Arbuscular Mycorrhizal Fungi-Mediated Modulation of Physiological, Biochemical, and Secondary Metabolite Responses in Hemp (Cannabis sativa L.) under Salt and Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Determination of Colonization Rate, Mycorrhizal Dependence, Biomass, and Relative Water Content
2.3. Determination of Malondialdehyde, Proline, Soluble Sugar, and Soluble Protein Content
2.4. Determination of SPAD and Photosynthetic Parameters
2.5. Determination of Fluorescence Parameters
2.6. Measurement of Ion Content
2.7. Determination of Secondary Metabolite Content
2.8. Statistical Analysis
3. Results
3.1. AMF Colonization Rate and Mycorrhizal Dependence in Plant Roots
3.2. Growth Parameter
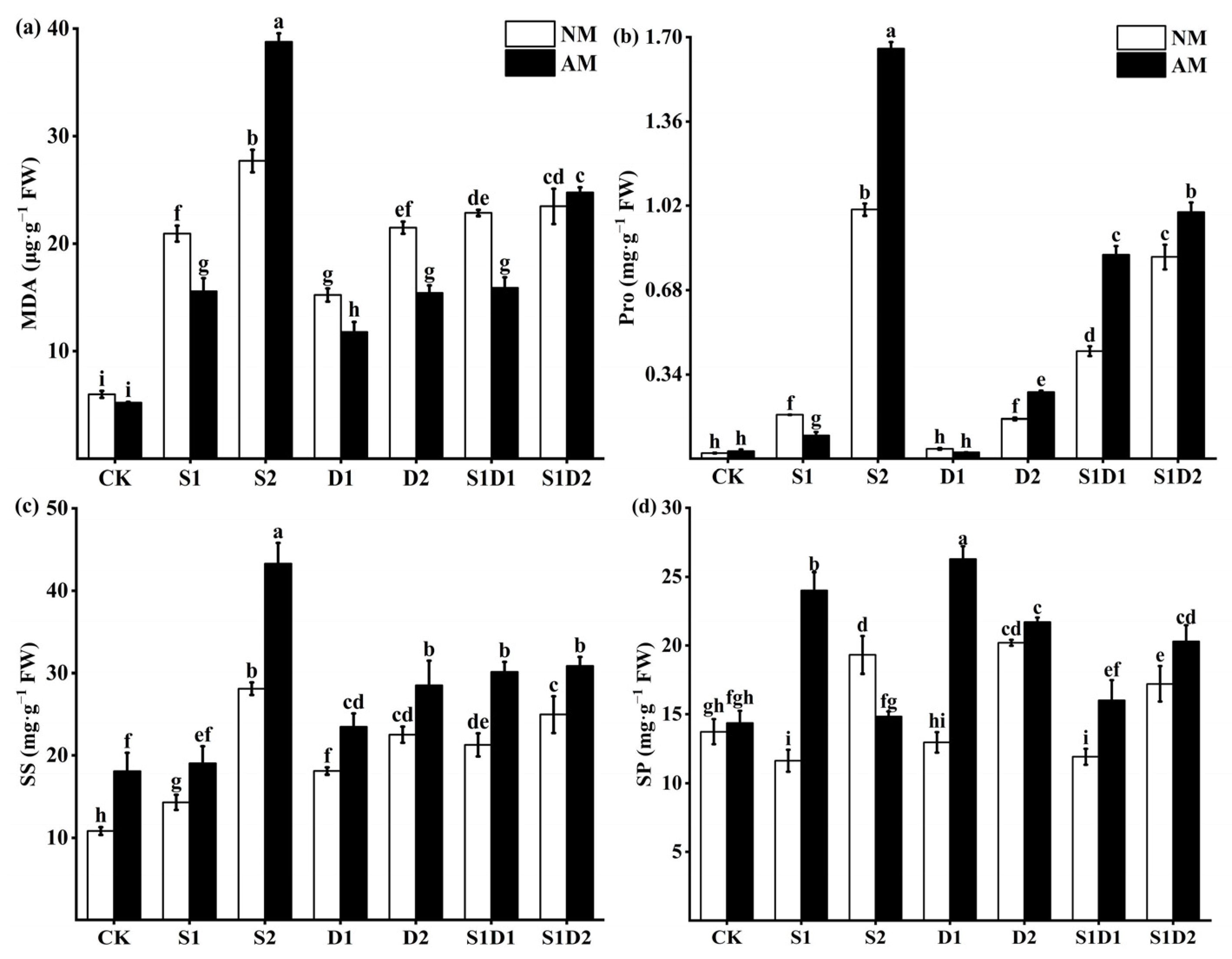
3.3. MDA, Proline, Soluble Sugar, and Soluble Protein Content
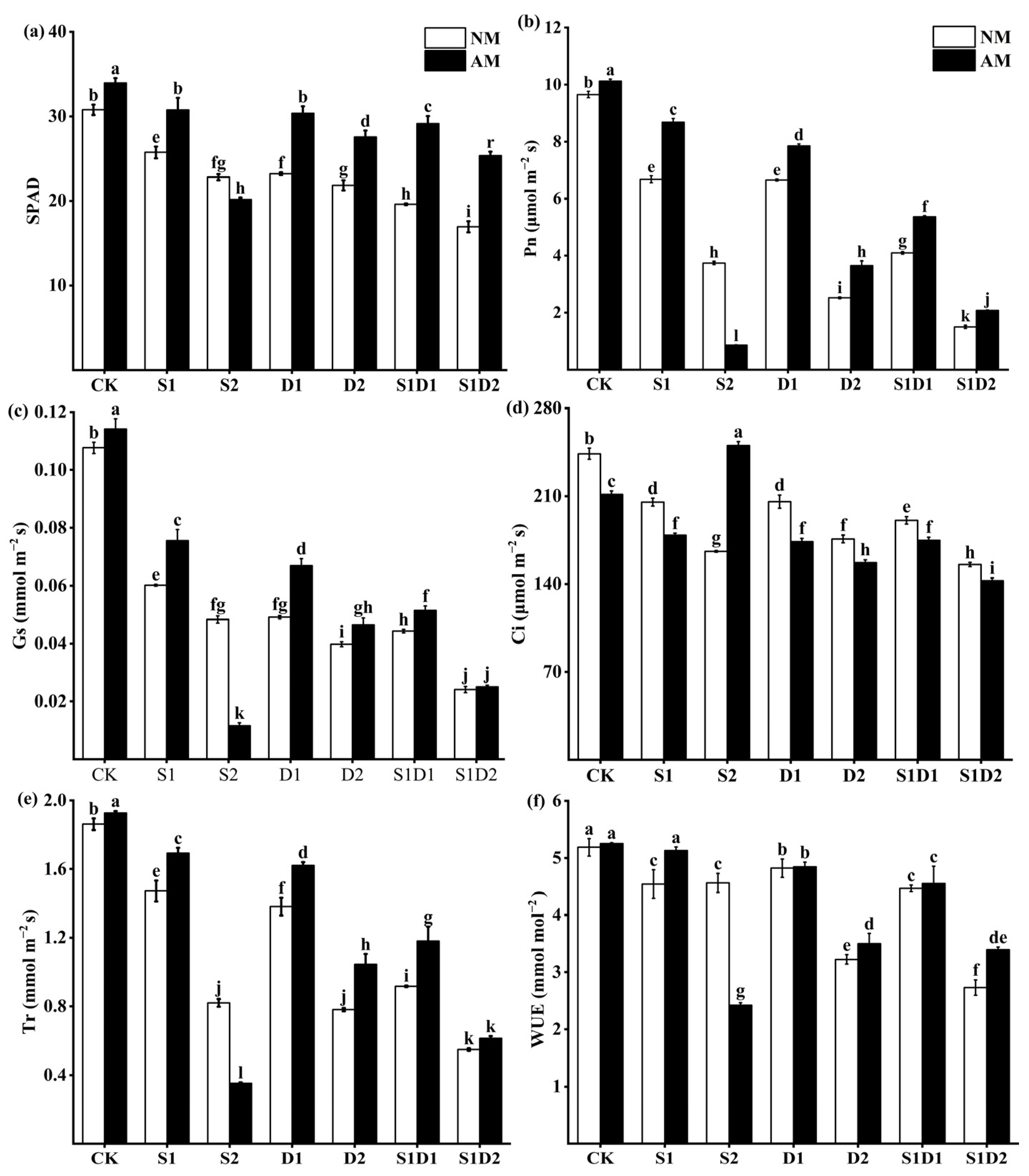
3.4. SPAD Values and Photosynthesis Parameters
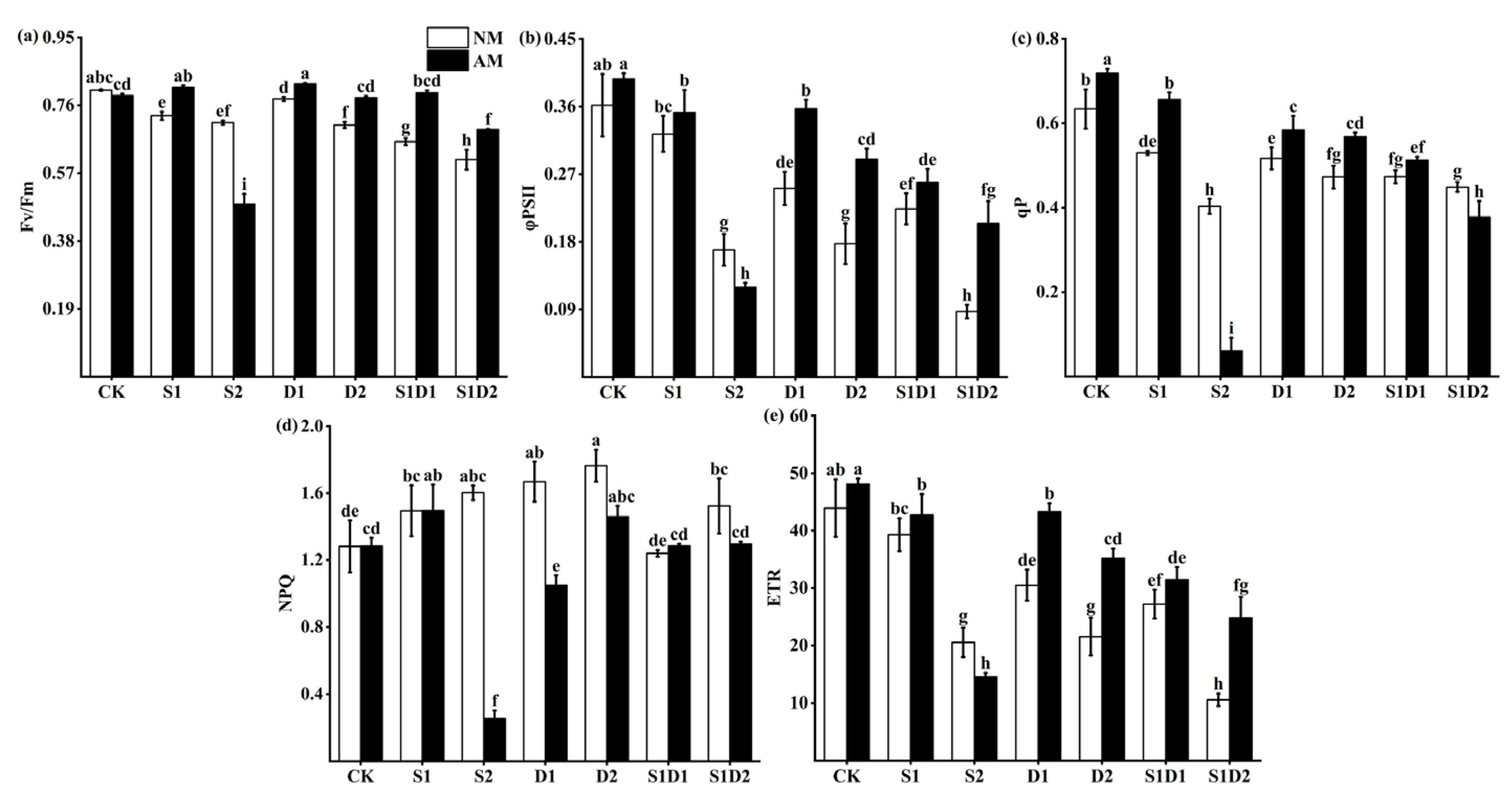
3.5. Fluorescence Parameters
3.6. Ionic Content
3.7. Secondary Metabolite Content
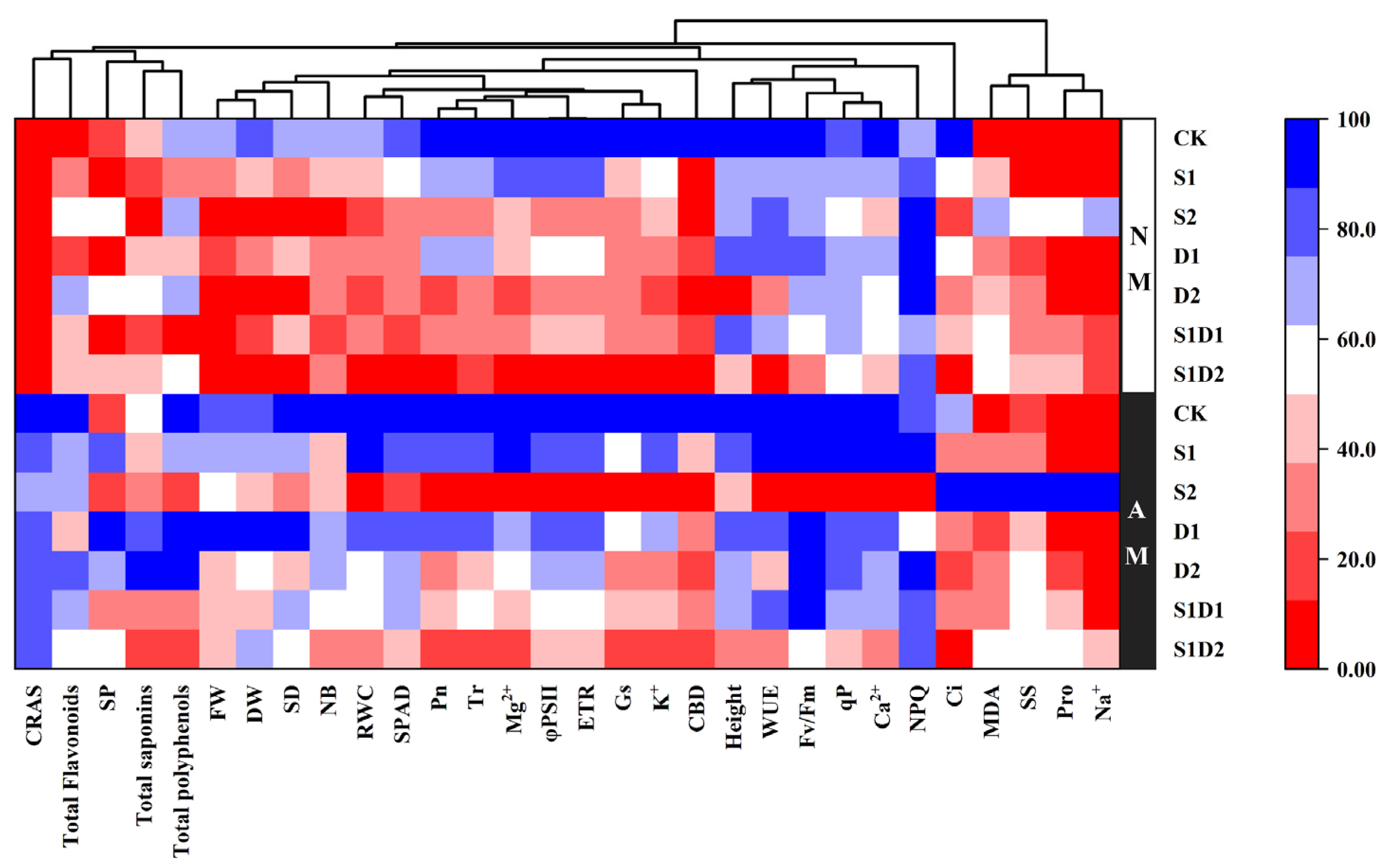
