Multi-Locus Microsatellite Typing of Colonising and Invasive Aspergillus fumigatus Isolates from Patients Post Lung Transplantation and with Chronic Lung Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Isolates and Patient Population
2.2. Isolation of Genomic DNA from A. fumigatus Cultures
2.3. Molecular Identification of Isolates
2.4. Microsatellite Genotyping
2.5. Clinical Data
2.6. Study Definitions
2.7. Statistical Analysis
3. Results
3.1. Isolates
3.2. Patient Characteristics and Classification of A. fumigatus Isolation
3.3. Rejection Episodes in Lung Transplant Recipients
3.4. Co-Infection
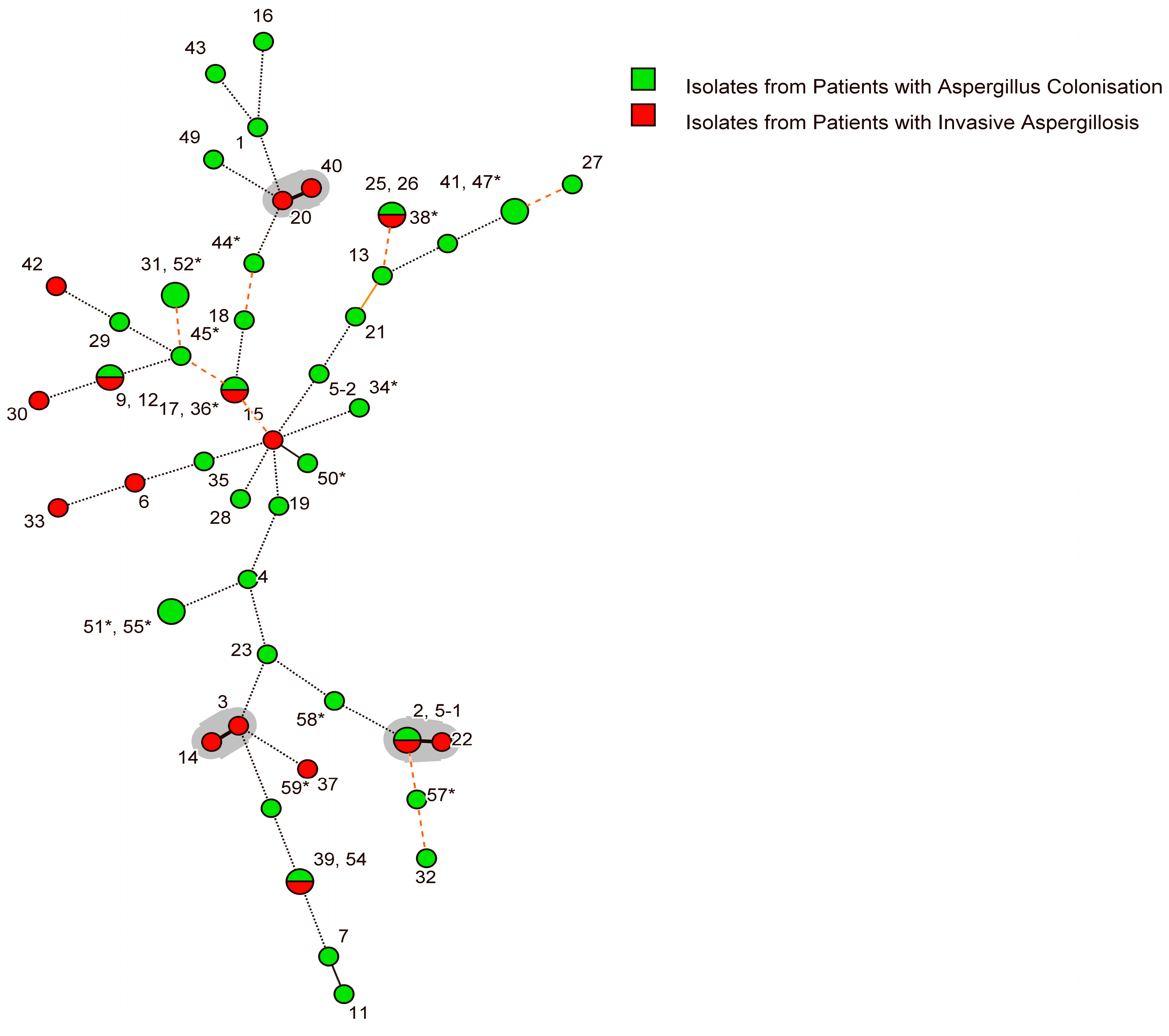
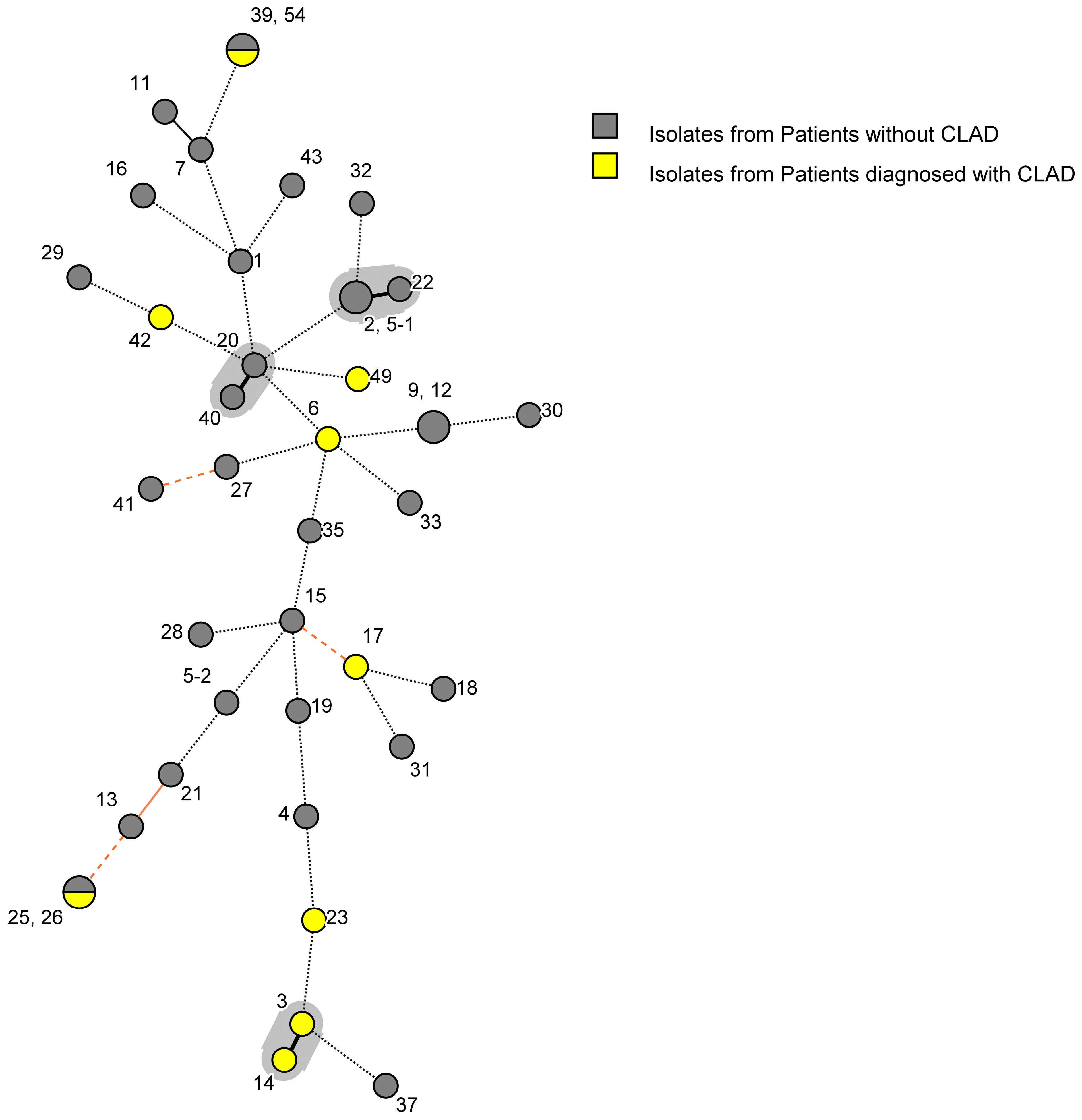
3.5. Genotypic Diversity of A. fumigatus Isolates
3.6. Relationship between Strain Type and Clinical Characteristics
3.7. Antifungal Therapy and Treatment Outcomes
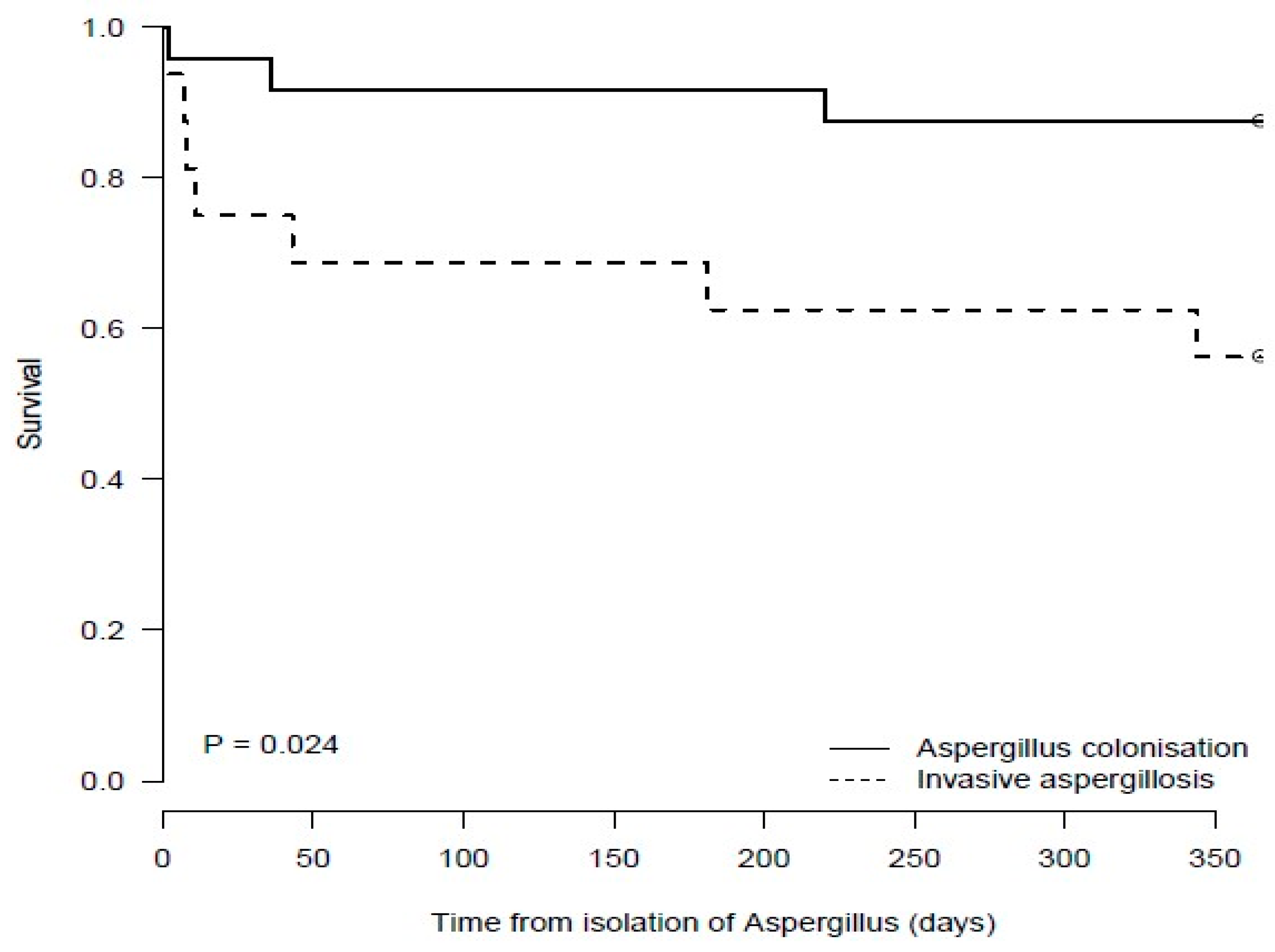
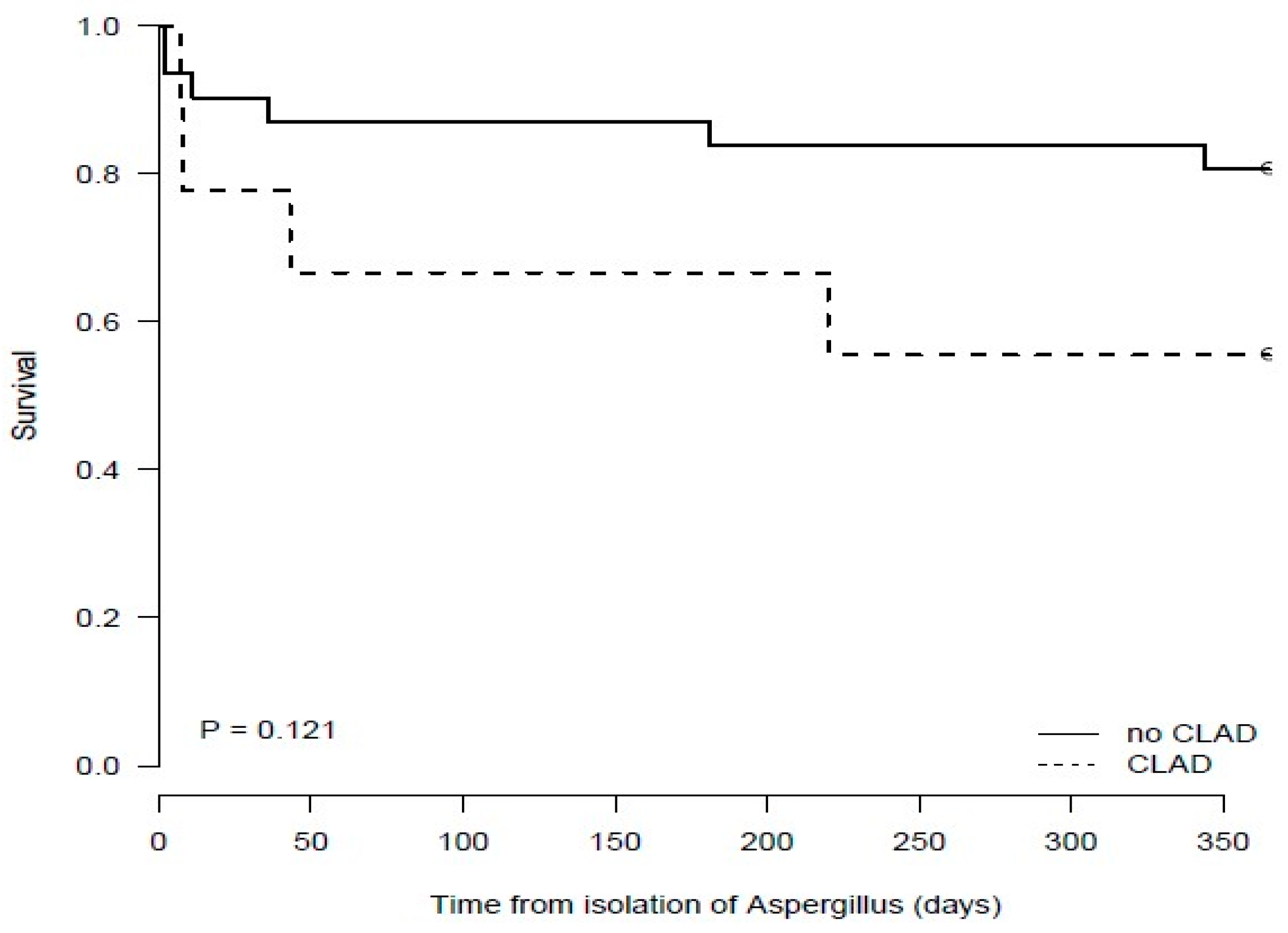
3.8. Mortality at 6 and 12 Months Post Aspergillus fumigatus Isolation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hope, W.W.; Walsh, T.J.; Denning, D.W. The invasive and saprophytic syndromes due to Aspergillus spp. Med. Mycol. 2005, 43 (Suppl. S1), S207–S238. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Bhaskaran, A.; Rotstein, C.; Li, Y.; Bhimji, A.; Pavan, R.; Kumar, D.; Humar, A.; Keshavjee, S.; Singer, L.G. A strategy for prevention of fungal infections in lung transplantation: Role of bronchoalveolar lavage fluid galactomannan and fungal culture. J. Heart Lung Transplant. 2018, 37, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Solé, A.; Morant, P.; Salavert, M.; Pemán, J.; Morales, P. Aspergillus infections in lung transplant recipients: Risk factors and outcome. Clin. Microbiol. Infect. 2005, 11, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Agbetile, J.; Fairs, A.; Desai, D.; Mistry, V.; Morley, J.P.; Pancholi, M.; Pavord, I.D.; Wardlaw, A.J.; et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur. Respir. J. 2014, 43, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Torres-Narbona, M.; Gijón, P.; Muñoz, P.; Pozo, F.; Peláez, T.; de Miguel, J.; Bouza, E. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin. Microbiol. Infect. 2010, 16, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Hazarika, B.; Gupta, D.; Aggarwal, A.N.; Chakrabarti, A.; Jindal, S.K. Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med. Mycol. 2010, 48, 988–994. [Google Scholar] [CrossRef]
