Properties and Fungal Communities of Different Soils for Growth of the Medicinal Asian Water Plantain, Alisma orientale, in Fujian, China
Abstract
1. Introduction
2. Materials and Methods
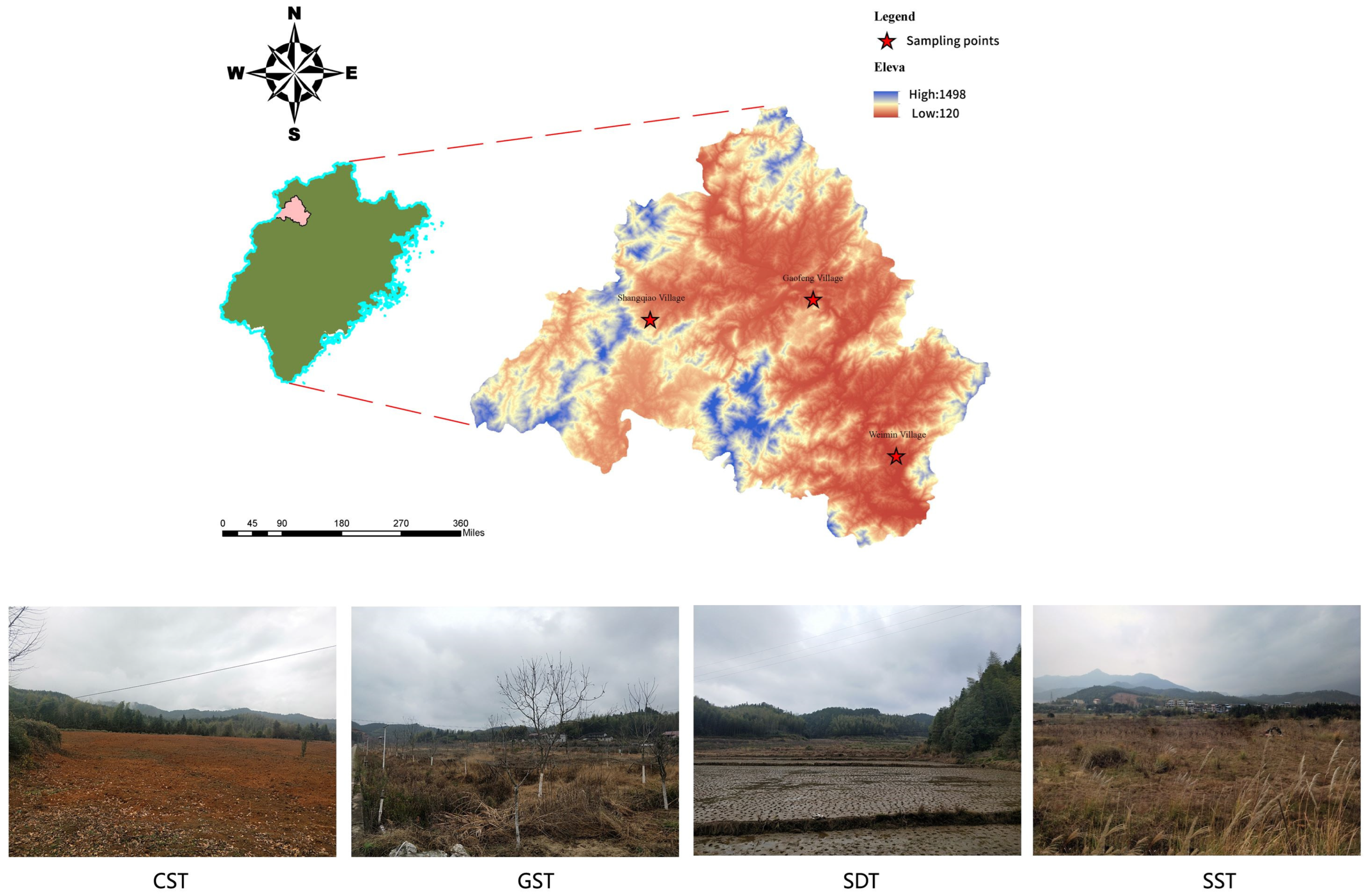
2.1. Site Description and Soil Sampling
2.2. Soil Chemical Properties Analysis
2.3. Heavy Metal Analysis and Pesticide Residue Estimation
2.4. PacBio Long-Read Sequencing of Soil Fungi
2.5. Bioinformatic Analysis
2.6. Field Planting and Experimental Design
2.7. HPLC-MS Analysis for the Triterpenoid Content
2.8. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Heavy Metal Analysis and Pesticide Residue Estimation
3.3. PacBio Long-Read Sequencing Data
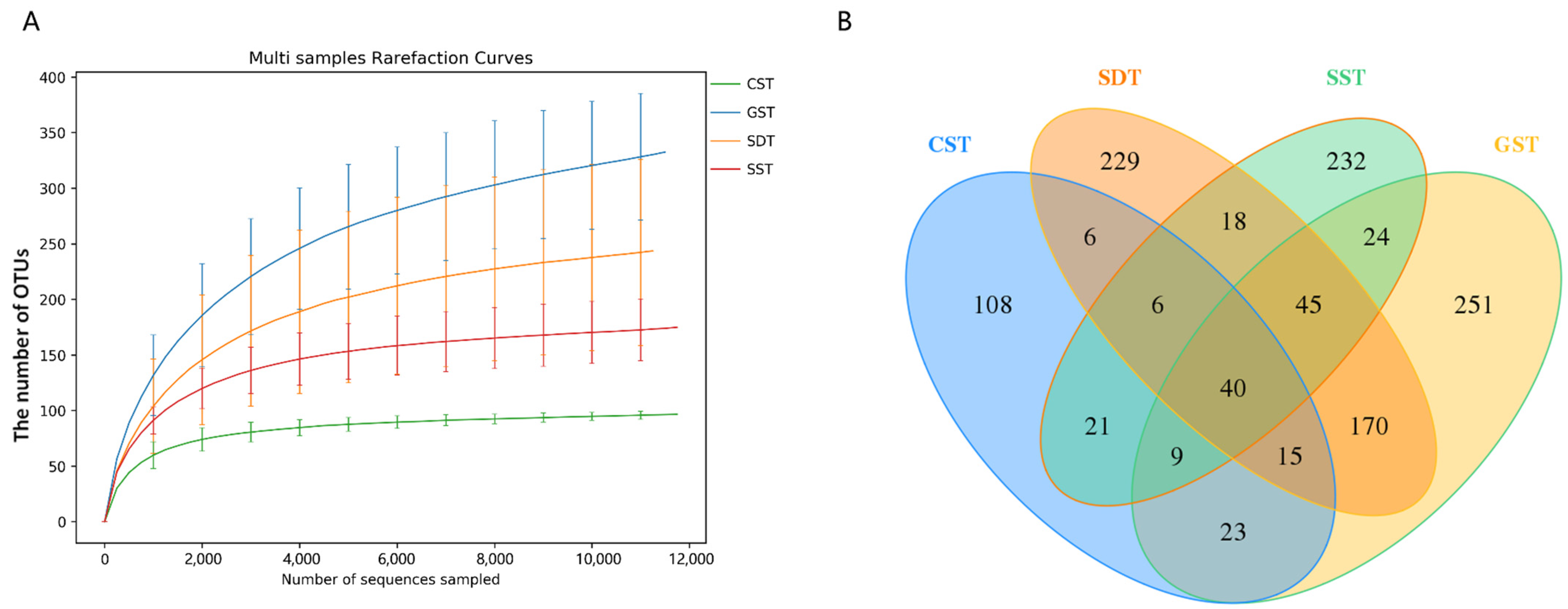
3.4. Soil Fungal Community Diversity
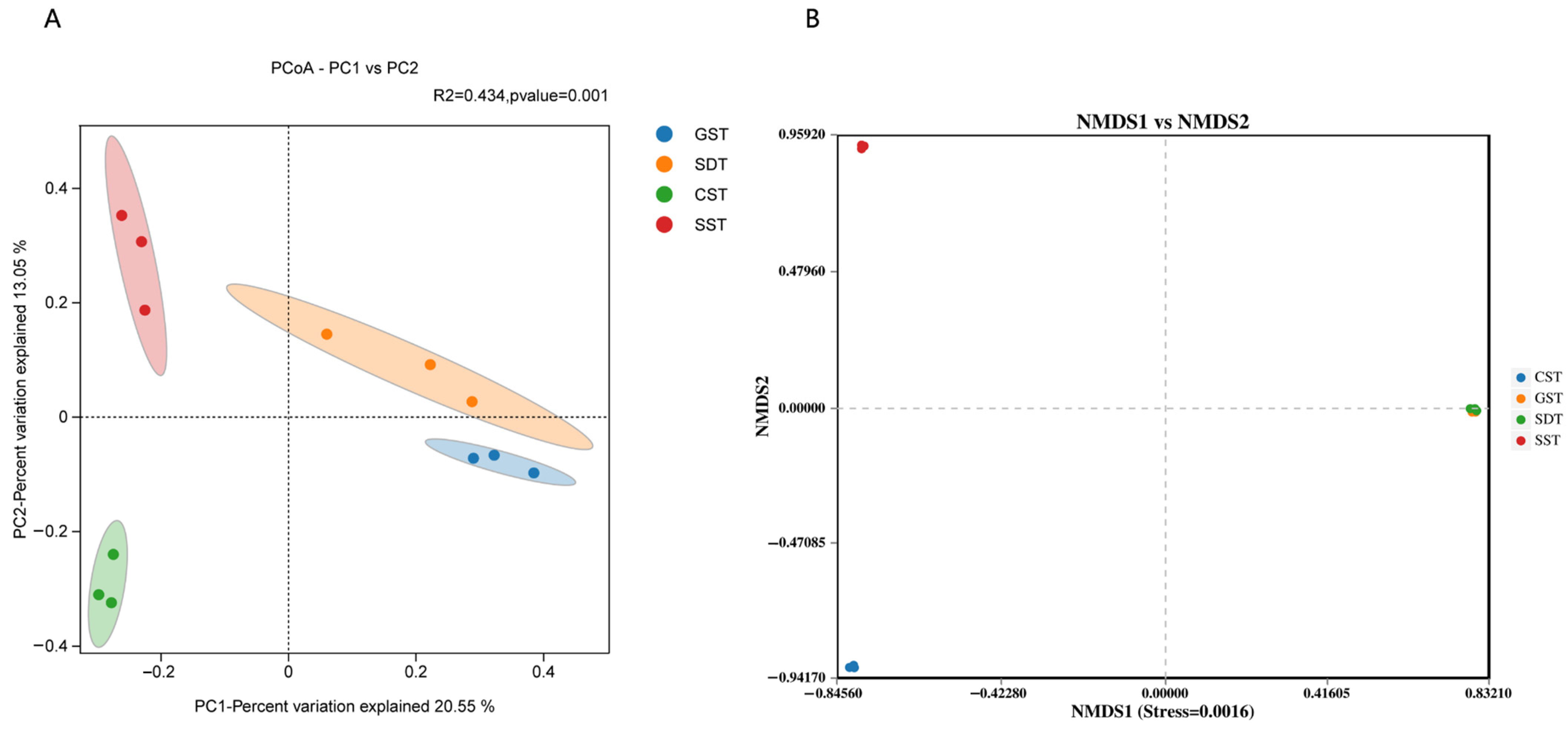
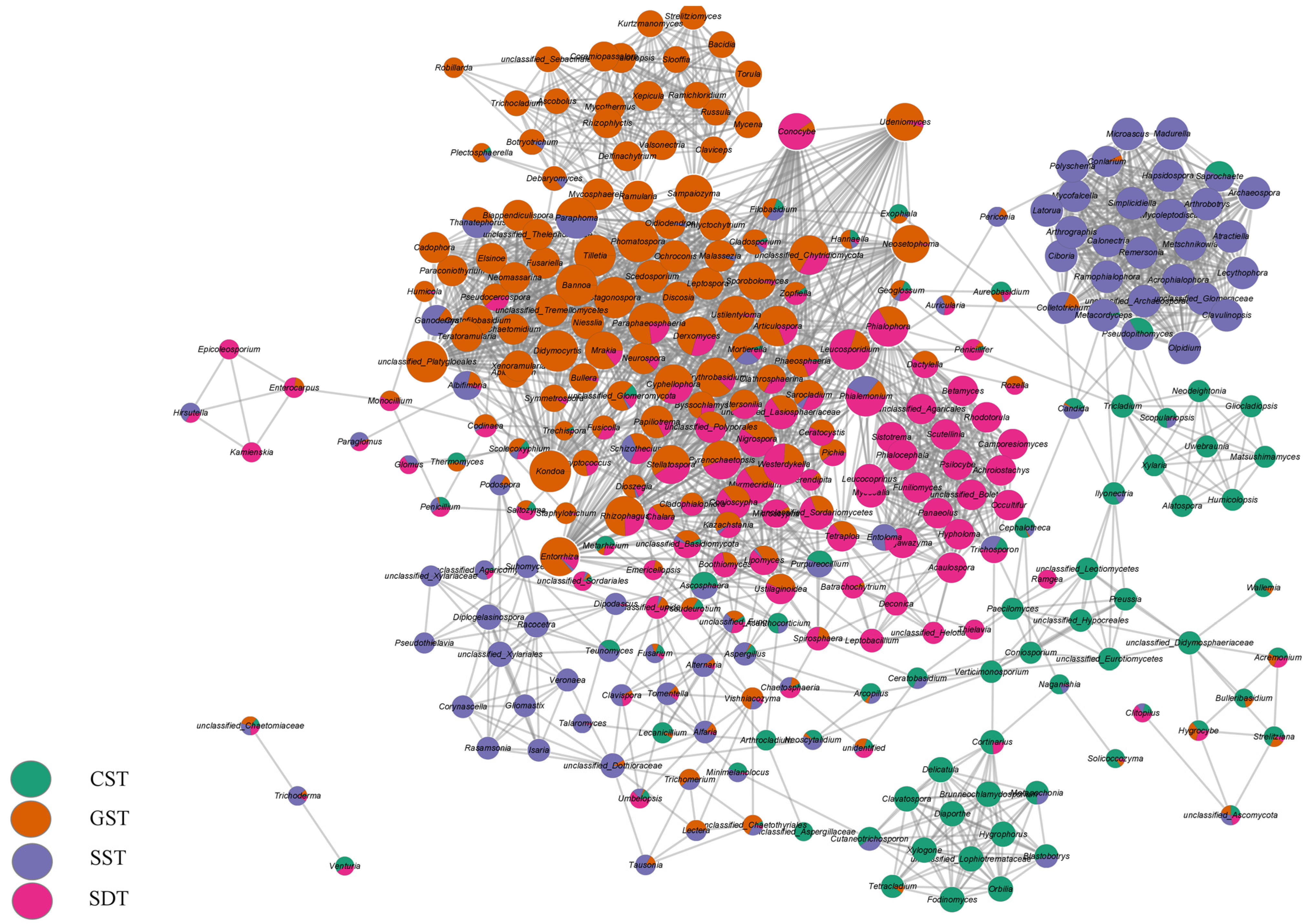
3.5. Soil Fungal Community Distribution
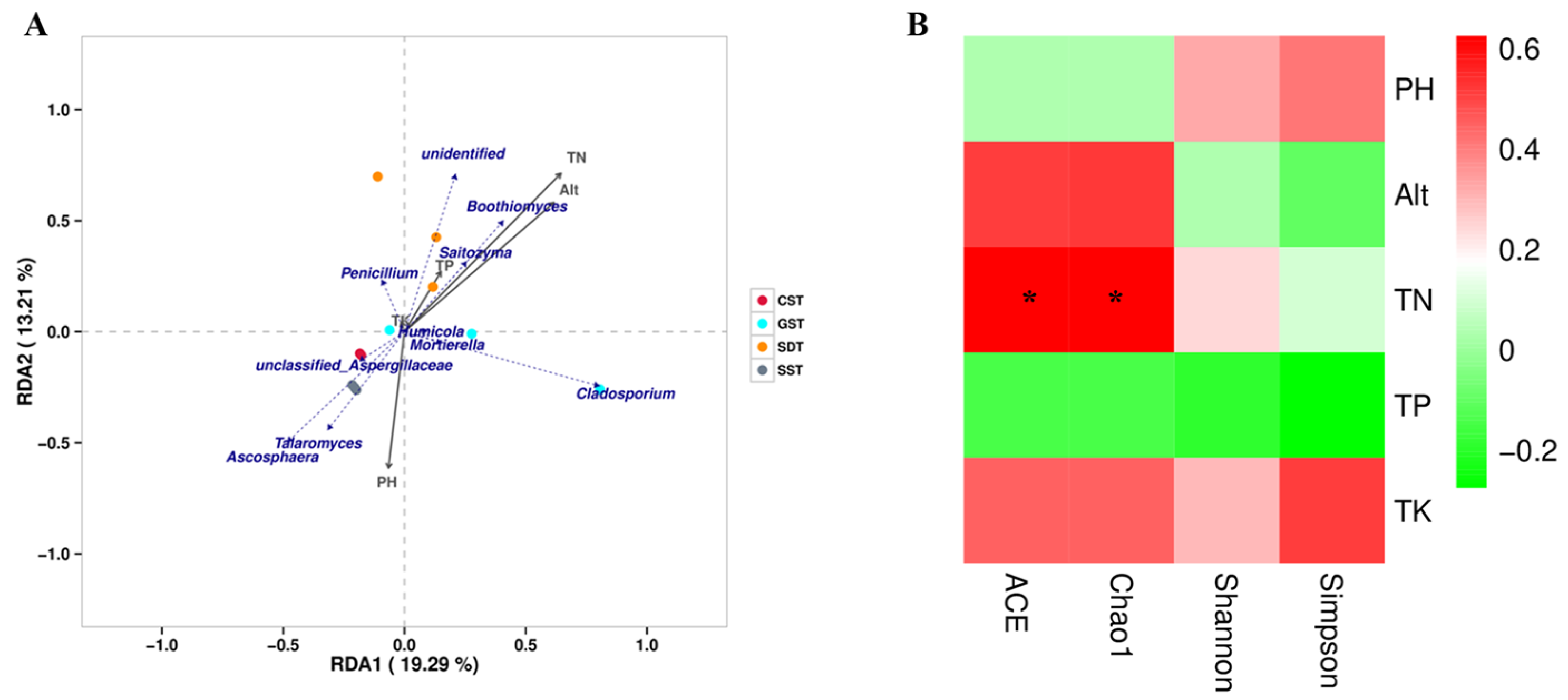
3.6. Relationship between Abundant Fungal Genera and Diversity Indices, and Soil Chemical Parameters
3.7. Functional Prediction of Fungal Phenotype by FUNGuild
3.8. The Morphological Characteristics and Quality of the Alismatis Rhizoma Planted in Soils of Different Plots
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenda, M.; Kocevar Glavac, N.; Nagy, M.; Sollner Dolenc, M. Medicinal Plants Used for Anxiety, Depression, or Stress Treatment: An Update. Molecules 2022, 27, 6021. [Google Scholar] [CrossRef]
- Akkol, E.K.; Dereli, F.T.G.; Sobarzo-Sanchez, E.; Khan, H. Roles of Medicinal Plants and Constituents in Gynecological Cancer Therapy: Current Literature and Future Directions. Curr. Top. Med. Chem. 2020, 20, 1772–1790. [Google Scholar] [CrossRef]
