Abstract
This review article explores the effectiveness of antibacterial drugs that inhibit protein synthesis in treating pythiosis, a difficult-to-treat infection caused by Pythium insidiosum. The article highlights the susceptibility of P. insidiosum to antibacterial drugs, such as macrolides, oxazolidinones, and tetracyclines. We examine various studies, including in vitro tests, experimental infection models, and clinical case reports. Based on our synthesis of these findings, we highlight the potential of these drugs in managing pythiosis, primarily when combined with surgical interventions. The review emphasizes the need for personalized treatment strategies and further research to establish standardized testing protocols and optimize therapeutic approaches.
1. Introduction
Pythium insidiosum, the causative agent of pythiosis, represents a significant threat to human and animal health due to its aggressive and destructive nature. This pathogen, a member of the oomycetes, is particularly concerning because of its capacity to infect and induce life-threatening conditions in otherwise healthy individuals [1,2].
Pythiosis is a significant concern for animal health, especially for horses, dogs, and, to a lesser extent, cats and other mammals [3]. In horses, the disease manifests as chronic, debilitating subcutaneous lesions, leading to systemic illness and significant tissue damage in the limbs, abdomen, and face. In dogs, it causes ulcerations, lymph node involvement, and severe gastrointestinal issues, such as tumor-like masses in the intestines, resulting in severe diarrhea, lethargy, and potentially fatal outcomes if left untreated [2].
In humans, the impact of pythiosis can be profoundly severe, necessitating procedures like enucleation for ocular infections [4] and limb amputation in cases of arteritis due to vascular commitment [1]. The disease’s severity is further heightened by its ability to spread systemically. For instance, untreated vascular pythiosis can extend through the arteries, affecting the iliac and renal arteries and even the abdominal aorta, leading to disseminated pythiosis, which is often fatal [5].
Treating P. insidiosum infections presents significant challenges, but growing research suggests promise for antibacterial agents that specifically target protein synthesis [6,7,8,9]. This approach could improve treatment strategies for specific forms of pythiosis. This review focuses on treating pythiosis, specifically examining the role of antibacterials that inhibit protein synthesis against P. insidiosum. It evaluates their effectiveness by analyzing in vitro susceptibility data, experimental infection models, and clinical case studies.
2. Overview of Pythiosis Treatment
The treatment of pythiosis has seen considerable advancements, yet it remains a complex challenge, primarily due to P. insidiosum’s resistance to traditional antifungal therapies (Table 1). Surgery is considered a key strategy, particularly effective for localized, smaller, and superficial lesions, though its success can be constrained [5]. Antifungal drugs, initially used based on the microorganism’s misclassification as a fungus, have demonstrated limited efficacy [10,11]. Immunotherapy emerges as a promising alternative, particularly beneficial in equine and some human cases, although its success varies depending on factors like lesion duration and antigen preparation methods [11,12]. Moreover, exploring adjuvant treatments and plant-derived compounds has introduced new possibilities for pythiosis management, yet their safety and effectiveness warrant further investigation [11].

Table 1.
Critical aspects in pythiosis treatment.
3. P. insidiosum Cell Structure and Susceptibility to Antibacterial Drugs
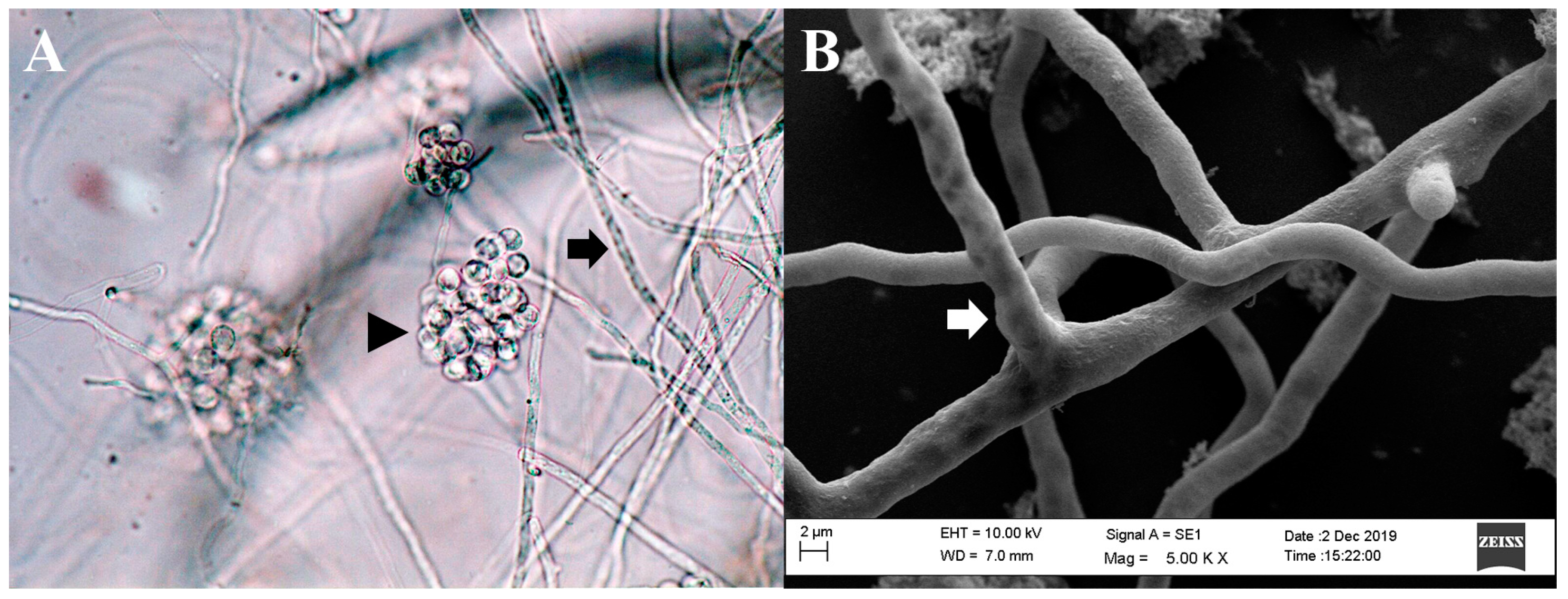
Although P. insidiosum shares morphological traits with filamentous fungi, it is more closely related to organisms such as brown algae and diatoms. As a member of the Stramenopiles-Alveolata–Rhizaria supergroup, P. insidiosum is characterized by its broad hyphae, perpendicular branching, and the production of biflagellate zoospores in aquatic environments (Figure 1) [42,43,44].
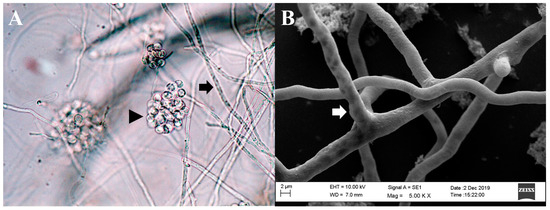
Figure 1.
Microscopic morphology of Pythium insidiosum. (A). Hyphae of P. insidiosum (black arrow) and a cluster of encysted zoospores (black arrowhead) (light microscopy, 400× magnification). (B). Image from a scanning electron microscope depicting the three-dimensional structure of P. insidiosum mycelium (white arrow).
The biochemical distinction of oomycetes from fungi is evident in its cell wall composition, which contains minimal chitin but is rich in cellulose and β-glucans [45,46,47]. Other differences include its mitochondrial structure, actin cytoskeleton, and protein repertoire [48,49,50]. Notably, P. insidiosum has an incomplete sterol biosynthesis pathway, relying on external sterol sources for physiological functions, which contributes to its resistance or reduced susceptibility to sterol biosynthesis inhibitors and sterol-binding drugs [21,51,52,53].
Pythium spp. are distinguished from true fungi due to their heightened sensitivity to antibacterials that act on protein synthesis in the 70S ribosome, such as tetracycline, chloramphenicol, streptomycin, and erythromycin [54,55,56]. It is plausible that these antimicrobials inhibit the growth of the microorganism by interfering with cytoplasmic and mitochondrial protein synthesis [55]. However, the addition of sterols (ergosterol, cholesterol, beta-sitosterol, or stigmasterol) to the culture medium shields Pythium spp. against the action of these antibacterials [57] and other anti-Pythium drugs [53], possibly by altering cell membrane permeability and reducing the entry of these drugs into the cell.
The clinical implications of these findings regarding tetracycline, chloramphenicol, streptomycin, and erythromycin are nuanced by the potential interaction between Pythium spp. and host-derived sterols. This protective mechanism suggests that the observed in vitro sensitivity may not directly translate to in vivo efficacy, as the possible incorporation of host-derived sterols could alter the susceptibility of Pythium to these drugs. However, further scientific research is required to support or refute this hypothesis.
