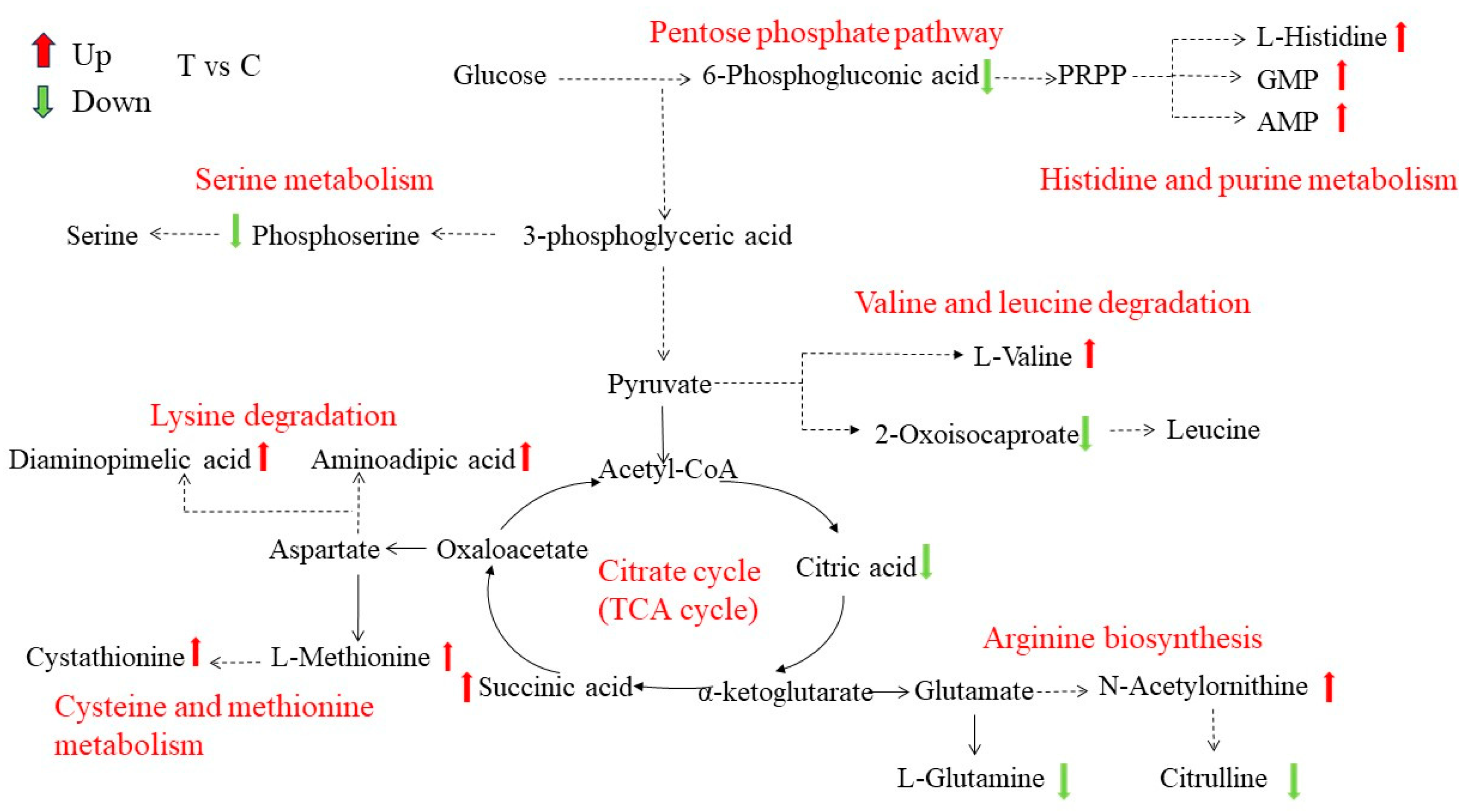
3.5.4. Differential Metabolite Network Analysis
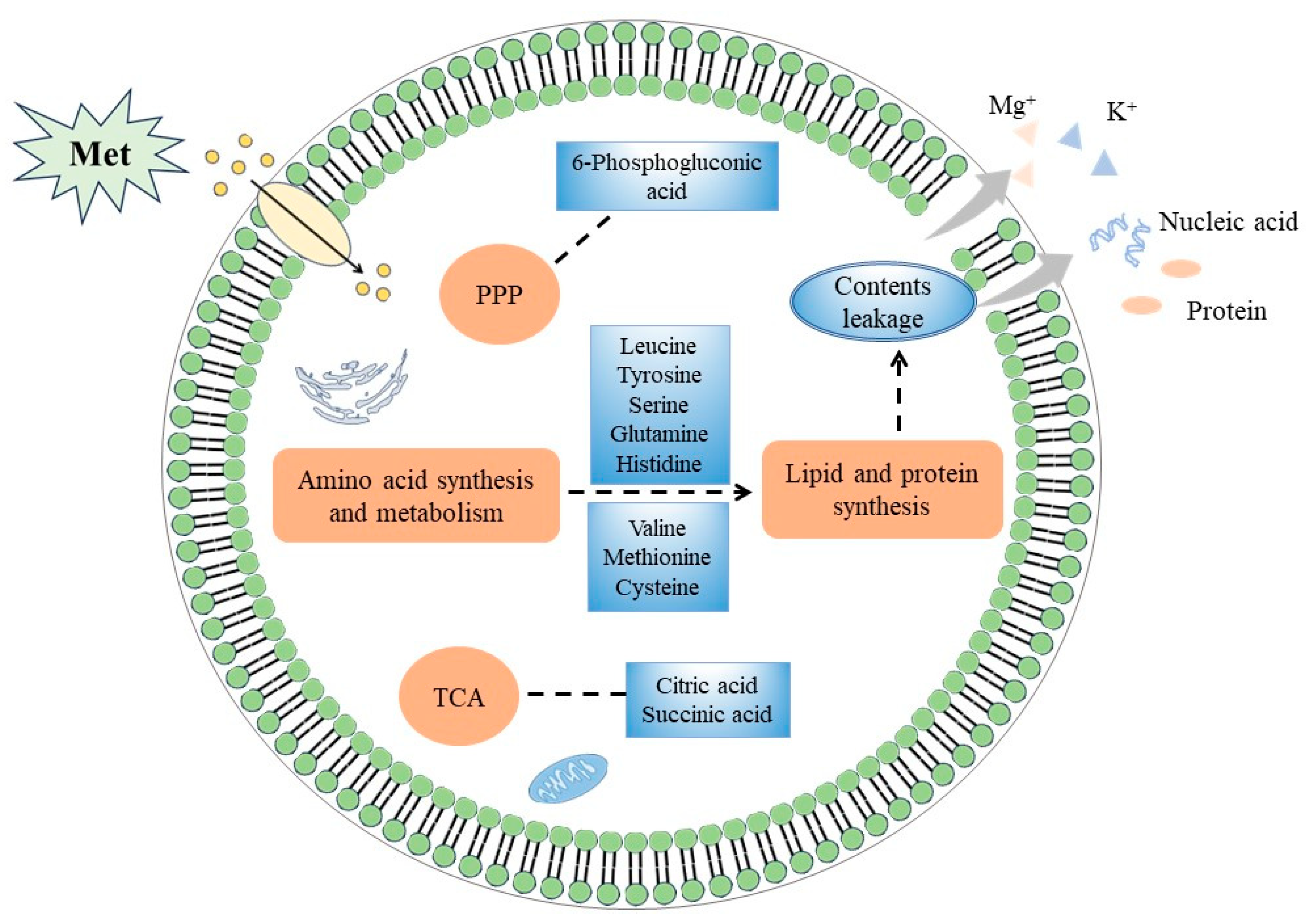
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Qiao, B.; Ji, X.; Wang, X.; Zhu, E. Antifungal activity and possible mechanisms of submicron chitosan dispersions against Alteraria alternata. Postharvest Biol. Technol. 2020, 161, 110883. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, S.; Wang, C.; Ding, X.; Cao, J.; Jiang, W. Enhanced resistance of jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit against postharvest Alternaria rot by β-aminobutyric acid dipping. Sci. Hortic. 2015, 186, 108–114. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.J.; Xiao, C.-L. Postharvest use of natamycin to control Alternaria rot on blueberry fruit caused by Alternaria alternata and A. arborescens. Postharvest Biol. Technol. 2021, 172, 111383. [Google Scholar] [CrossRef]
- Gur, L.; Reuveni, M.; Cohen, Y. β-Aminobutyric Acid Induced Resistance against Alternaria Fruit Rot in Apple Fruits. J. Fungi 2021, 7, 564. [Google Scholar] [CrossRef]
- Meng, J.; Guo, W.; Zhao, Z.; Zhang, Z.; Nie, D.; Tangni, E.K.; Han, Z. Production of Alternaria Toxins in Yellow Peach (Amygdalus persica) upon Artificial Inoculation with Alternaria alternate. Toxins 2021, 13, 656. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria Mycotoxins: An Overview of Toxicity, Metabolism, and Analysis in Food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef] [PubMed]
- Aloi, F.; Riolo, M.; Sanzani, S.M.; Mincuzzi, A.; Ippolito, A.; Siciliano, I.; Pane, A.; Gullino, M.L.; Cacciola, S.O. Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit. J. Fungi 2021, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Rovetto, E.I.; Luz, C.; La Spada, F.; Meca, G.; Riolo, M.; Cacciola, S.O. Diversity of Mycotoxins and Other Secondary Metabolites Recovered from Blood Oranges Infected by Colletotrichum, Alternaria, and Penicillium Species. Toxins 2023, 15, 407. [Google Scholar] [CrossRef] [PubMed]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crops Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Guo, Y.; Kang, L.; Yu, Y. Carbon monoxide enhances the resistance of jujube fruit against postharvest Alternaria rot. Postharvest Biol. Technol. 2020, 168, 111268. [Google Scholar] [CrossRef]