- Moraes, R.M.; Cerdeira, A.L.; Lourenco, M.V. Using Micropropagation to Develop Medicinal Plants into Crops. Molecules 2021, 26, 1752. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Kong, Y.; Chen, W.; Wang, X.; Jia, Z.; Dai, Y.; Yang, X. The quality of wild Salvia miltiorrhiza from Dao Di area in China and its correlation with soil parameters and climate factors. Phytochem. Anal. PCA 2021, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Q.; Liu, W.; Ma, L.; Lv, Z.; Qin, H.; Guo, J. Experimental and Numerical Calculation Study on the Slope Stability of the Yellow River Floodplain from Wantan Town to Liuyuankou. Toxics 2023, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Hong, C.; Tong, W.; Xu, M.; Huang, C.; Yin, H.; Lin, Y.; Fu, Q. Health risk assessment of heavy metal pollution in a soil-rice system: A case study in the Jin-Qu Basin of China. Sci. Rep. 2020, 10, 11490. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef]
- Jiang, X.; Wright, A.L.; Wang, J.; Li, Z. Long-term tillage effects on the distribution patterns of microbial biomass and activities within soil aggregates. Catena 2011, 87, 276–280. [Google Scholar] [CrossRef]
- Bissett, A.; Richardson, A.; Baker, G.; Thrall, P. Long term land use effects on the structure and function of soil microbial communities. Appl. Soil Ecol. 2011, 51, 66–78. [Google Scholar] [CrossRef]
- Pandey, D.; Agrawal, M.; Bohra, J.S. Effects of conventional tillage and no tillage permutations on extracellular soil enzyme activities and microbial biomass under rice cultivation. Soil Tillage Res. 2014, 136, 51–60. [Google Scholar] [CrossRef]
- Ren, C.; Kang, D.; Wu, J.P.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Ma, C.; Wu, F.; Jin, X.; Dini-Andreote, F.; Wei, Z. Biochar amendment reduces cadmium uptake by stimulating cadmium-resistant PGPR in tomato rhizosphere. Chemosphere 2022, 307, 136138. [Google Scholar] [CrossRef]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Sadowsky, M.J. Associations between soil bacterial community structure and nutrient cycling functions in long-term organic farm soils following cover crop and organic fertilizer amendment. Sci. Total Environ. 2016, 566–567, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Ren, T.; Tian, Z.; Wang, G.; He, X.; Tian, C. Short-term effect of tillage and crop rotation on microbial community structure and enzyme activities of a clay loam soil. Biol. Fertil. Soils 2014, 50, 1077–1085. [Google Scholar] [CrossRef]