4. In Vitro Anti-Pythium Antimicrobial Activity of Protein Synthesis-Inhibiting Antibacterials
It is crucial to recognize that, to date, a standardized susceptibility testing protocol specifically for P. insidiosum has yet to be established. The susceptibility assessments are adapted mainly from established protocols for fungi and bacteria. Investigations in this domain have examined diverse culture media, varying inoculum concentrations, and various methodologies [58]. These investigations have also extended to analyzing pathogenic microorganisms isolated from animal and human hosts. The heterogeneity in these testing methodologies underscores the intricate challenges and complexities that P. insidiosum presents in clinical microbiology and infectious disease research.
4.1. Anti-Pythium Antimicrobial Activity Determined by Reduction in Mycelial Weight
Marchant and Smith [54] described that chloramphenicol exerted an inhibitory effect on the growth rate of Pythium ultimum. The maximum inhibitory response was observed at 100 µg/mL. Rawn and Van Etten [55] investigated the sensitivity of a P. ultimum isolate to several antibiotics over a 24 h treatment period. They found that cycloheximide, an eukaryotic protein synthesis inhibitor, inhibited 98% of P. ultimum growth at a concentration of 1 µg/mL. Tetracycline showed 83% inhibition at 10 µg/mL and 99% at 100 µg/mL. Chloramphenicol resulted in 62% growth inhibition at 100 µg/mL, while erythromycin achieved 70% inhibition at 10 µg/mL and 91% at 100 µg/mL.
McMeekin [59] reported that 100 µg/mL of streptomycin could stimulate the growth of a P. aphanidermatum isolate, in contrast to 200 µg/mL of streptomycin, which inhibited the growth of this microorganism. Similarly, McMeekin and Mendoza [60] found varying effects of streptomycin on the in vitro growth of two P. insidiosum isolates, with one isolate inhibited and the other stimulated by this aminoglycoside.
4.2. Anti-Pythium Antimicrobial Activity Determined by Linear or Radial Growth Inhibition
Marchant and Smith [54] found that while chloramphenicol at 100 µg/mL had a lesser impact on the P. ultimum linear growth rate compared to its effect on dry weight production, it significantly altered the morphology, resulting in a lower density of hyphae and reduced aerial mycelium.
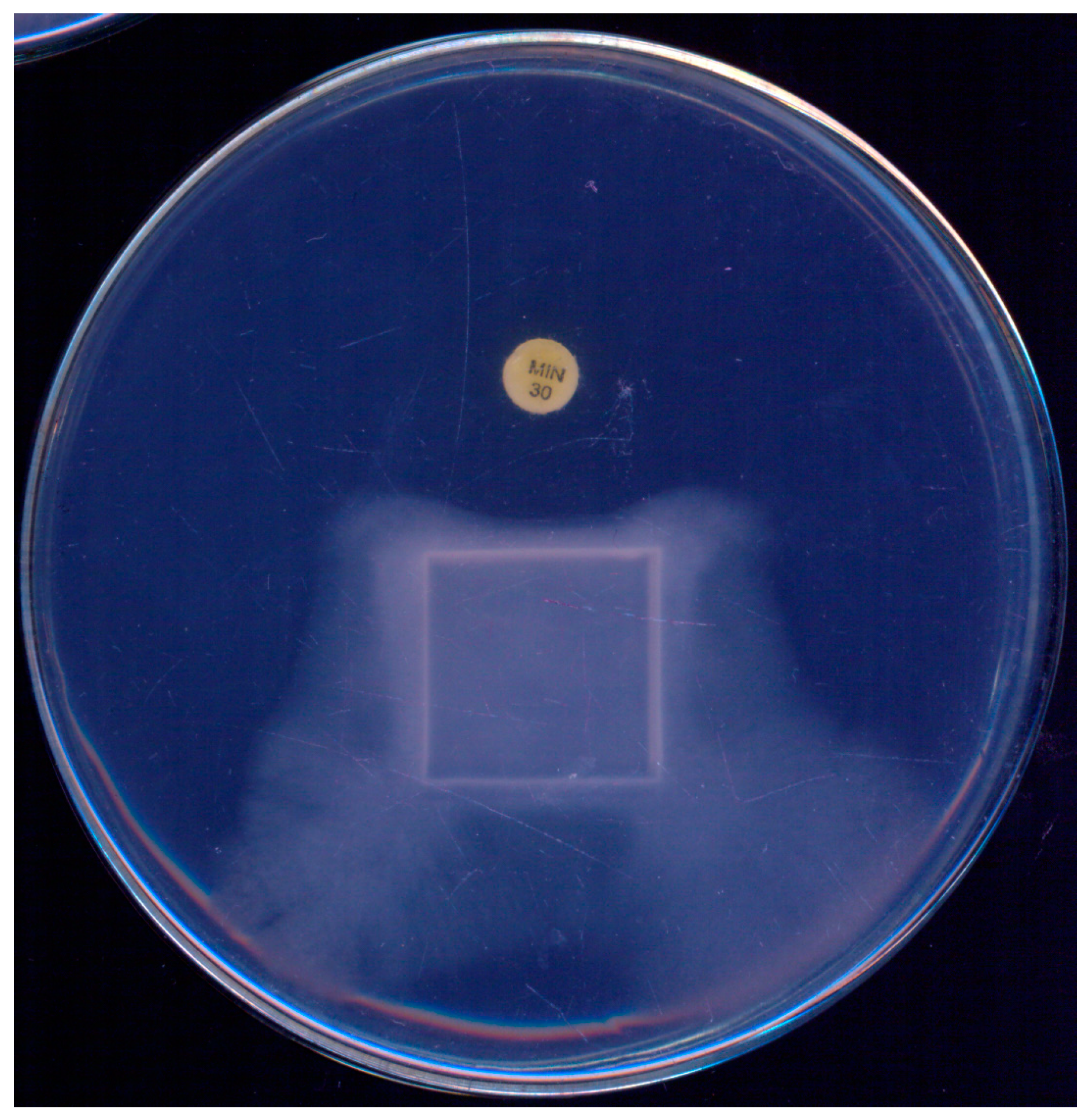
During the initial standardization of a disk-diffusion test to assess the susceptibility of P. insidiosum to antibacterials, Tondolo et al. [61] observed a unique response to minocycline (30 µg). Not only did the disks inhibit the growth of P. insidiosum, they also induced a phenomenon of mycelial “escape” from the antibacterial drug, as illustrated in Figure 2. Given its simplicity, the authors proposed this technique as a screening tool to distinguish P. insidiosum from true fungi, highlighting that true fungi exhibited no inhibition in radial growth at this minocycline concentration.
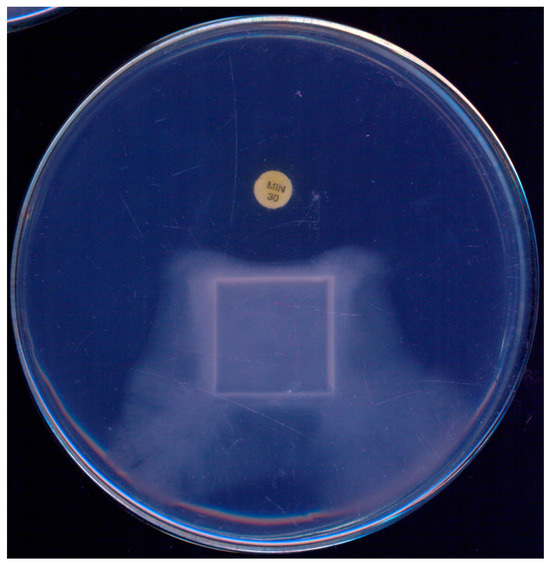
Figure 2.
Pythium insidiosum growth observed on a single plate after 48 h incubation at 35 °C on Muller–Hinton agar, demonstrating the effect of a minocycline (30 µg) disk. A marked growth inhibition is noticeable in the area surrounding the minocycline disk, illustrating its antibacterial activity against P. insidiosum.