- Rapaka, R.R.; Kolls, J.K. Pathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis: Current understanding and future directions. Med. Mycol. 2009, 47 (Suppl. S1), S331–S337. [Google Scholar] [CrossRef][Green Version]
- Saunders, R.V.; Modha, D.E.; Claydon, A.; Gaillard, E.A. Chronic Aspergillus fumigatus colonization of the pediatric cystic fibrosis airway is common and may be associated with a more rapid decline in lung function. Med. Mycol. 2016, 54, 537–543. [Google Scholar] [CrossRef]
- King, J.; Brunel, S.F.; Warris, A. Aspergillus infections in cystic fibrosis. J. Infect. 2016, 72, S50–S55. [Google Scholar] [CrossRef]
- Brandt, C.; Roehmel, J.; Rickerts, V.; Melichar, V.; Niemann, N.; Schwarz, C. Aspergillus Bronchitis in Patients with Cystic Fibrosis. Mycopathologia 2018, 183, 61–69. [Google Scholar] [CrossRef]
- Elphick, H.E.; Southern, K.W. Antifungal therapies for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst. Rev. 2016, 11, Cd002204. [Google Scholar] [CrossRef]
- Mondon, P.; De Champs, C.; Donadille, A.; Ambroise-Thomas, P.; Grillot, R. Variation in virulence of Aspergillus fumigatus strains in a murine model of invasive pulmonary aspergillosis. J. Med. Microbiol. 1996, 45, 186–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bart-Delabesse, E.; Sarfati, J.; Debeaupuis, J.P.; van Leeuwen, W.; van Belkum, A.; Bretagne, S.; Latge, J.P. Comparison of restriction fragment length polymorphism, microsatellite length polymorphism, and random amplification of polymorphic DNA analyses for fingerprinting Aspergillus fumigatus isolates. J. Clin. Microbiol. 2001, 39, 2683–2686. [Google Scholar] [CrossRef][Green Version]
- Lasker, B.A. Evaluation of performance of four genotypic methods for studying the genetic epidemiology of Aspergillus fumigatus isolates. J. Clin. Microbiol. 2002, 40, 2886–2892. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Valk, H.A.; Meis, J.F.; Curfs, I.M.; Muehlethaler, K.; Mouton, J.W.; Klaassen, C.H. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J. Clin. Microbiol. 