- Wu, J.R.; Xu, F.J.; Cao, W.; Zhang, W.; Dai, C.C. Fungal endophyte Phomopsis liquidambari B3 enriches the diversity of nodular culturable endophytic bacteria associated with continuous cropping of peanut. Arch. Agron. Soil Sci. 2018, 65, 240–252. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, W.; Li, S.; Hao, J.; Su, Z.; Min, S.; Gao, Z.; Zhang, C. Variation of Bacterial Community Diversity in Rhizosphere Soil of Sole-Cropped versus Intercropped Wheat Field after Harvest. PLoS ONE 2016, 11, e0150618. [Google Scholar] [CrossRef]
- Navarro-Noya, Y.E.; Gómez-Acata, S.; Montoya-Ciriaco, N.; Rojas-Valdez, A.; Dendooven, L. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol. Biochem. 2013, 65, 86–95. [Google Scholar] [CrossRef]
- Ji, N.N.; Gao, C.; Sandel, B.; Zheng, Y.; Chen, L.; Wu, B.W.; Li, X.C.; Wang, Y.L.; Lu, P.P.; Sun, X.; et al. Late Quaternary climate change explains soil fungal community composition rather than fungal richness in forest ecosystems. Ecol. Evol. 2019, 9, 6678–6692. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ramesh, A.; Sharma, M.P.; Joshi, O.P.; Karlen, D.L. Microbial Community Structure and Diversity as Indicators for Evaluating Soil Quality; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Liu, R.; Tang, M.; Chen, Y. Recent advances in the study of mycorrhizal fungi and stress resistance of plants. J. Fungal Res. 2017, 15, 70–88. [Google Scholar]
- Ren, J.L.; Zhang, A.H.; Wang, X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liang, P.; Ma, Y.; Sun, Q.; Yang, S. Research Progress of Traditional Chinese Medicine Against COVID-19. Biomed. Pharmacother. 2021, 137, 111310. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, L.; Zhang, Y.; Lu, X.; Lin, W.; Wu, S.; Qin, X.; Xu, R. Hypolipidemic effect of Alisma orientale (Sam.) Juzep on gut microecology and liver transcriptome in diabetic rats. PLoS ONE 2020, 15, e0240616. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Pharmacological Properties and Molecular Targets of Alisol Triterpenoids from Alismatis Rhizoma. Biomedicines 2022, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Judge, J.; Bongiovanni, T.; Rangarajan, A.; Ranka, S. Spatial Scaling Using Temporal Correlations and Ensemble Learning to Obtain High-Resolution Soil Moisture. IEEE Trans. Geosci. Remote Sens. 2018, 56, 1238–1250. [Google Scholar] [CrossRef]
- GB15618; Risk Control Standard for Soil Contamination of Agricultural Land. Standards Press of China: Beijing, China, 2018; Volume 189, pp. 528–536.
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, Z.; Fu, G. Geo-Distribution Patterns of Soil Fungal Community of Pennisetum flaccidum in Tibet. J. Fungi 2022, 8, 1230. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Zhou, H.; Ganjurjav, H.; Wang, X. Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016, 562, 353–363. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.F.; Zhou, H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020.
- Lin, W.; Sun, F.; Zhang, Y.; Xu, X.; Lu, X.; Li, L.; Xu, R. Comparative transcriptome and metabolite profiling of four tissues from Alisma orientale (Sam.) Juzep reveals its inflorescence developmental and medicinal characteristics. Sci. Rep. 2019, 9, 12310. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, S.; Shi, Q.; Xiang, X.; Jin, Y.; Wei, S.; Zhang, L.; Yang, M.; Song, C.; Huang, R.; et al. Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS. Molecules 2021, 27, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Qian, Y.; Zhao, X.; Wang, Q. Ternary toxicological interactions of insecticides, herbicides, and a heavy metal on the earthworm Eisenia fetida. J. Hazard. Mater. 2015, 284, 233–240. [Google Scholar] [CrossRef]