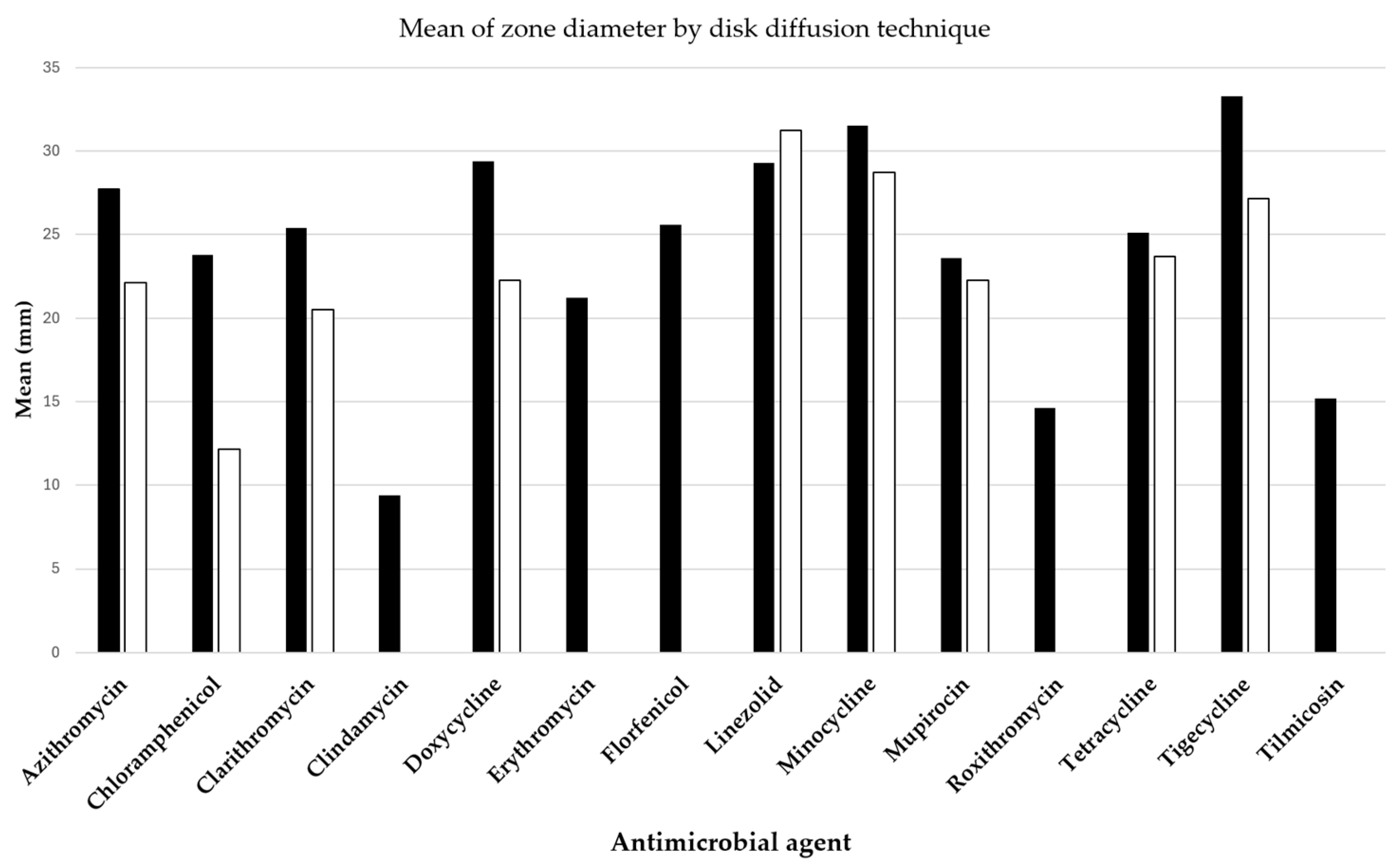
Two pivotal studies by Loreto et al. [7] and Bagga et al. [8] have provided insightful data on the antibacterial efficacy against P. insidiosum, assessed through the disk diffusion method and detailed in Figure 3. The antibacterial drugs evaluated included azithromycin, clarithromycin, linezolid, mupirocin, doxycycline, minocycline, tetracycline, and tigecycline, all exhibiting varying extents of inhibition zones.
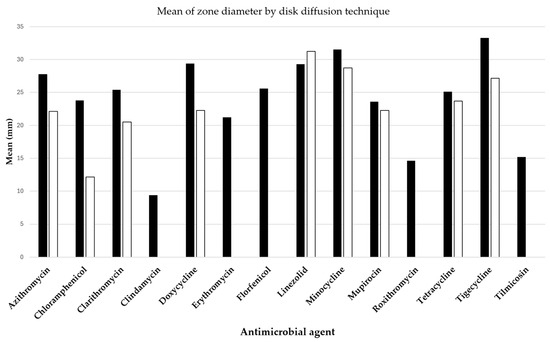
Figure 3.
Mean diameters of Pythium insidiosum growth inhibition zones around disks containing antibacterial drugs, with black bars representing results from Loreto et al. [7] and white bars indicating findings from Bagga et al. [8].
4.3. Anti-Pythium Antimicrobial Activity Determined by Broth Microdilution and Gradient Strip Susceptibility Tests
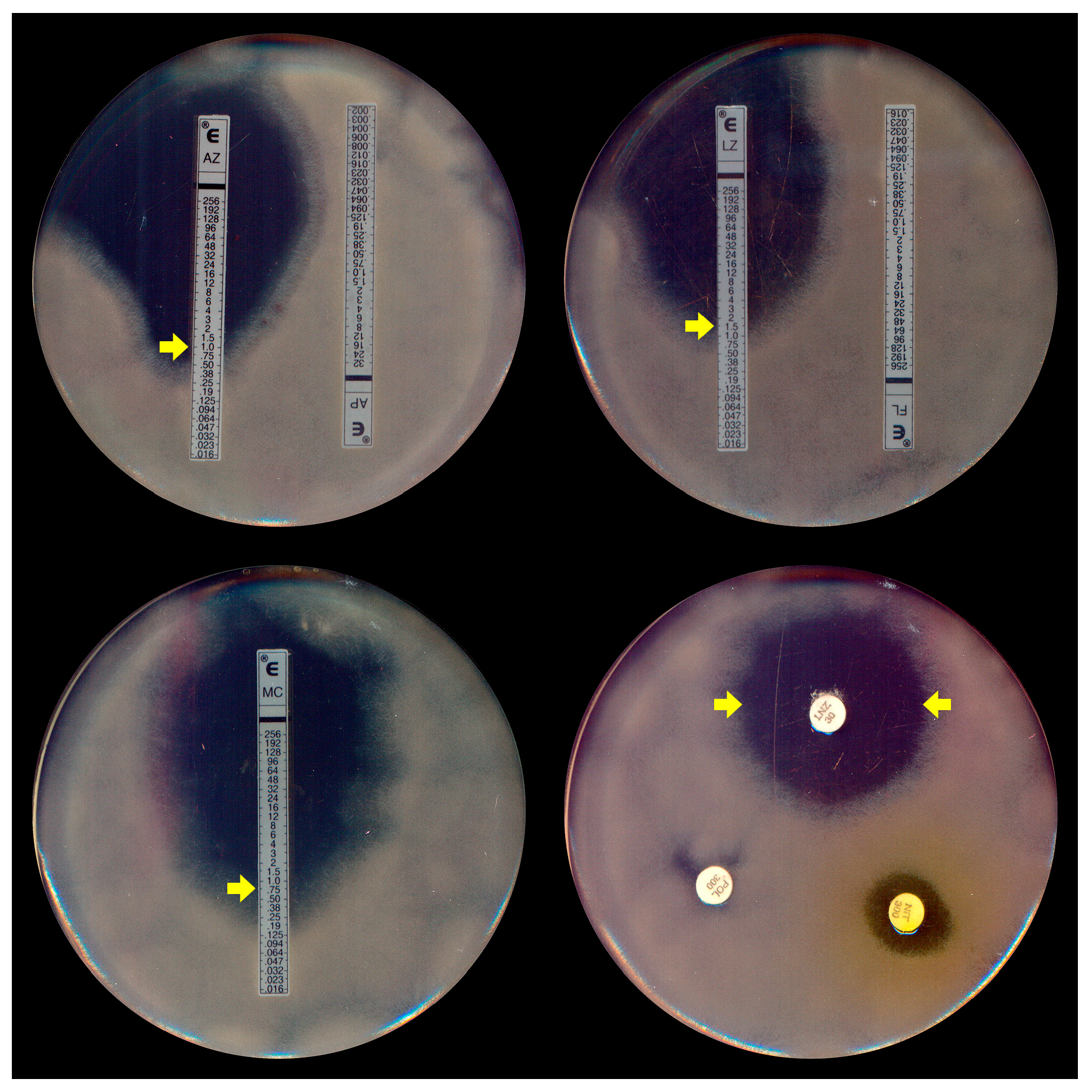
Due to the absence of a specific protocol for P. insidiosum susceptibility assays, most broth microdilution tests for this oomycete are conducted following the most recent guidelines of the Clinical and Laboratory Standards Institute’s (CLSI) M38-A2 protocol [62,63], which was initially designed for filamentous fungi. In addition to these standard microdilution methods, susceptibility testing for P. insidiosum was conducted using gradient strip tests (Etest® and Liofilchem®) (Figure 4). A key distinction, however, is the use of zoospore inocula (Figure 1A), generated in vitro through zoosporogenesis techniques [64], in these tests.
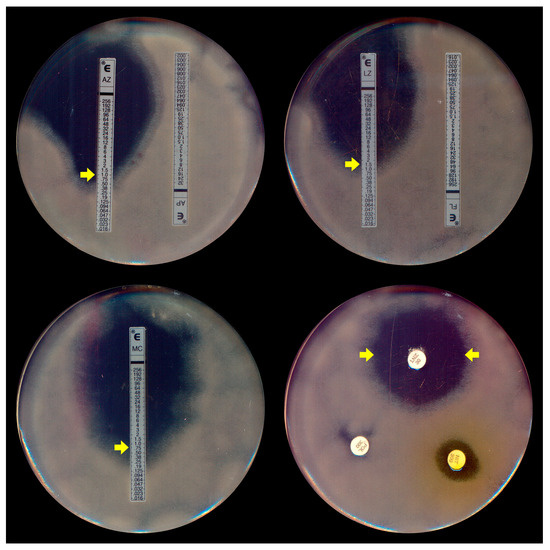
Figure 4.