2005, 43, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- de Valk, H.A.; Klaassen, C.H.; Yntema, J.B.; Hebestreit, A.; Seidler, M.; Haase, G.; Müller, F.M.; Meis, J.F. Molecular typing and colonization patterns of Aspergillus fumigatus in patients with cystic fibrosis. J. Cyst. Fibros. 2009, 8, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, L.M.; Symoens, F.; Bouchara, J.P.; Nelis, H.J.; Coenye, T. High-resolution genotyping of Aspergillus fumigatus isolates recovered from chronically colonised patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1005–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Valk, H.A.; Meis, J.F.; de Pauw, B.E.; Donnelly, P.J.; Klaassen, C.H. Comparison of two highly discriminatory molecular fingerprinting assays for analysis of multiple Aspergillus fumigatus isolates from patients with invasive aspergillosis. J. Clin. Microbiol. 2007, 45, 1415–1419. [Google Scholar] [CrossRef][Green Version]
- Martin-Gandul, C.; Mueller, N.J.; Pascual, M.; Manuel, O. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am. J. Transplant. 2015, 15, 3024–3040. [Google Scholar] [CrossRef]
- Gregson, A.L. Infectious Triggers of Chronic Lung Allograft Dysfunction. Curr. Infect. Dis. Rep. 2016, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.S.; Elashoff, R.M.; Huang, C.; Ardehali, A.; Gregson, A.L.; Kubak, B.; Fishbein, M.C.; Saggar, R.; Keane, M.P.; Saggar, R.; et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am. J. Transplant. 2009, 9, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.S.; Copeland, C.A.F.; Derhovanessian, A.; Shino, M.Y.; Davis, W.A.; Snyder, L.D.; Gregson, A.L.; Saggar, R.; Lynch, J.P., 3rd; Ross, D.J.; et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: A two-center validation study. Am. J. Transplant. 2013, 13, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lee, Y.K.; Wickes, B.L. Simple chemical extraction method for DNA isolation from Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 2004, 42, 4293–4296. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Hong, S.B.; Go, S.J.; Shin, H.D.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 2005, 97, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Rougeron, A.; Giraud, S.; Razafimandimby, B.; Meis, J.F.; Bouchara, J.P.; Klaassen, C.H. Different colonization patterns of Aspergillus terreus in patients with cystic fibrosis. Clin. Microbiol. Infect. 2014, 20, 327–333. [Google Scholar] [CrossRef][Green Version]