- Catanesi, M.; Brandolini, L.; d’Angelo, M.; Benedetti, E.; Tupone, M.G.; Alfonsetti, M.; Cabri, E.; Iaconis, D.; Fratelli, M.; Cimini, A.; et al. L-Methionine Protects against Oxidative Stress and Mitochondrial Dysfunction in an In Vitro Model of Parkinson’s Disease. Antioxidants 2021, 10, 1467. [Google Scholar] [CrossRef]
- Boubakri, H.; Wahab, M.A.; Chong, J.; Gertz, C.; Gandoura, S.; Mliki, A.; Bertsch, C.; Soustre-Gacougnolle, I. Methionine elicits H2O2 generation and defense gene expression in grapevine and reduces Plasmopara viticola infection. J. Plant Physiol. 2013, 170, 1561–1568. [Google Scholar] [CrossRef]
- Kang, N.J. Inhibition of powdery mildew development and activation of antioxidant enzymes by induction of oxidative stress with foliar application of a mixture of riboflavin and methionine in cucumber. Sci. Hortic. 2008, 118, 181–188. [Google Scholar] [CrossRef]
- El-Bauome, H.A.; Abdeldaym, E.A.; Abd El-Hady, M.A.M.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; et al. Exogenous Proline, Methionine, and Melatonin Stimulate Growth, Quality, and Drought Tolerance in Cauliflower Plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Freedman, N.D.; Ren, J.; Hollenbeck, A.R.; Abnet, C.C.; Park, Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br. J. Cancer 2014, 110, 1328–1333. [Google Scholar] [CrossRef]
- Che, J.; Chen, Y.; Wu, Y.; Li, L.; Tao, N. Metabolomics analysis reveals that myrcene stimulates the spore germination of Penicillium digitatum via the upregulation of central carbon and energy metabolism. Postharvest Biol. Technol. 2020, 170, 111329. [Google Scholar] [CrossRef]
- Wang, N.; Shao, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H. Quantitative proteomics reveals that tea tree oil effects Botrytis cinerea mitochondria function. Pestic. Biochem. Physiol. 2020, 164, 156–164. [Google Scholar] [CrossRef]
- Yu, S.; Zhen, C.; Zhao, P.; Li, J.; Qin, Z.; Gao, H. Antifungal mechanisms of γ-aminobutyric acid against the postharvest pathogen Alternaria alternata. LWT 2023, 173, 114314. [Google Scholar] [CrossRef]
- Ji, D.; Chen, T.; Ma, D.; Liu, J.; Xu, Y.; Tian, S. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.; Zhu, X.; Yuan, P.; Gong, B.; Ding, S.; Shan, Y. Inhibitory mechanisms of cinnamic acid on the growth of Geotrichum citri-aurantii. Food Control 2022, 131, 108459. [Google Scholar] [CrossRef]