Etest assay (top left, right, and bottom left) demonstrating the elliptical inhibition zones (MIC, indicated by yellow arrows) of Pythium insidiosum induced by azithromycin (AZ), linezolid (LZ), and minocycline (MC), respectively. Disk diffusion (bottom right) exhibits the halo of linezolid (LNZ) (yellow arrows). Note the absence of inhibition with the antifungal agents amphotericin B (AP) and fluconazole (FL) in the top left and the right plates.
The effectiveness of various antibacterial drugs against P. insidiosum has been the subject of several recent research studies revealing varying levels of susceptibility and efficacy. Notably, macrolides (such as azithromycin and clarithromycin) and tetracyclines (including doxycycline, minocycline, and tigecycline) have consistently shown promising in vitro antimicrobial activity against this pathogen, as demonstrated in the studies of Loreto et al. [65], Mahl et al. [66], Worasilchai et al. [9], and Torvorapanit et al. [67], particularly highlighting their low minimum inhibitory concentrations (MICs) in comparison to other evaluated antibacterial drugs, as comprehensively detailed in Table 2. Furthermore, in vitro synergy between tetracyclines and macrolides has also been described, suggesting an enhanced antimicrobial effect when these two classes of antibacterials are combined against P. insidiosum [9,67].

Table 2.
Minimum inhibitory concentrations (MICs) of antibacterials inhibiting protein synthesis against Pythium spp.
Linezolid exhibited similar or slightly higher MICs than macrolides and tetracyclines and was also highlighted as an effective drug inhibiting the in vitro growth of P. insidiosum [7,8]. Additionally, Loreto et al. [68] expanded the research to include other oxazolidinones like sutezolid and tedizolid, which demonstrated varying levels of effectiveness. In contrast, aminoglycosides, as studied by Mahl et al. [66], showed less effectiveness due to their higher MICs, a finding further supported by research from Loreto et al. [7], Loreto et al. [6], Worasilchai et al. [9], and Torvorapanit et al. [67].
The research on P. insidiosum inhibition by mupirocin and drugs from the pleuromutilin class has revealed some interesting findings. Mupirocin, primarily used as a topical drug, has significantly inhibited Pythium growth in vitro [7,8]. Moreover, all evaluated pleuromutilins, common drugs used in veterinary medicine, showed inhibitory activity against this pathogen [68].
Amphenicols, fusidic acid, lincosamides, and streptogramins exhibited higher MICs, indicating a reduced efficacy in inhibiting the in vitro growth of P. insidiosum. This variation ranged from moderately elevated MICs to a complete lack of inhibition in some cases [6,7,8].
5. Evaluating Protein Synthesis-Inhibiting Antibacterials in Experimental Models of Pythiosis
In the realm of antibacterial treatments, Jesus et al. [72] delved into the in vivo efficacy of azithromycin, clarithromycin, minocycline, and tigecycline against P. insidiosum, particularly in the context of subcutaneous pythiosis in a rabbit model. This investigation highlighted that azithromycin, when administered at a dosage of 20 mg/kg/day on a bi-daily schedule, either as a standalone treatment or in conjunction with minocycline at 10 mg/kg/day, led to a significant diminution in microbial load. This reduction was statistically significant and manifested in clinical cures of some animals.
Furthering this line of inquiry, Loreto et al. [73] scrutinized the efficacy of azithromycin in an experimental model involving vascular/disseminated pythiosis in immunocompromised mice. This study specifically assessed the impact of azithromycin administered at 50 mg/kg bi-daily, uncovering a notable decrease in mortality rates. This finding underscores the potential clinical utility of azithromycin in managing this severe variant of pythiosis, with the treatment notably enhancing survival rates to 80% and extending mean survival to 32.4 days.
In 2020, Zimmermann et al. [74] conducted a study to evaluate the effectiveness of minocycline, Pitium-Vac® immunotherapy, and both in treating subcutaneous pythiosis in rabbits. The study found that the combined therapy was significantly more effective in reducing lesion size than using only one or no treatment. Interestingly, one rabbit in the combined treatment group showed complete lesion resolution, which highlights the potential of this approach.
A subsequent study in 2021 by Ahirwar et al. [75] involved the testing of linezolid (0.2%), azithromycin (1%), and tigecycline (1%) in the treatment of induced keratitis in rabbits. The findings of this study revealed that linezolid emerged as the most effective treatment, achieving a 50% success rate and a significant reduction in clinical scores. In contrast, azithromycin and tigecycline demonstrated lower efficacy, with 16.7% and 25% success rates, respectively. Moreover, the study noted adverse reactions in some animals within the azithromycin and tigecycline groups, whereas linezolid was devoid of such adverse effects.
6. Exploring the Use of Protein Synthesis-Inhibiting Antibacterials in the Clinical Treatment of Pythiosis
The treatment and management of Pythium infections, particularly keratitis, have evolved significantly (Table 3). This evolution is evidenced by a shift from the traditional use of antifungal agents to incorporating antibacterial regimens, especially linezolid and azithromycin [76]. A study by Ramappa et al. [77] exemplifies this shift, where a significant improvement was observed by the fourth day using a combination of topical linezolid, azithromycin, and atropine sulfate, along with oral azithromycin.

Table 3.
Summary of clinical cases detailing the efficacy of antibacterial drugs in treating Pythium infections.
The time-related aspects of these treatments are pivotal. Initial antifungal treatments often delayed resolution, necessitating more aggressive interventions such as therapeutic penetrating keratoplasty (TPK). In contrast, Bagga et al. [8] reported a favorable response within 5 to 6 days with the new antibacterial regimen, although a complete cure could take up to 45 days.
Surgical interventions have shown their efficacy in managing severe cases of Pythium keratitis and vascular pythiosis [15,18,97]. Studies such as those by Agarwal et al. [81] and Acharya et al. [90] have highlighted that combining TPK with antibacterial therapy and, in some instances, adjunctive procedures like cryotherapy resulted in better outcomes, including a lower recurrence rate and higher rate of globe salvage.
A multidisciplinary approach was more effective in systemic pythiosis, especially in patients with conditions like thalassemia. For instance, Manothummetha et al. [95] reported improved survival rates in such cases with a combination of surgical interventions and a cocktail of antimicrobials.
Collectively, these studies provide insights into the treatment of Pythium infections. The timing of treatment initiation and the choice of therapeutic agents are crucial for patient outcomes. However, the data underscore the need for continued research to refine treatment protocols, particularly in understanding the efficacy of antibacterial agents against Pythium infections and tailoring treatment plans for different patient demographics.
7. Antibacterial Drugs and Pythiosis: Challenges from In Vitro and Experimental Susceptibility to Clinical Insights
Given the structural similarity between Pythium species and fungi, initial attempts to standardize susceptibility testing for P. insidiosum were based on methods already standardized for fungi, such as broth microdilution assays [62,63] and disk diffusion [98] assays. However, while Pythium spp. mycelia can grow on conventional media like Sabouraud dextrose agar, RPMI, and Muller–Hinton broth and agar, zoospores are not produced in these media. To generate zoospores for in vitro susceptibility tests (inoculum), species-specific methodologies that mimic a microrganism’s natural aquatic environment involving water, salts, and plant substrates are required [68]. Additionally, after repeated subculturing in the laboratory, isolates may lose their ability to produce zoospores, which can compromise the reproducibility of susceptibility tests.
In the same way, the first fundamental challenge in the experimental reproduction of pythiosis lies in the necessity to induce the formation of zoospores as the infectious stage of Pythium spp. Secondly, the pathogenesis mechanisms of pythiosis, mainly why animals like rabbits—which are not natural hosts—are susceptible to experimental pythiosis while there is no reported success in inducing experimental pythiosis in natural hosts like horses, remain unclear. The disease’s development is presumably tied to an immunological response within the host [26]. Intriguingly, in natural environments where multiple horses are exposed to the same risk factors, only a subset may develop the disease, suggesting individual variations in susceptibility or immune response [99]. Furthermore, reinfection in clinically cured animals, when returned to their original natural environment [100], adds another layer of complexity, indicating a possible lack of lasting immunity against the pathogen. Thirdly, clinical forms of the disease are host-dependent; subcutaneous and ocular forms of pythiosis have only been described in rabbits, with vascular forms only in immunosuppressed mice.
Since the 2010s, many studies have focused on evaluating the susceptibility of Pythium species to various classes of antibacterial agents using in vitro susceptibility tests (Table 2) through microdilution, disk diffusion, and gradient strip methods. Pythium species have varied in susceptibility to different antibacterial classes inhibiting protein synthesis. Among these antibacterial agents, azithromycin, clarithromycin, linezolid, minocycline, and tigecycline have been evaluated in experimental models of pythiosis [72,74,75].