- Jensen, R.H.; Hagen, F.; Astvad, K.M.; Tyron, A.; Meis, J.F.; Arendrup, M.C. Azole-resistant Aspergillus fumigatus in Denmark: A laboratory-based study on resistance mechanisms and genotypes. Clin. Microbiol. Infect. 2016, 22, 570.e1–570.e9. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Husain, S.; Mooney, M.L.; Danziger-Isakov, L.; Mattner, F.; Singh, N.; Avery, R.; Ison, M.; Humar, A.; Padera, R.F.; Lawler, L.P.; et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J. Heart Lung Transplant. 2011, 30, 361–374. [Google Scholar] [CrossRef]
- Martín-Peña, A.; Aguilar-Guisado, M.; Espigado, I.; Parody, R.; Miguel Cisneros, J. Prospective study of infectious complications in allogeneic hematopoietic stem cell transplant recipients. Clin. Transplant. 2011, 25, 468–474. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R. An international ISHLT/ATS/ERS clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D., Jr.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.F.; Snell, G.I.; Levvey, B.; Kotsimbos, T.; Morrissey, C.O.; Slavin, M.A.; Stewart, K.; Kong, D.C. Preemptive treatment with voriconazole in lung transplant recipients. Transpl. Infect. Dis. 2013, 15, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef]
- Nivoix, Y.; Velten, M.; Letscher-Bru, V.; Moghaddam, A.; Natarajan-Amé, S.; Fohrer, C.; Lioure, B.; Bilger, K.; Lutun, P.; Marcellin, L.; et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin. Infect. Dis. 2008, 47, 1176–1184. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Guinea, J.; Garcia de Viedma, D.; Pelaez, T.; Escribano, P.; Munoz, P.; Meis, J.F.; Klaassen, C.H.; Bouza, E. Molecular epidemiology of Aspergillus fumigatus: An in-depth genotypic analysis of isolates involved in an outbreak of invasive aspergillosis. J. Clin. Microbiol. 2011, 49, 3498–3503. [Google Scholar] [CrossRef]
- Escribano, P.; Pelaez, T.; Bouza, E.; Guinea, J. Microsatellite (STRAf) genotyping cannot differentiate between invasive and colonizing Aspergillus fumigatus isolates. J. Clin. Microbiol. 2015, 53, 667–670. [Google Scholar] [CrossRef][Green Version]