- Shaban, N.S.; Abdou, K.A.; Hassan, N.E.H.Y. Impact of toxic heavy metals and pesticide residues in herbal products. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 102–106. [Google Scholar] [CrossRef]
- Sherer, T.B.; Richardson, J.R.; Testa, C.M.; Seo, B.B.; Panov, A.V.; Yagi, T.; Matsuno-Yagi, A.; Miller, G.W.; Greenamyre, J.T. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 2010, 100, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, X.; Yao, X.; Wu, X. Safety Evaluation of Heavy Metal Contamination and Pesticide Residues in Coix Seeds in Guizhou Province, China. Foods 2022, 11, 2286. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2019, 10, 1741. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.; Chen, L.; Wang, J.; Zhang, L. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef]
- Yu, C.; Han, F.; Fu, G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Sci. Total Environ. 2019, 655, 814–822. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Jarvis, S.G.; Woodward, S.; Taylor, A.F.S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300m) elevation gradient. New Phytol. 2015, 206, 1145–1155. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Nevarez, L.; Vasseur, V.; Madec, A.L.; Bras, M.A.L.; Coroller, L.; Leguérinel, I.; Barbier, G. Physiological traits of Penicillium glabrum strain LCP 08.5568, a filamentous fungus isolated from bottled aromatised mineral water. Int. J. Food Microbiol. 2009, 130, 166–171. [Google Scholar] [CrossRef]
- Klimek, B.; Chodak, M.; Jaźwa, M.G.; Niklińska, M. Functional diversity of soil microbial communities in boreal and temperate Scots pine forests. Eur. J. For. Res. 2016, 135, 731–742. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, F.; Peng, S.; Lu, M. Relationship between soil fungal community structure and physical and chemical properties of different seasons swamp meadow in Napahai wetland. Chin. Agric. Sci. Bull. 2018, 34, 69–75. [Google Scholar]