In experimental models of subcutaneous pythiosis using rabbits, the disease presents as a chronic condition, with subcutaneous lesions expanding over months without evolving into lethal forms. Research by Jesus et al. [72] and Zimmermann et al. [74] demonstrated that tigecycline, minocycline, and azithromycin inhibited lesion progression and achieved clinical cures in some animals, showcasing their potential as effective treatments against P. insidiosum. These studies further emphasized that combining or using these drugs with the immunotherapeutic agent PitiumVac® could improve treatment outcomes. In humans, there has been only one reported case of subcutaneous pythiosis, characterized by a right deltoid mass developing over six months, where a regimen incorporating antibacterials was used. In this case, administering itraconazole, azithromycin, and terbinafine led to a gradual lesion regression after three months of follow-up [96].
Ocular pythiosis is clinically distinguished by its rapid onset, often manifesting within ten days or less after exposure to risk factors, and progresses more swiftly than subcutaneous pythiosis. Symptoms resembling a corneal ulcer, including pain, redness, watering, discharge, photophobia, and blurred vision, necessitate immediate diagnosis and intervention to avert severe complications and vision loss [85,97]. The use of antibacterials in treating ocular patients, as outlined in Table 3, underscores their relevance, despite the complexity of discerning their standalone efficacy due to concurrent treatments like therapeutic penetrating keratoplasty (TPK) and other pharmacological drugs that are necessary to manage this clinical condition. However, an experimental study on ocular pythiosis in rabbits assessed the efficacy and safety profiles of azithromycin, linezolid, and tigecycline, suggesting their consideration for trials in human disease [75]. Additionally, review studies propose therapeutic protocols for ocular pythiosis, which now include antibacterial drugs [76,97]. Further research is necessary to evaluate the impact of diagnostic timing and the efficacy of these therapeutic protocols in treating the disease.
Vascular pythiosis is a rare but severe infection that can be life-threatening and often leads to limb loss. Due to its uncommon nature, diagnosis is often delayed, making treatment even more challenging. Amputation is currently the primary course of action, though antimicrobial medications and immunotherapy may be used alongside it [1]. This severity and aggressiveness was observed in an experimental model using immunosuppressed mice, in which the subcutaneous inoculation of zoospores resulted in extremely devastating vascular and systemic impairment, leading to unilateral or bilateral paralysis of the hind limbs and even death of the animals as quickly as 24 to 48 h after disease induction. In this study, azithromycin treatment, initiated 3 h after zoospore inoculation, demonstrated a markedly reduced mortality rate [73].
Azithromycin, doxycycline, and clarithromycin are used as part of the treatment for vascular pythiosis in combination with above-knee amputation, immunotherapy, antifungal drugs, and iron chelator treatments [67,93,94,95] (Table 3). Although radical surgery is considered the primary method of managing this condition, recent multicenter studies by Torvorapanit et al. [67] and Manothummetha et al. [95] described that the addition of azithromycin and doxycycline improved the survival rates in patients with vascular pythiosis who have residual disease, when used in conjunction with surgical and antifungal interventions.
The progression of pythiosis disease is heavily impacted by immunomodulation, which occurs when the pathogen manipulates the immune response of the host to its advantage [26]. This manipulation can often make it difficult to clear the infection, which is why targeted interventions are needed. One potential strategy is the selection of antibacterial drugs that target P. insidiosum cells and have a beneficial immunomodulatory effect on the host [10]. Several studies have described the impact of antibacterial drugs on the immune system, including their effects on cellular accumulation, chemotaxis, the microbicidal activity of phagocytic cells, nitric oxide production, cytokine profiles, superoxide anion scavenging, and inflammatory profiles [101,102]. In this context, future research should aim to elucidate the precise mechanisms of action of antibacterial drugs against P. insidiosum and consider their potential immunomodulatory role in enhancing strategies to treat pythiosis.
8. Conclusions
In conclusion, exploring protein synthesis-inhibiting antibacterials in treating pythiosis offers a nuanced advancement in managing this complex infection. The evidence from in vitro studies, experimental models, and clinical observations suggests a potential benefit of using antibacterials like macrolides, oxazolidinones, and tetracyclines against P. insidiosum infections. These findings underscore the necessity of understanding the unique biological characteristics of this pathogen, particularly its distinct susceptibility profile, which sets it apart from fungi.
The clinical application of these findings, however, requires careful consideration. The variability in response to different antibacterials and the potential impact of host-derived sterols on drug efficacy suggests that treatment strategies must be tailored to individual cases. This approach is particularly relevant in severe pythiosis cases, where timely and effective intervention is critical. The promising results from combining surgical interventions with antibacterial therapy in cases like Pythium keratitis also point towards a more integrated treatment approach.
9. Future Directions
Future research needs to establish standardized protocols for susceptibility testing and further explore the synergistic effects of different antibacterial classes. Additionally, conducting more extensive clinical trials is crucial, especially considering the evolving understanding of pythiosis. For many years, P. insidiosum was considered the sole species affecting mammals. However, recent cases have identified other species, such as P. aphanidermatum [94,103,104] and Pythium flevoense [105], as well as other genera of oomycetes [106,107], as causative agents in mammalian infections. This emerging diversity necessitates a broader scope in the exploration of effective treatments. Such efforts are essential to develop more effective, targeted therapies for pythiosis across its various causative species, ultimately improving patient outcomes and advancing our understanding of this complex and often devastating disease.
Author Contributions
Conceptualization, E.S.L.; writing—original draft preparation, E.S.L.; writing—review and editing, J.S.M.T. and R.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arworn, S.; Reanpang, T.; Apichartpiyakul, P.; Orrapin, S.; Rerkasem, K. Retrospective review of management and overall survival rate of patients with vascular pythiosis of the lower extremity: 20 years experience. Int. J. Low Extrem. Wounds 2023, 15347346231214291. [Google Scholar] [CrossRef]
- Yolanda, H.; Krajaejun, T. Global distribution and clinical features of pythiosis in humans and animals. J. Fungi 2022, 8, 182. [Google Scholar] [CrossRef]
- Nguyen, D.; Vilela, R.; Miraglia, B.M.; Vilela, G.; Jasem-Alali, N.; Rohn, R.; Glass, R.; Hansen, R.D.; Mendoza, L. Geographic distribution of Pythium insidiosum infections in the United States. J. Am. Vet. Med. Assoc. 2021, 260, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Kaur, K.; Agarwal, S.; Lalgudi, V.G.; Shekhawat, N.S.; Venugopal, A.; Tripathy, K.; Srinivasan, B.; Iyer, G.; Gubert, J. Pythium insidiosum keratitis: Past, present, and future. Ophthalmol. Ther. 2022, 11, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Permpalung, N.; Worasilchai, N.; Chindamporn, A. Human pythiosis: Emergence of fungal-like organism. Mycopathologia 2020, 185, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Loreto, E.S.; Tondolo, J.S.M.; Santurio, J.M.; Alves, S.H. Screening of antibacterial drugs for antimicrobial activity against Pythium insidiosum. Med. Mycol. 2019, 57, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Loreto, E.S.; Tondolo, J.S.; Pilotto, M.B.; Alves, S.H.; Santurio, J.M. New insights into the in vitro susceptibility of Pythium insidiosum. Antimicrob. Agents Chemother. 2014, 58, 7534–7537. [Google Scholar] [CrossRef]