- Ben-Ami, R.; Lamaris, G.A.; Lewis, R.E.; Kontoyiannis, D.P. Interstrain variability in the virulence of Aspergillus fumigatus and Aspergillus terreus in a Toll-deficient Drosophila fly model of invasive aspergillosis. Med. Mycol. 2010, 48, 310–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi-Nakaguchi, A.; Muraosa, Y.; Hagiwara, D.; Sakai, K.; Toyotome, T.; Watanabe, A.; Kawamoto, S.; Kamei, K.; Gonoi, T.; Takahashi, H. Genome sequence comparison of Aspergillus fumigatus strains isolated from patients with pulmonary aspergilloma and chronic necrotizing pulmonary aspergillosis. Med. Mycol. 2015, 53, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ballard, E.; Melchers, W.J.G.; Zoll, J.; Brown, A.J.P.; Verweij, P.E.; Warris, A. In-host microevolution of Aspergillus fumigatus: A phenotypic and genotypic analysis. Fungal Genet. Biol. 2018, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Desnos-Ollivier, M.; Garcia-Hermoso, D.; Bretagne, S. Investigating Clinical Issues by Genotyping of Medically Important Fungi: Why and How? Clin. Microbiol. Rev. 2017, 30, 671–707. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Takahashi, H.; Watanabe, A.; Takahashi-Nakaguchi, A.; Kawamoto, S.; Kamei, K.; Gonoi, T. Whole-genome comparison of Aspergillus fumigatus strains serially isolated from patients with aspergillosis. J. Clin. Microbiol. 2014, 52, 4202–4209. [Google Scholar] [CrossRef] [PubMed]
- Husni, R.N.; Gordon, S.M.; Longworth, D.L.; Arroliga, A.; Stillwell, P.C.; Avery, R.K.; Maurer, J.R.; Mehta, A.; Kirby, T. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin. Infect. Dis. 1998, 26, 753–755. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]

| (a) | |||||
| Characteristic | Overall N: 52 (%) | 1 November 2006–31 March 2009 N: 25 (%) | 1 November 2015–30 June 2017 N: 27 (%) | p-Value 1 | |
| Age in years, median (IQR) 2 | 47 (29.0) | 40 (21.0) | 53 (32.5) | 0.07 | |
| Sex (Female) | 27 (51.9) | 15 (60.0) | 12 (44.4) | 0.27 | |
| Underlying disease | |||||
| COPD | 15 (28.8) | 9 (36.0) | 6 (22.2) | 0.28 | |
| CF | 18 (34.6) | 12 (48.0) | 6 (22.2) | 0.05 | |
| Interstitial Lung Disease | 4 (7.7) | 0 (0.0) | 4 (14.8) | 0.11 | |