3.8. Heat Map Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, M.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M.A. Salt stress under the scalpel-dissecting the genetics of salt tolerance. Plant J. 2018, 97, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kongkadee, K.; Wisuitiprot, W.; Ingkaninan, K.; Waranuch, N. Anti-inflammation and gingival wound healing activities of Cannabis sativa L. Subsp. Sativa (hemp) extract and cannabidiol: An in vitro study. Arch. Oral Biol. 2022, 140, 105464. [Google Scholar]
- Tang, K.L.; Wang, J.Y.; Yang, Y.; Deng, G.; Yu, J.; Hu, W.Q.; Guo, L.; Du, G.H.; Liu, F.H. Fiber hemp (Cannabis sativa L.) yield and its response to fertilization and planting density in China. Ind. Crop. Prod. 2022, 177, 114542. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Gill, A.R.; Loveys, B.R.; Cowley, J.M.; Hall, T.; Cavagnaro, T.R.; Burton, R.A. Physiological and morphological responses of industrial hemp (Cannabis sativa L.) to water deficit. Ind. Crop. Prod. 2022, 187, 115331. [Google Scholar] [CrossRef]
- Liu, J.H.; Xia, J.B.; Fang, Y.M.; Li, T.; Liu, J.T. Effects of salt-drought stress on growth and physiobiochemical characteristics of Tamarix chinensis seedlings. Sci. World J. 2014, 2014, 765840. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, J.G.; Syvertsen, J.P.; Botia, P.; Garcia-Sanchez, F. Leaf water relations and net gas exchange responses of salinized carrizo citrange seedlings during drought stress and recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, F.; Zheng, X.; Pan, H.Y.; Wen, Y.Q.; Song, F.Q. Effects of amf compound inoculants on growth, ion homeostasis, and salt tolerance-related gene expression in Oryza sativa L. under salt treatments. Rice 2023, 16, 18. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, Y.H.; Liu, B.W.; Liu, Q.; Wen, S.Y.; Ao, B.; Lin, Z.Q.; Zheng, Y.L.; Yang, W.Z.; Chu, X.T.; et al. Arbuscular mycorrhiza fungus improved growth, antioxidant defense, and endogenous hormones in tall fescue under low-light stress. S. Afr. J. Bot. 2019, 127, 43–50. [Google Scholar] [CrossRef]
- Begum, N.; Xiao, Y.T.; Wang, L.; Li, D.M.; Irshad, A.; Zhao, T.J. Arbuscular mycorrhizal fungus Rhizophagus irregularis alleviates drought stress in soybean with overexpressing the gmspl9d gene by promoting photosynthetic apparatus and regulating the antioxidant system. Microbiol. Res. 2023, 273, 127398. [Google Scholar] [CrossRef] [PubMed]