- Bagga, B.; Sharma, S.; Madhuri Guda, S.J.; Nagpal, R.; Joseph, J.; Manjulatha, K.; Mohamed, A.; Garg, P. Leap forward in the treatment of Pythium insidiosum keratitis. Br. J. Ophthalmol. 2018, 102, 1629–1633. [Google Scholar] [CrossRef]
- Worasilchai, N.; Chindamporn, A.; Plongla, R.; Torvorapanit, P.; Manothummetha, K.; Chuleerarux, N.; Permpalung, N. In vitro susceptibility of Thai Pythium insidiosum isolates to antibacterial agents. Antimicrob. Agents Chemother. 2020, 64, e02099-19. [Google Scholar] [CrossRef]
- Medhasi, S.; Chindamporn, A.; Worasilchai, N. A review: Antimicrobial therapy for human pythiosis. Antibiotics 2022, 11, 450. [Google Scholar] [CrossRef]
- Chitasombat, M.N.; Jongkhajornpong, P.; Lekhanont, K.; Krajaejun, T. Recent update in diagnosis and treatment of human pythiosis. PeerJ 2020, 8, e8555. [Google Scholar] [CrossRef] [PubMed]
- Yolanda, H.; Krajaejun, T. History and perspective of immunotherapy for pythiosis. Vaccines 2021, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L. Pythium insidiosum and mammalian hosts. In Oomycete Genetics and Genomics: Diversity, Interactions and Research Tools; Lamour, K., Kamoun, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 387–405. [Google Scholar]
- Sermsathanasawadi, N.; Praditsuktavorn, B.; Hongku, K.; Wongwanit, C.; Chinsakchai, K.; Ruangsetakit, C.; Hahtapornsawan, S.; Mutirangura, P. Outcomes and factors influencing prognosis in patients with vascular pythiosis. J. Vasc. Surg. 2016, 64, 411–417. [Google Scholar] [CrossRef]
- Permpalung, N.; Worasilchai, N.; Plongla, R.; Upala, S.; Sanguankeo, A.; Paitoonpong, L.; Mendoza, L.; Chindamporn, A. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: A retrospective study of 18 patients. J. Antimicrob. Chemother. 2015, 70, 1885–1892. [Google Scholar] [CrossRef]
- Krajaejun, T.; Sathapatayavongs, B.; Pracharktam, R.; Nitiyanant, P.; Leelachaikul, P.; Wanachiwanawin, W.; Chaiprasert, A.; Assanasen, P.; Saipetch, M.; Mootsikapun, P.; et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 2006, 43, 569–576. [Google Scholar] [CrossRef]
- Barequet, I.S.; Lavinsky, F.; Rosner, M. Long-term follow-up after successful treatment of Pythium insidiosum keratitis in Israel. Semin. Ophthalmol. 2013, 28, 247–250. [Google Scholar] [CrossRef]
- Vishwakarma, P.; Bagga, B. Pythium insidiosum keratitis: Review of literature of 5 years’ clinical experience at a tertiary eye care center. Semin. Ophthalmol. 2023, 38, 190–200. [Google Scholar] [CrossRef]
- McMullan, W.C.; Joyce, J.R.; Hanselka, D.V.; Heitmann, J.M. Amphotericin B for the treatment of localized subcutaneous phycomycosis in the horse. J. Am. Vet. Med. Assoc. 1977, 170, 1293–1298. [Google Scholar]
- Thianprasit, M.; Chaiprasert, A.; Imwidthaya, P. Human pythiosis. Curr. Top. Med. Mycol. 1996, 7, 43–54. [Google Scholar]
- Lerksuthirat, T.; Sangcakul, A.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Krajaejun, T. Evolution of the sterol biosynthetic pathway of Pythium insidiosum and related oomycetes contributes to antifungal drug resistance. Antimicrob. Agents Chemother. 2017, 61, e02352-16. [Google Scholar] [CrossRef] [PubMed]
- Shenep, J.L.; English, B.K.; Kaufman, L.; Pearson, T.A.; Thompson, J.W.; Kaufman, R.A.; Frisch, G.; Rinaldi, M.G. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 1998, 27, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Grooters, A.; Davidson, G.; Jennings, S.; Nicklas, J.; Birkenheuer, A. Successful management of gastrointestinal pythiosis in a dog using itraconazole, terbinafine, and mefenoxam. Med. Mycol. 2011, 49, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Dória, R.G.S.; Carvalho, M.B.; Freitas, S.H.; Laskoski, L.M.; Colodel, E.M.; Mendonca, F.S.; Silva, M.A.; Grigoletto, R.; Fantinato Neto, P. Evaluation of intravenous regional perfusion with amphotericin B and dimethylsulfoxide to treat horses for pythiosis of a limb. BMC Vet. Res. 2015, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Loreto, É.S. Update on pythiosis immunobiology and immunotherapy. World J. Immunol. 2014, 4, 88–97. [Google Scholar] [CrossRef]
- Mendoza, L.; Newton, J.C. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Med. Mycol. 2005, 43, 477–486. [Google Scholar] [CrossRef]
- Bridges, C.H.; Emmons, C.W. A phycomycosis of horses caused by Hyphomyces destruens. J. Am. Vet. Med. Assoc. 1961, 138, 579–589. [Google Scholar]
- Costa, R.O.; Macedo, P.M.; Carvalhal, A.; Bernardes-Engemann, A.R. Use of potassium iodide in dermatology: Updates on an old drug. An. Bras. Dermatol. 2013, 88, 396–402. [Google Scholar] [CrossRef]
- Sterling, J.B.; Heymann, W.R. Potassium iodide in dermatology: A 19th century drug for the 21st century-uses, pharmacology, adverse effects, and contraindications. J. Am. Acad. Dermatol. 2000, 43, 691–697. [Google Scholar] [CrossRef]
- Hutchins, D.R.; Johnston, K.G. Phycomycosis in the horse. Aust. Vet. J. 1972, 48, 269–278. [Google Scholar] [CrossRef]
- Murray, D.R.; Ladds, P.W.; Johnson, R.H.; Pott, B.W. Metastatic phycomycosis in a horse. J. Am. Vet. Med. Assoc. 1978, 172, 834–836. [Google Scholar]
- Ader, P.L. Phycomycosis in fifteen dogs and two cats. J. Am. Vet. Med. Assoc. 1979, 174, 1216–1223. [Google Scholar] [PubMed]
- Rodrigues, V.S.; Trevisan, L.A.C.; Cintra, B.S.; Pires, R.H.; Ribeiro, A.B.; Tavares, D.C.; Oberhaus, E.; Ferreira, J.C. Effectiveness of photo-ozone therapy against equine Pythium insidiosum. J. Equine Vet. Sci. 2024, 134, 105030. [Google Scholar] [CrossRef]
- Sedrish, S.A.; Moore, R.M.; ValdesVasquez, M.A.; Haynes, P.F.; Vicek, T. Adjunctive use of a neodymium:yttrium-aluminum-garnet laser for treatment of pythiosis granulomas in two horses. J. Am. Vet. Med. Assoc. 1997, 211, 464–465. [Google Scholar] [CrossRef]
- Pires, L.; Bosco Sde, M.; Baptista, M.S.; Kurachi, C. Photodynamic therapy in Pythium insidiosum—An in vitro study of the correlation of sensitizer localization and cell death. PLoS ONE 2014, 9, e85431. [Google Scholar] [CrossRef]
- Pires, L.; Bosco Sde, M.; da Silva, N.F., Jr.; Kurachi, C. Photodynamic therapy for pythiosis. Vet. Dermatol. 2013, 24, 130–136.e30. [Google Scholar] [CrossRef]
- Zanette, R.A.; Alves, S.H.; Pilotto, M.B.; Weiblen, C.; Fighera, R.A.; Wolkmer, P.; Flores, M.M.; Santurio, J.M. Iron chelation therapy as a treatment for Pythium insidiosum in an animal model. J. Antimicrob. Chemother. 2013, 68, 1144–1147. [Google Scholar] [CrossRef]
- Sriphana, U.; Thongsri, Y.; Ardwichai, P.; Poopasit, K.; Prariyachatigul, C.; Simasathiansophon, S.; Yenjai, C. New lignan esters from Alyxia schlechteri and antifungal activity against Pythium insidiosum. Fitoterapia 2013, 91, 39–43. [Google Scholar] [CrossRef]
- Fonseca, A.O.; Pereira, D.I.; Botton, S.A.; Potter, L.; Sallis, E.S.; Junior, S.F.; Filho, F.S.; Zambrano, C.G.; Maroneze, B.P.; Valente, J.S.; et al. Treatment of experimental pythiosis with essential oils of Origanum vulgare and Mentha piperita singly, in association and in combination with immunotherapy. Vet. Microbiol. 2015, 178, 265–269. [Google Scholar] [CrossRef]
- Trolezi, R.; Azanha, J.M.; Paschoal, N.R.; Chechi, J.L.; Dias Silva, M.J.; Fabris, V.E.; Vilegas, W.; Kaneno, R.; Fernandes Junior, A.; Bosco, S.M. Stryphnodendron adstringens and purified tannin on Pythium insidiosum: In vitro and in vivo studies. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 7. [Google Scholar] [CrossRef]