| Non-CF Bronchiectasis | 3 (5.8) | 1 (4.0) | 2 (7.4) | 0.61 | |
| Alpha-1 Antitrypsin Deficiency | 3 (5.8) | 0 (0.0) | 3 (11.1) | 0.24 | |
| Pulmonary Hypertension | 5 (9.6) | 0 (0.0) | 5 (18.5) | 0.05 | |
| Other 3a | 4 (7.7) | 3 (12.0) | 1 (3.7) | 0.26 | |
| Comorbidities 4 | |||||
| Diabetes mellitus | 17 (32.7) | 8 (32.0) | 9 (33.3) | 0.92 | |
| Insulin Dependent | 14 (26.9) | 5 (20.0) | 9 (33.3) | 0.29 | |
| Oral hypoglycaemics only | 3 (5.8) | 3 (12.0) | 0 (0.0) | 0.07 | |
| Chronic renal failure | 30 (57.7) | 8 (32.0) | 22 (81.5) | <0.001 | |
| Lymphopenia | 25 (48.1) | 7 (28.0) | 18 (66.7) | <0.01 | |
| Neutropenia | 4 (7.7) | 0 (0.0) | 4 (14.8) | 0.11 | |
| GORD | 16 (30.8) | 9 (36.0) | 7 (25.9) | 0.44 | |
| Hypogammaglobinaemia | 5 (9.6) | 1 (4.0) | 4 (14.8) | 0.19 | |
| (b) | |||||
| Characteristics of Lung Transplant Recipients | Overall N: 39 (%) | 1 November 2006–31 March 2009 N: 12 (%) | 1 November 2015–30 June 2017 N: 27 (%) | p-Value 1 | |
| Age in years, median (IQR) 2 | 50 (30.0) | 49 (16.0) | 50 (33.0) | 0.41 | |
| Sex (Female) | 19 (48.7) | 7 (58.3) | 12 (44.4) | 0.43 | |
| Underlying disease | |||||
| COPD | 13 (33.3) | 7 (58.3) | 6 (22.2) | 0.03 | |
| CF | 9 (23.1) | 3 (25.0) | 6 (22.2) | 0.85 | |
| Interstitial Lung Disease | 4 (10.3) | 0 (0.0) | 4 (14.8) | 0.28 | |
| Non-CF Bronchiectasis | 2 (5.1) | 0 (0.0) | 2 (7.4) | 1 | |
| Alpha-1 Antitrypsin Deficiency | 3 (7.7) | 0 (0.0) | 3 (11.1) | 0.54 | |
| Pulmonary Hypertension | 5 (12.8) | 0 (0.0) | 5 (18.5) | 0.15 | |
| Other 3b | 3 (7.7) | 2 (16.7) | 1 (3.7) | 0.16 | |
| Co-morbidities 4 | |||||
| Diabetes mellitus | 14 (35.9) | 5 (41.6) | 9 (33.3) | 0.62 | |
| Insulin Dependent/Requiring | 13 (33.3) | 4 (33.3) | 9 (33.3) | 1 | |
| Oral hypoglycaemics only | 1 (2.6) | 1 (8.3) | 0 (0.0) | 0.13 | |
| Chronic renal failure | 29 (74.4) | 7 (58.3) | 22 (81.5) | 0.13 | |
| Lymphopenia | 24 (61.5) | 6 (50.0) | 18 (66.7) | 0.33 | |
| Neutropenia | 4 (10.3) | 0 (0.0) | 4 (14.8) | 0.28 | |
| GORD | 12 (30.8) | 5 (41.6) | 7 (25.9) | 0.33 | |
| Hypogammaglobinaemia | 5 (12.8) | 1 (8.3) | 4 (14.8) | 0.58 | |
| Type of transplant | |||||
| BSLT | 35 (89.7) | 8 (66.6) | 27 (100) | <0.01 | |
| SLT | 4 (10.3) | 4 (33.3) | 0 (0.0) | <0.01 | |