- Gravuer, K.; Eskelinen, A.; Winbourne, J.B.; Harrison, S.P. Vulnerability and resistance in the spatial heterogeneity of soil microbial communities under resource additions. Proc. Natl. Acad. Sci. USA 2020, 117, 7263–7270. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Wang, Y.; Xiong, S.; Yu, H.; Zhao, X.; Tan, W.; Cui, D.; Xi, B. Untangling the response of fungal community structure, composition and function in soil aggregate fractions to food waste compost addition. Sci. Total Environ. 2021, 769, 145248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, Z.; Shi, W.; Li, L.; Tian, R.; Huang, J.; Lin, R.; Wang, B.; Zhou, B. Material conversion, microbial community composition and metabolic functional succession during green soybean hull composting. Bioresour. Technol. 2020, 316, 123823. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal biogeography. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Muneer, M.A.; Huang, X.; Hou, W.; Zhang, Y.; Cai, Y.; Munir, M.Z.; Wu, L.; Zheng, C. Response of Fungal Diversity, Community Composition, and Functions to Nutrients Management in Red Soil. J. Fungi 2021, 7, 554. [Google Scholar] [CrossRef]
- Kaling, M.; Schmidt, A.; Moritz, F.; Rosenkranz, M.; Witting, M.; Kasper, K.; Janz, D.; Schmitt-Kopplin, P.; Schnitzler, J.-P.; Polle, A. Mycorrhiza-Triggered Transcriptomic and Metabolomic Networks Impinge on Herbivore Fitness. Plant Physiol. 2018, 176, 2639–2656. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Zabalgogeazcoa, I.; Aldana, B.R.V.D.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcón-Aguilar, C.; Pozo, M.J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef] [PubMed]


| Taxon | CST | GST | SDT | SST |
|---|---|---|---|---|
| pH | 4.49 ± 0.10 b | 4.83 ± 0.10 ab | 4.55 ± 0.10 ab | 5.36 ± 0.11 a |
| Alt (m) | 343.17 ± 1.02 ab | 483.80 ± 2.0 a | 464.10 ± 8.89 ab | 151.53 ± 2.77 b |
| TN (%) | 0.08 ± 0.001 ab | 0.133 ± 0.02 ab | 0.15 ± 0.03 a | 0.03 ± 0.001 b |