- Li, W.; Yuan, S.; Li, Q.; Sang, W.; Cao, J.; Jiang, W. Methyl p-coumarate inhibits black spot rot on jujube fruit through membrane damage and oxidative stress against Alternaria alternata. Postharvest Biol. Technol. 2018, 145, 230–238. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, X.; Chang, L.; Zhang, L.; Zhang, S. Combined treatments of chitosan and sodium silicate to inhibit Alternaria alternata pathogens of postharvest winter jujube. Food Sci. Biotechnol. 2021, 30, 589–597. [Google Scholar] [CrossRef]
- Ma, D.; Ji, D.; Liu, J.; Xu, Y.; Chen, T.; Tian, S. Efficacy of methyl thujate in inhibiting Penicillium expansum growth and possible mechanism involved. Postharvest Biol. Technol. 2020, 161, 111070. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Wei, M.; Li, X.; Tang, Q.; Duan, B. Effect of trisodium phosphate treatment on black spot of apple fruit and the roles of anti-oxidative enzymes. Physiol. Mol. Plant Pathol. 2019, 106, 226–231. [Google Scholar] [CrossRef]
- Kong, H.; Fu, X.; Chang, X.; Ding, Z.; Yu, Y.; Xu, H.; Wang, R.; Shan, Y.; Ding, S. The ester derivatives of ferulic acid exhibit strong inhibitory effect on the growth of Alternaria alternata in vitro and in vivo. Postharvest Biol. Technol. 2023, 196, 112158. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.; Xu, W.; Fan, S.; Wan, T.; Zhang, J.; Cai, Y. Methyl jasmonate induces postharvest disease resistance to decay caused by Alternaria alternata in sweet cherry fruit. Sci. Hortic. 2022, 292, 110624. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Limami, A.M.; Van den Ende, W. Spermine and Spermidine Priming against Botrytis cinerea Modulates ROS Dynamics and Metabolism in Arabidopsis. Biomolecules 2021, 11, 223. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, X.; Deng, B.; Chen, O.; Deng, L.; Zeng, K. Methionine enhances disease resistance of jujube fruit against postharvest black spot rot by activating lignin biosynthesis. Postharvest Biol. Technol. 2022, 190, 111935. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, F.; Xi, P.; Lin, Z.; Jiang, Z.; Romanazzi, G.; Gao, L. Efficacy of pterostilbene suppression of postharvest gray mold in table grapes and potential mechanisms. Postharvest Biol. Technol. 2022, 183, 111745. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zheng, X.; Wang, X.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Zong, L.; Wang, J. Mode of action of plectasin-derived peptides against gas gangrene-associated Clostridium perfringens type A. PLoS ONE 2017, 12, e0185215. [Google Scholar] [CrossRef]