| Pre-transplant Aspergillus isolation | 2 (5.1) | 1 (8.3) | 1 (3.7) | 1 | |
| Immunosuppressants 5,6 | |||||
| Cyclosporin | 8 (20.5) | 6 (50.0) | 2 (7.4) | <0.01 | |
| Methylprednisolone | 11 (28.2) | 0 (0.0) | 11 (40.7) | 0.01 | |
| Mycophenolate | 15 (38.5) | 4 (33.3) | 11 (40.7) | 0.66 | |
| Tacrolimus | 32 (82.1) | 6 (50.0) | 26 (96.3) | <0.01 | |
| Prednisolone | 36 (92.3) | 11 (91.7) | 25 (92.6) | 0.92 | |
| Azathioprine | 21 (53.8) | 6 (50.0) | 15 (55.6) | 0.75 | |
| Everolimus | 1 (2.6) | 1 (8.3) | 0 (0.0) | 0.13 | |
| Basiliximab | 7 (17.9) | 0 (0.0) | 7 (25.9) | 0.06 | |
| Category | Overall N: 52 (%) | Lung Transplant N: 39 (%) | Non-LT CRD N: 13 (%) | p-Value 1 | 1 November 2006–31 March 2009 N: 25 (%) | 1 November 2015–30 June 2017 N: 27 (%) | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Colonisation | 36 (69.2) | 23 (58.9) | 13 (100.0) | <0.01 | 20 (80.0) | 16 (59.3) | 0.11 |
| TB/BA infection | 6 (11.5) | 6 (15.4) | 0 (0.0) | 0.14 | 1 (4.0) | 5 (18.5) | 0.11 |
| IA/Aspergillus Pneumonia Proven Probable | 10 (19.2) 7 (13.5) 3 (5.8) | 10 (25.6) 7 (17.9) 3 (7.7) | 0 (0.0) 0 (0.0) 0 (0.0) | 0.04 | 4 (16.0) 3 (12.0) 1 (4.0) | 6 (22.2) 4 (14.8) 2 (7.4) | 0.57 |
| Variable | Exposure N: 39 (%) | OR (95% CI) | |
|---|---|---|---|
| Age | |||
| 12 to <40 | 13 (33.3) | Reference | |
| ≥40 to <50 | 7 (17.9) | 0.9 (0.1–6.57) | |
| ≥50 to <60 | 9 (23.1) | 1.12 (0.17–7.06) | |
| ≥60 to 70 | 10 (25.6) | 3.94 (0.76–24.03) | |
| Sex (Female) | 19 (48.7) | 0.56 (0.15–1.98) | |
| Underlying disease | |||
| CF | 9 (23.1) | Reference | |
| COPD | 13 (33.3) | 1.71 (0.3–11.1) | |
| Other 1 | 17 (43.6) | 1.27 (0.24–7.62) | |
| Pre-transplant Aspergillus | 2 (5.1) | 1.53 (0.06–40.8) | |
| Time period | |||
| 1 November 2006–31 March 2009 | 12 (30.8) | Reference | |
| 1 November 2015–30 June 2017 | 27 (69.2) | 1.1 (0.29–4.48) | |
| Diabetes | 14 (35.9) | 1.56 (0.42–5.83) | |
| Lymphopenia | 24 (61.5) | 3.00 (0.79–13.24) | |
| GORD | 12 (30.8) | 1.10 (0.27–4.37) | |
| Hypogammaglobulinaemia | 5 (12.8) | 0.33 (0.22–2.55) | |
| Chronic kidney disease | 29 (74.4) | 2.17 (0.51–11.45) | |
| Immunosuppressive agents | |||
| Cyclosporin | 8 (20.5) | 4.20 (0.91–23.35) | |
| Mycophenolate | 15 (38.5) | 1.00 (0.26–3.69) | |