| TP (g/kg) | 0.71 ± 0.01 b | 0.48 ± 0.10 ab | 0.54 ± 0.13 ab | 0.29 ± 0.01 a |
| TK (g/kg) | 13.6 ± 0.03 a | 25.7 ± 7.57 a | 19.93 ± 4.56 a | 24.3 ± 1.04 a |
| Group | Standard Values a | CST | GST | SDT | SST |
|---|---|---|---|---|---|
| Cd (μg/g) | ≤0.30 | 0.03 ± 0.002 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.14 ± 0.04 |
| Cr (mg/kg) | ≤250 | 70 ± 3.00 | 16 ± 2.52 | 25 ± 14.11 | 20 ± 2.01 |
| Hg (mg/kg) | ≤0.50 | 0.38 ± 0.10 | 0.20 ± 0.05 | 0.17 ± 0.03 | 0.03 ± 0.004 |
| Ni (μg/g) | ≤60 | 35.90 ± 1.00 | 8.50 ± 1.53 | 13.2 ± 6.93 | 8.10 ± 0.02 |
| Pb (μg/g) | ≤80 | 19.70 ± 0.30 | 24.20 ± 6.01 | 40.60 ± 4.98 | 35.8 ± 2.30 |
| As (mg/kg) | ≤30 | 11.9 ± 0.70 | 2.92 ± 0.41 | 1.93 ± 0.50 | 1.49 ± 0.30 |
| Cu (μg/g) | ≤50 | 29.6 ± 0.40 | 14.50 ± 0.91 | 13.00 ± 3.58 | 9.20 ± 0.51 |
| Zn (μg/g) | ≤200 | 117 ± 2.00 | 41.10 ± 5.45 | 46.70 ± 4.47 | 71.6 ± 1.03 |
| DDT (mg/kg) | ≤0.10 | ND (<0.01) | ND (<0.01) | ND (<0.01) | ND (<0.01) |
| BHC (mg/kg) | ≤0.10 | ND (<0.01) | ND (<0.01) | ND (<0.01) | ND (<0.01) |
| Group | ACE | Chao1 | Simpson | Shannon |
|---|---|---|---|---|
| CST | 99.65 ± 5.98 a | 100.89 ± 8.17 a | 0.75 ± 0.10 a | 3.34 ± 0.87 a |
| GST | 374.12 ± 80.36 b | 377.14 ± 79.83 b | 0.90 ± 0.07 a | 5.02 ± 1.47 a |
| SDT | 263.60 ± 140.90 abc | 264.08 ± 141.7 abc | 0.83 ± 0.16 a | 4.33 ± 1.69 a |
| SST | 182.05 ± 44.07 c | 189.88 ± 31.45 c | 0.91 ± 0.05 a | 4.73 ± 0.69 a |
| Group | CST | GST | SDT | SST |
|---|---|---|---|---|
| Diameter (mm) | 36.87 ± 2.67 b | 44.97 ± 4.21 a | 42.77 ± 1.62 a | 38.80 ± 1.41 ab |
| Wet weight (mg) | 37.29 ± 0.71 c | 81.2 ± 11.41 a | 63.42 ± 4.76 b | 55.54 ± 6.22 b |
| Dry weight (mg) | 10.24 ± 1.59 b | 25.20 ± 7.44 a | 22.32 ± 0.72 a | 14.69 ± 5.50 ab |
| AlisolB23-acetate (mg/g) | 0.7150 ± 0.0168 b | 2.1314 ± 0.5424 a | 1.5233 ± 0.1264 c | 0.8595 ± 0.0266 b |
| Alisol B (mg/g) | 0.1211 ± 0.006 b | 0.2680 ± 0.030 a | 0.1613 ± 0.011 c | 0.1470 ± 0.011 bc |