- Fang, W.; Sanz, A.B.; Bartual, S.G.; Wang, B.; Ferenbach, A.T.; Farkaš, V.; Hurtado-Guerrero, R.; Arroyo, J.; van Aalten, D.M.F. Mechanisms of redundancy and specificity of the Aspergillus fumigatus Crh transglycosylases. Nat. Commun. 2019, 10, 1669. [Google Scholar] [CrossRef]
- Shu, C.; Cui, K.; Li, Q.; Cao, J.; Jiang, W. Epsilon-poly-l-lysine (ε-PL) exhibits multifaceted antifungal mechanisms of action that control postharvest Alternaria rot. Int. J. Food Microbiol. 2021, 348, 109224. [Google Scholar] [CrossRef]
- Kloska, S.M.; Pałczyński, K.; Marciniak, T.; Talaśka, T.; Miller, M.; Wysocki, B.J.; Davis, P.; Wysocki, T.A. Queueing theory model of pentose phosphate pathway. Sci. Rep. 2022, 12, 4601. [Google Scholar] [CrossRef]
- Hakansson, A.P.; Kim, J.; Kim, G.-L.; Norambuena, J.; Boyd, J.M.; Parker, D. Impact of the pentose phosphate pathway on metabolism and pathogenesis of Staphylococcus aureus. PLoS Pathog. 2023, 19, e1011531. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. UHPLC-Q-TOF/MS-Based Metabolomics Approach Reveals the Antifungal Potential of Pinocembroside against Citrus Green Mold Phytopathogen. Plants 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Diebold, L.P.; Kong, H.; Schieber, M.; Huang, H.; Hensley, C.T.; Mehta, M.M.; Wang, T.; Santos, J.H.; Woychik, R.; et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol. Cell 2016, 61, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H. Metabolic regulatory mechanisms and physiological roles of functional amino acids and their applications in yeast. Biosci. Biotechnol. Biochem. 2019, 83, 1449–1462. [Google Scholar] [CrossRef]
- Xu, J.; Shao, X.; Li, Y.; Wei, Y.; Xu, F.; Wang, H. Metabolomic Analysis and Mode of Action of Metabolites of Tea Tree Oil Involved in the Suppression of Botrytis cinerea. Front. Microbiol. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Amich, J.; Bignell, E. Amino acid biosynthetic routes as drug targets for pulmonary fungal pathogens: What is known and why do we need to know more? Curr. Opin. Microbiol. 2016, 32, 151–158. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Dorai, T.; Pinto, J.T.; Denton, T.T. Metabolic Heterogeneity, Plasticity, and Adaptation to “Glutamine Addiction” in Cancer Cells: The Role of Glutaminase and the GTωA [Glutamine Transaminase—ω-Amidase (Glutaminase II)] Pathway. Biology 2023, 12, 1131. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wu, X.; Jiang, M.; Jin, H.; Tao, K.; Hou, T. Unraveling the polypharmacology of a natural antifungal product, eugenol, against Rhizoctonia solani. Pest Manag. Sci. 2021, 77, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Fani, R.; Brilli, M.; Fondi, M.; Lió, P. The role of gene fusions in the evolution of metabolic pathways: The histidine biosynthesis case. BMC Evol. Biol. 2007, 7, S4. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Bou Zeidan, M.; Zara, G.; Viti, C.; Decorosi, F.; Mannazzu, I.; Budroni, M.; Giovannetti, L.; Zara, S. L-Histidine Inhibits Biofilm Formation and FLO11-Associated Phenotypes in Saccharomyces cerevisiae Flor Yeasts. PLoS ONE 2014, 9, e0118167. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Zhou, Z. Exploring the antifungal mechanism of limonin-loaded eugenol emulsion against Penicillium italicum: From the perspective of microbial metabolism. Postharvest Biol. Technol. 2021, 182, 111704. [Google Scholar] [CrossRef]




| Time (h) | Control | 5 mmolL−1 | 25 mmolL−1 | 50 mmolL−1 | 100 mmolL−1 |
|---|---|---|---|---|---|
| 24 | 17.73 ± 0.251 a | 16.50 ± 0.500 b | 16.43 ± 0.513 b | 15.86 ± 0.230 bc | 15.40 ± 0.529 c |
| 48 | 32.60 ± 0.360 a | 28.36 ± 0.321 b | 27.66 ± 0.577 bc | 26.56 ± 0.513 c | 27.90 ± 1.014 b |
| 72 | 47.26 ± 0.250 a | 43.60 ± 0.529 b | 41.26 ± 1.101 c | 40.66 ± 1.258 c | 42.26 ± 0.873 bc |
| 96 | 62.29 ± 0.611 a | 55.33 ± 0.577 b | 53.90 ± 1.014 b | 51.23 ± 0.680 c | 53.66 ± 1.154 b |
| 120 | 74.46 ± 0.503 a | 66.06 ± 0.404 b | 61.73 ± 1.750 c | 59.66 ± 0.577 d | 60.50 ± 0.500 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhang, S.; Yu, Y.; Li, S.; Yang, C.; Chang, Y. Inhibitory Effect of L-Methionine on Alternaria alternata Based on Metabolomics Analysis. J. Fungi 2024, 10, 151. https://doi.org/10.3390/jof10020151
Zhu X, Zhang S, Yu Y, Li S, Yang C, Chang Y. Inhibitory Effect of L-Methionine on Alternaria alternata Based on Metabolomics Analysis. Journal of Fungi. 2024; 10(2):151. https://doi.org/10.3390/jof10020151
Chicago/Turabian StyleZhu, Xianran, Shaoying Zhang, Youwei Yu, Shengwang Li, Chao Yang, and Yuan Chang. 2024. "Inhibitory Effect of L-Methionine on Alternaria alternata Based on Metabolomics Analysis" Journal of Fungi 10, no. 2: 151. https://doi.org/10.3390/jof10020151