- Sriphana, U.; Thongsri, Y.; Prariyachatigul, C.; Pakawatchai, C.; Yenjai, C. Clauraila E from the roots of Clausena harmandiana and antifungal activity against Pythium insidiosum. Arch. Pharm. Res. 2013, 36, 1078–1083. [Google Scholar] [CrossRef]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The new tree of eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef]
- McCarthy, C.G.P.; Fitzpatrick, D.A. Phylogenomic reconstruction of the oomycete phylogeny derived from 37 genomes. mSphere 2017, 2, e00095-17. [Google Scholar] [CrossRef]
- Thines, M. Oomycetes. Curr. Biol. 2018, 28, R812–R813. [Google Scholar] [CrossRef]
- Aronson, J.M.; Cooper, B.A.; Fuller, M.S. Glucans of Oomycete cell walls. Science 1967, 155, 332–335. [Google Scholar] [CrossRef]
- Judelson, H.S.; Blanco, F.A. The spores of Phytophthora: Weapons of the plant destroyer. Nat. Rev. Microbiol. 2005, 3, 47–58. [Google Scholar] [CrossRef]
- Tondolo, J.S.M.; Ledur, P.C.; Loreto, E.S.; Verdi, C.M.; Bitencourt, P.E.R.; de Jesus, F.P.K.; Rocha, J.P.; Alves, S.H.; Sassaki, G.L.; Santurio, J.M. Extraction, characterization and biological activity of a (1,3)(1,6)-beta-d-glucan from the pathogenic oomycete Pythium insidiosum. Carbohydr. Polym. 2017, 157, 719–727. [Google Scholar] [CrossRef]
- Meijer, H.J.; Hua, C.; Kots, K.; Ketelaar, T.; Govers, F. Actin dynamics in Phytophthora infestans; rapidly reorganizing cables and immobile, long-lived plaques. Cell Microbiol. 2014, 16, 948–961. [Google Scholar] [CrossRef]
- Seidl, M.F.; Van den Ackerveken, G.; Govers, F.; Snel, B. A domain-centric analysis of oomycete plant pathogen genomes reveals unique protein organization. Plant Physiol. 2011, 155, 628–644. [Google Scholar] [CrossRef]
- Powell, M.J.; Lehnen, L.P., Jr.; Bortnick, R.N. Microbody-like organelles as taxonomic markers among oomycetes. Biosystems 1985, 18, 321–334. [Google Scholar] [CrossRef]
- Gaulin, E.; Bottin, A.; Dumas, B. Sterol biosynthesis in oomycete pathogens. Plant Signal. Behav. 2010, 5, 258–260. [Google Scholar] [CrossRef]
- Hendrix, J.W. Sterol induction of reproduction and stimulation of growth of Pythium and Phytophthora. Science 1964, 144, 1028–1029. [Google Scholar] [CrossRef]
- Ferrarini, E.; De Roo, V.; Geudens, N.; Martins, J.C.; Hofte, M. Altering in vivo membrane sterol composition affects the activity of the cyclic lipopeptides tolaasin and sessilin against Pythium. Biochim. Biophys. Acta (BBA)-Biomembr. 2022, 1864, 184008. [Google Scholar] [CrossRef]
- Marchant, R.; Smith, D.G. The effect of chloramphenicol on growth and mitochondrial structure of Pythium ultimum. J. Gen. Microbiol. 1968, 50, 391–397. [Google Scholar] [CrossRef]
- Rawn, C.D.; Van Etten, J.L. Mechanism of antibacterial antibiotic sensitivity in Pythium ultimum. J. Gen. Microbiol. 1978, 108, 133–139. [Google Scholar] [CrossRef]
- Guo, L.Y.; Ko, W.H. Growth rate and antibiotic sensitivities of conidium and selfed-oospore progenies of heterothallic Pythium splendens. Can. J. Bot. 1994, 72, 1709–1712. [Google Scholar] [CrossRef]
- Rawn, C.D.; Schwarz, M. Protection of Pythium species against antibacterial antibiotics by cholesterol. Phytopathology 1987, 77, 319–323. [Google Scholar] [CrossRef]
- Yolanda, H.; Krajaejun, T. Review of methods and antimicrobial agents for susceptibility testing against Pythium insidiosum. Heliyon 2020, 6, e03737. [Google Scholar] [CrossRef]
- McMeekin, D. Inhibition and stimulation of growth of Pythium by streptomycin. Mycologia 1978, 70, 880–883. [Google Scholar] [CrossRef]
- McMeekin, D.; Mendoza, L. In vitro effect of streptomycin on clinical isolates of Pythium insidiosum. Mycologia 2000, 92, 371–373. [Google Scholar] [CrossRef]
- Tondolo, J.S.; Loreto, E.S.; Denardi, L.B.; Mario, D.A.; Alves, S.H.; Santurio, J.M. A simple, rapid and inexpensive screening method for the identification of Pythium insidiosum. J. Microbiol. Methods 2013, 93, 52–54. [Google Scholar] [CrossRef]
- CLSI Document M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard; 2nd ed. Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2008.
- CLSI Standard M38; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; 3rd ed. Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2017.
- Tondolo, J.S.M.; Loreto, E.S.; Ledur, P.C.; Jesus, F.P.K.; Silva, T.M.; Kommers, G.D.; Alves, S.H.; Santurio, J.M. Chemically induced disseminated pythiosis in BALB/c mice: A new experimental model for Pythium insidiosum infection. PLoS ONE 2017, 12, e0177868. [Google Scholar] [CrossRef] [PubMed]
- Loreto, E.S.; Mario, D.A.; Denardi, L.B.; Alves, S.H.; Santurio, J.M. In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob. Agents Chemother. 2011, 55, 3588–3590. [Google Scholar] [CrossRef] [PubMed]
- Mahl, D.L.; de Jesus, F.P.; Loreto, E.; Zanette, R.A.; Ferreiro, L.; Pilotto, M.B.; Alves, S.H.; Santurio, J.M. In vitro susceptibility of Pythium insidiosum isolates to aminoglycoside antibiotics and tigecycline. Antimicrob. Agents Chemother. 2012, 56, 4021–4023. [Google Scholar] [CrossRef] [PubMed]
- Torvorapanit, P.; Chuleerarux, N.; Plongla, R.; Worasilchai, N.; Manothummetha, K.; Thongkam, A.; Langsiri, N.; Diewsurin, J.; Kongsakpaisan, P.; Bansong, R.; et al. Clinical outcomes of radical surgery and antimicrobial agents in vascular pythiosis: A multicenter prospective study. J. Fungi 2021, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Loreto, E.S.; Tondolo, J.S.M.; Oliveira, D.C.; Santurio, J.M.; Alves, S.H. In vitro activities of miltefosine and antibacterial agents from the macrolide, oxazolidinone, and pleuromutilin classes against Pythium insidiosum and Pythium aphanidermatum. Antimicrob. Agents Chemother. 2018, 62, e01678-17. [Google Scholar] [CrossRef] [PubMed]
- Itaqui, S.R.; Verdi, C.M.; Tondolo, J.S.; da Luz, T.S.; Alves, S.H.; Santurio, J.M.; Loreto, E.S. In vitro synergism between azithromycin or terbinafine and topical antimicrobial agents against Pythium insidiosum. Antimicrob. Agents Chemother. 2016, 60, 5023–5025. [Google Scholar] [CrossRef] [PubMed]
- Ianiski, L.B.; Stibbe, P.C.; Denardi, L.B.; Weiblen, C.; Soares, M.P.; Valente, J.S.S.; Sangioni, L.A.; Pereira, D.I.B.; Santurio, J.M.; Botton, S.A. In vitro anti-Pythium insidiosum activity of amorolfine hydrochloride and azithromycin, alone and in combination. Med. Mycol. 2021, 59, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ianiski, L.B.; Maciel, A.F.; Colla, A.C.N.; Braga, C.Q.; Sangioni, L.A.; Pal, M.; Pereira, D.I.B.; Santurio, J.M.; Botton, S.d.A. Pythium insidiosum: In vitro oomicidal evaluation of telithromycin and interactions with azithromycin and amorolfine hydrochloride. J. Med. Mycol. 2024, 34, 101460. [Google Scholar] [CrossRef]
- Jesus, F.P.; Loreto, E.S.; Ferreiro, L.; Alves, S.H.; Driemeier, D.; Souza, S.O.; Franca, R.T.; Lopes, S.T.; Pilotto, M.B.; Ludwig, A.; et al. In vitro and in vivo antimicrobial activities of minocycline in combination with azithromycin, clarithromycin, or tigecycline against Pythium insidiosum. Antimicrob. Agents Chemother. 2016, 60, 87–91. [Google Scholar] [CrossRef]
- Loreto, E.S.; Tondolo, J.S.M.; de Jesus, F.P.K.; Verdi, C.M.; Weiblen, C.; de Azevedo, M.I.; Kommers, G.D.; Santurio, J.M.; Zanette, R.A.; Alves, S.H. Efficacy of azithromycin and miltefosine in experimental systemic pythiosis in immunosuppressed mice. Antimicrob. Agents Chemother. 2019, 63, e01385-18. [Google Scholar] [CrossRef]
- Zimmermann, C.E.P.; Jesus, F.P.K.; Schlemmer, K.B.; Loreto, E.S.; Tondolo, J.S.M.; Driemeier, D.; Alves, S.H.; Ferreiro, L.; Santurio, J.M. In vivo effect of minocycline alone and in combination with immunotherapy against Pythium insidiosum. Vet. Microbiol. 2020, 243, 108616. [Google Scholar] [CrossRef]