| AlisolC23-acetate (mg/g) | 0.0315 ± 0.0016 b | 0.0862 ± 0.0065 a | 0.0721 ± 0.0034 c | 0.0444 ± 0.0011 d |
| AlisolG (mg/g) | 0.000 | 0.0072 ± 0.001 | 0.0030 ± 0.0003 | 0.0021 ± 0.0004 |
| Alisol F (mg/g) | 0.000 | 0.0005 | 0.0001 | 0.000 |
| AlisolF 24-acetate (mg/g) | 0.000 | 0.0003 | 0.000 | 0.000 |
| AlisolA24-acetate (mg/g) | 0.0002 ± 0.0001 | 0.0025 ± 0.0006 | 0.0005 ± 0.0003 | 0.0001 ± 0.0002 |
| AlisolA (mg/g) | 0.0003 ± 0.0001 | 0.0031 ± 0.0009 | 0.0014 ± 0.0006 | 0.0015 ± 0.0008 |
| Total triterpenoids (mg/g) | 0.8685 ± 0.0145 c | 2.4991 ± 0.5450 a | 1.7613 ± 0.1190 b | 1.0547 ± 0.0267 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Lin, W.; Keyhani, N.O.; Liu, S.; Li, L.; Zhang, Y.; Lu, X.; Wei, Q.; Wei, D.; Huang, S.; et al. Properties and Fungal Communities of Different Soils for Growth of the Medicinal Asian Water Plantain, Alisma orientale, in Fujian, China. J. Fungi 2024, 10, 187. https://doi.org/10.3390/jof10030187
Xu X, Lin W, Keyhani NO, Liu S, Li L, Zhang Y, Lu X, Wei Q, Wei D, Huang S, et al. Properties and Fungal Communities of Different Soils for Growth of the Medicinal Asian Water Plantain, Alisma orientale, in Fujian, China. Journal of Fungi. 2024; 10(3):187. https://doi.org/10.3390/jof10030187
Chicago/Turabian StyleXu, Xiaomei, Wenjin Lin, Nemat O. Keyhani, Sen Liu, Lisha Li, Yamin Zhang, Xuehua Lu, Qiuran Wei, Daozhi Wei, Shuaishuai Huang, and et al. 2024. "Properties and Fungal Communities of Different Soils for Growth of the Medicinal Asian Water Plantain, Alisma orientale, in Fujian, China" Journal of Fungi 10, no. 3: 187. https://doi.org/10.3390/jof10030187
APA StyleXu, X., Lin, W., Keyhani, N. O., Liu, S., Li, L., Zhang, Y., Lu, X., Wei, Q., Wei, D., Huang, S., Cao, P., Tian, L., & Qiu, J. (2024). Properties and Fungal Communities of Different Soils for Growth of the Medicinal Asian Water Plantain, Alisma orientale, in Fujian, China. Journal of Fungi, 10(3), 187. https://doi.org/10.3390/jof10030187