| Tacrolimus | 32 (82.1) | 0.60 (0.12–2.96) | |
| Prednisolone | 36 (92.3) | 1.36 (0.12–30.91) | |
| Methylprednisolone | 11 (28.2) | 1.36 (0.32–5.63) | |
| Azathioprine | 21 (53.8) | 1.09 (0.3–3.97) | |
| Basiliximab | 7 (17.9) | 2.33 (0.44–13.59) | |
| Everolimus | 1 (2.6) | NA | |
| Other infection | |||
| Pseudomonas aeruginosa | 6 (15.4) | 0.71 (0.09–4.2) | |
| Staphylococcus aureus | 7 (17.9) | 13.8 (2.01–279.23) | |
| CMV | 0 (0) | NA | |
| Respiratory viruses | 2 (5) | NA | |
| Rejection | |||
| Acute | 6 (1.35) | 1.53 (0.06–40.8) | |
| Chronic | 9 (23.1) | 3.67 (0.62–29.36) | |
| Time from Tx to Aspergillus isolation, days | |||
| 1 to <30.75 | 9(25) | Reference | |
| ≥30.75 to <180 | 10 (25) | 2.33 (0.38–16.28) | |
| ≥180 to <803.75 | 10 (25) | 1.00 (0.14–7.16) | |
| ≥803.75 to <3314 | 10 (25) | 2.33 (0.38–16.28) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birnie, J.D.; Ahmed, T.; Kidd, S.E.; Westall, G.P.; Snell, G.I.; Peleg, A.Y.; Morrissey, C.O. Multi-Locus Microsatellite Typing of Colonising and Invasive Aspergillus fumigatus Isolates from Patients Post Lung Transplantation and with Chronic Lung Disease. J. Fungi 2024, 10, 95. https://doi.org/10.3390/jof10020095
Birnie JD, Ahmed T, Kidd SE, Westall GP, Snell GI, Peleg AY, Morrissey CO. Multi-Locus Microsatellite Typing of Colonising and Invasive Aspergillus fumigatus Isolates from Patients Post Lung Transplantation and with Chronic Lung Disease. Journal of Fungi. 2024; 10(2):95. https://doi.org/10.3390/jof10020095
Chicago/Turabian StyleBirnie, Joshua D., Tanveer Ahmed, Sarah E. Kidd, Glen P. Westall, Gregory I. Snell, Anton Y. Peleg, and Catherine Orla Morrissey. 2024. "Multi-Locus Microsatellite Typing of Colonising and Invasive Aspergillus fumigatus Isolates from Patients Post Lung Transplantation and with Chronic Lung Disease" Journal of Fungi 10, no. 2: 95. https://doi.org/10.3390/jof10020095
APA StyleBirnie, J. D., Ahmed, T., Kidd, S. E., Westall, G. P., Snell, G. I., Peleg, A. Y., & Morrissey, C. O. (2024). Multi-Locus Microsatellite Typing of Colonising and Invasive Aspergillus fumigatus Isolates from Patients Post Lung Transplantation and with Chronic Lung Disease. Journal of Fungi, 10(2), 95. https://doi.org/10.3390/jof10020095