- Soussani, F.E.; Boutasknit, A.; Ben-Laouane, R.; Benkirane, R.; Baslam, M.; Meddich, A. Arbuscular mycorrhizal fungi and compost-based biostimulants enhance fitness, physiological responses, yield, and quality traits of drought-stressed tomato plants. Plants 2023, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, L.; Jia, X.; Zhao, Y.H.; Zhang, C.Y. Adaptation of antioxidant enzymes in Robinia pseudoacacia L. grown in cadmium-contaminated soils under elevated CO2 to arbuscular mycorrhizal symbiosis. J. Soil Sci. Plant Nutr. 2023, 23, 2451–2464. [Google Scholar] [CrossRef]
- Carrara, J.E.; Reddivari, L.; Lehotay, S.J.; Zinati, G.; Heller, W.P. Arbuscular mycorrhizal fungi increase the yield and nutritional quality of yellow and purple fleshed potatoes (Solanum tuberosum). Am. J. Potato Res. 2023, 100, 210–220. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.F.; Yuan, D.; Hu, X.C.; Zhang, D.J.; Li, Y.Y. Effects of mycorrhizal fungi on plant growth, nutrient absorption and phytohormones levels in tea under shading condition. Not. Bot. Horti Agrobot. Cluj-Na. 2020, 48, 2006–2020. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, S.X.; Yang, X.Q.; Xia, G.D.; Wang, B.C.; Gu, B.J. Arbuscular mycorrhizal fungi alter rhizosphere bacterial community characteristics to improve cr tolerance of Acorus calamus. Ecotoxicol. Environ. Saf. 