- Ahirwar, L.K.; Kalra, P.; Sharma, S.; Mohamed, A.; Mittal, R.; Das, S.; Bagga, B. Linezolid shows high safety and efficacy in the treatment of Pythium insidiosum keratitis in a rabbit model. Exp. Eye Res. 2021, 202, 108345. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Predicting prognosis based on regional prevalence, ulcer morphology and treatment strategy in vision-threatening Pythium insidiosum keratitis. Clin. Ophthalmol. 2023, 17, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, M.; Nagpal, R.; Sharma, S.; Chaurasia, S. Successful medical management of presumptive Pythium insidiosum keratitis. Cornea 2017, 36, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Ros Castellar, F.; Sobrino Jimenez, C.; del Hierro Zarzuelo, A.; Herrero Ambrosio, A.; Boto de Los Bueis, A. Intraocular minocycline for the treatment of ocular pythiosis. Am. J. Health Syst. Pharm. 2017, 74, 821–825. [Google Scholar] [CrossRef]
- Chatterjee, S.; Agrawal, D. Azithromycin in the management of Pythium insidiosum keratitis. Cornea 2018, 37, e8–e9. [Google Scholar] [CrossRef]
- Raghavan, A.; Bellamkonda, P.; Mendoza, L.; Rammohan, R. Pythium insidiosum and Acanthamoeba keratitis in a contact lens user. BMJ Case Rep. 2018, 11, bcr-2018-226386. [Google Scholar] [CrossRef]
- Agarwal, S.; Iyer, G.; Srinivasan, B.; Benurwar, S.; Agarwal, M.; Narayanan, N.; Lakshmipathy, M.; Radhika, N.; Rajagopal, R.; Krishnakumar, S.; et al. Clinical profile, risk factors and outcome of medical, surgical and adjunct interventions in patients with Pythium insidiosum keratitis. Br. J. Ophthalmol. 2019, 103, 296–300. [Google Scholar] [CrossRef]
- Maeno, S.; Oie, Y.; Sunada, A.; Tanibuchi, H.; Hagiwara, S.; Makimura, K.; Nishida, K. Successful medical management of Pythium insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol. Am. J. Ophthalmol. Case Rep. 2019, 15, 100498. [Google Scholar] [CrossRef]
- Ravindran, R.; Harwani, A.A.; Natarajan, R. Pythium keratitis: Clinical course of an emerging scourge. Asian J. Ophthalmol. 2020, 17, 318–323. [Google Scholar] [CrossRef]
- Bagga, B.; Kate, A.; Mohamed, A.; Sharma, S.; Das, S.; Mitra, S. Successful strategic management of Pythium insidiosum keratitis with antibiotics. Ophthalmology 2021, 128, 169–172. [Google Scholar] [CrossRef]
- Gurnani, B.; Christy, J.; Narayana, S.; Rajkumar, P.; Kaur, K.; Gubert, J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J. Ophthalmol. 2021, 69, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wang, Y.; Tian, L.; Wang, F.; Sun, Z.; Chen, Z. Pythium insidiosum keratitis reported in China, raising the alertness to this fungus-like infection: A case series. J. Med. Case Rep. 2021, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Puangsricharern, V.; Chotikkakamthorn, P.; Tulvatana, W.; Kittipibul, T.; Chantaren, P.; Reinprayoon, U.; Kasetsuwan, N.; Satitpitakul, V.; Worasilchai, N.; Chindamporn, A. Clinical characteristics, histopathology, and treatment outcomes of Pythium keratitis: A retrospective cohort study. Clin. Ophthalmol. 2021, 15, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.S.; Madduri, B.; Mohan, N.; Mittal, R.; Raghava, J.V.; Fernandes, M. Improved outcome of Pythium keratitis with a combined triple drug regimen of linezolid and azithromycin. Cornea 2021, 40, 888–893. [Google Scholar] [CrossRef]
- Gurnani, B.; Narayana, S.; Christy, J.; Rajkumar, P.; Kaur, K.; Gubert, J. Successful management of pediatric Pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur. J. Ophthalmol. 2022, 32, NP87–NP91. [Google Scholar] [CrossRef]
- Acharya, M.; Singh, A.; Nidhi, V.; Tiwari, A.; Gandhi, A.; Chaudhari, I. Outcomes of keratoplasty in a cohort of Pythium insidiosum keratitis cases at a tertiary eye care center in India. Preprints 2023, 2023060900. [Google Scholar] [CrossRef]
- Gurnani, B.; Christy, J.; Kaur, K.; Moutappa, F.; Gubert, J. Successful management of Pythium insidiosum keratitis masquerading as dematiaceous fungal keratitis in an immunosuppressed asian male. Ocul. Immunol. Inflamm. 2023, 1–4. [Google Scholar] [CrossRef]
- Kate, A.; Thigale, U.; Ponnapati, L.P.; Chaudhary, S.; Vishwakarma, P.; Sharma, S.; Bagga, B. Outcomes of therapeutic penetrating keratoplasty in Pythium insidiosum keratitis managed with a combination of antibiotics. Indian J. Ophthalmol. 2023, 71, 1868–1874. [Google Scholar] [CrossRef]
- Susaengrat, N.; Torvorapanit, P.; Plongla, R.; Chuleerarux, N.; Manothummetha, K.; Tuangsirisup, J.; Worasilchai, N.; Chindamporn, A.; Permpalung, N. Adjunctive antibacterial agents as a salvage therapy in relapsed vascular pythiosis patients. Int. J. Infect. Dis. 2019, 88, 27–30. [Google Scholar] [CrossRef]
- Thongsuk, P.; Plongla, R.; Thammahong, A.; Tiewsurin, J.; Worasilchai, N.; Chindamporn, A.; Suankratay, C. Vascular pythiosis caused by Pythium aphanidermatum: The first case report in Asia. Eur. J. Med. Res. 2021, 26, 132. [Google Scholar] [CrossRef]
- Manothummetha, K.; Torvorapanit, P.; Susaengrat, N.; Worasilchai, N.; Chindamporn, A.; Chuleerarux, N.; Bansong, R.; Wattanasoontornsakul, W.; Oranrigsupak, P.; Diewsurin, J.; et al. 374. A multicenter open-label single-arm clinical trial of combination therapy of surgery, itraconazole, doxycycline, and azithromycin for vascular pythiosis. Open Forum Infect. Dis. 2023, 10, ofad500.444. [Google Scholar] [CrossRef]
- Luangnara, A.; Chuamanochan, M.; Chiewchanvit, S.; Pattamapaspong, N.; Salee, P.; Chaiwarith, R. Pythiosis presenting with chronic swelling and painful subcutaneous lesion at right deltoid. IDCases 2023, 33, e01873. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K.; Venugopal, A.; Srinivasan, B.; Bagga, B.; Iyer, G.; Christy, J.; Prajna, L.; Vanathi, M.; Garg, P.; et al. Pythium insidiosum keratitis—A review. Indian J. Ophthalmol. 2022, 70, 1107–1120. [Google Scholar] [CrossRef]
- CLSI Document M51-A; Reference Method for Antifungal Disk Diffusion Susceptibility Testing of Non-Dermatophyte Filamentous Fungi; Approved Guideline; 1st ed. Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2010.
- Santos, C.E.P.; Ubiali, D.G.; Pescador, C.A.; Zanette, R.A.; Santurio, J.M.; Marques, L.C. Epidemiological survey of equine pythiosis in the Brazilian Pantanal and nearby areas: Results of 76 cases. J. Equine Vet. Sci. 2014, 34, 270–274. [Google Scholar] [CrossRef]
- Santos, C.E.; Marques, L.C.; Zanette, R.A.; Jesus, F.P.; Santurio, J.M. Does immunotherapy protect equines from reinfection by the oomycete Pythium insidiosum? Clin. Vaccine Immunol. 2011, 18, 1397–1399. [Google Scholar] [CrossRef]
- Labro, M.T. Immunomodulatory effects of antimicrobial agents. Part I: Antibacterial and antiviral agents. Expert Rev. Anti Infect. Ther. 2012, 10, 319–340. [Google Scholar] [CrossRef]
- Ruh, C.; Banjade, R.; Mandadi, S.; Marr, C.; Sumon, Z.; Crane, J.K. Immunomodulatory effects of antimicrobial drugs. Immunol. Investig. 2017, 46, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.R.; Murray, C.K.; Driscoll, I.R.; Wickes, B.L.; Wiederhold, N.; Sutton, D.A.; Sanders, C.; Mende, K.; Enniss, B.; Feig, J.; et al. Combat-related Pythium aphanidermatum invasive wound infection: Case report and discussion of utility of molecular diagnostics. J. Clin. Microbiol. 2015, 53, 1968–1975. [Google Scholar] [CrossRef]
- Calvano, T.P.; Blatz, P.J.; Vento, T.J.; Wickes, B.L.; Sutton, D.A.; Thompson, E.H.; White, C.E.; Renz, E.M.; Hospenthal, D.R. Pythium aphanidermatum infection following combat trauma. J. Clin. Microbiol. 2011, 49, 3710–3713. [Google Scholar] [CrossRef]
- Veldhuis Kroeze, E.J.B.; van Elk, C.E.; van de Bildt, M.W.G.; van Run, P.; Foster, G.; Abou-Chakra, N.; Hare, R.K.; Kuiken, T. Infection with Pythium flevoense in a harbour porpoise (Phocoena phocoena) as a novel cause of dermatitis in marine mammals. Vet. Res. 2023, 54, 102. [Google Scholar] [CrossRef]
- White, A.G.; Smart, K.; Hathcock, T.; Tillson, D.M.; Poudel, A.; Rynders, P.; Wang, C. Successful management of cutaneous paralagenidiosis in a dog treated with mefenoxam, minocycline, prednisone, and hyperbaric oxygen therapy. Med. Mycol. Case Rep. 2020, 29, 38–42. [Google Scholar] [CrossRef]
- Spies, C.F.J.; Grooters, A.M.; Levesque, C.A.; Rintoul, T.L.; Redhead, S.A.; Glockling, S.L.; Chen, C.Y.; de Cock, A. Molecular phylogeny and taxonomy of Lagenidium-like oomycetes pathogenic to mammals. Fungal Biol. 2016, 120, 931–947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).