2023, 253, 114652. [Google Scholar]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Amani Machiani, M.; Javanmard, A.; Ostadi, A.; Alizadeh, K. Improvement in essential oil quantity and quality of thyme (Thymus vulgaris L.) by integrative application of chitosan nanoparticles and arbuscular mycorrhizal fungi under water stress conditions. Plants 2023, 12, 1422. [Google Scholar] [CrossRef]
- Rasouli, F.; Hassanpouraghdam, M.B.; Pirsarandib, Y.; Aazami, M.A.; Asadi, M.; Ercişli, S.; Mehrabani, L.V.; Puglisi, I.; Baglieri, A. Improvements in the biochemical responses and Pb and Ni phytoremediation of lavender (Lavandula angustifolia L.) plants through Funneliformis mosseae inoculation. BMC Plant Biol. 2023, 23, 252. [Google Scholar] [CrossRef]
- Rojas-Andrade, R.; Cerda-García-Rojas, C.M.; Frias-Hernandez, J.T.; Dendooven, L.; Olalde-Portugal, V.; Ramos-Valdivia, A.C. Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza 2003, 13, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.F.; Wang, Y.; Li, Y.M. Plant secondary metabolism and its response to environment. Acta Ecol. Sin. 2007, 6, 2554–2562. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Lin, J.X.; Wang, Y.N.; Sun, S.N.; Mu, C.S.; Yan, X.F. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Takács, T.; Vörös, I. Effect of metal non-adapted arbuscular mycorrhizal fungi on Cd, Ni and Zn uptake by ryegrass. Acta Agron. Hung. 2003, 51, 347–354. [Google Scholar] [CrossRef]
- Fan, S.Y.; Wu, H.; Gong, H.J.; Guo, J. The salicylic acid mediates selenium-induced tolerance to drought stress in tomato plants. Sci. Hortic. 2022, 300, 111092. [Google Scholar] [CrossRef]
- Wang, Y.L.; Gao, S.S.; He, X.Y.; Li, Y.; Li, P.Y.; Zhang, Y.; Chen, W. Growth, secondary metabolites and enzyme activity responses of two edible fern species to drought stress and rehydration in northeast China. Agronomy 2019, 9, 137. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jie, W.G.; Peng, X.Y.; Hua, X.Y.; Yan, X.F.; Zhou, Z.Q.; Lin, J.X. Physiological adaptive strategies of oil seed crop Ricinus communis early seedlings (cotyledon vs. True leaf) under salt and alkali stresses: From the growth, photosynthesis and chlorophyll fluorescence. Front. Plant Sci. 2019, 9, 1939. [Google Scholar] [CrossRef]
- Wang, Y.N.; Lin, J.X.; Huang, S.C.; Zhang, L.; Zhao, W.N.; Yang, C.X. Isobaric tags for relative and absolute quantification-based proteomic analysis of Puccinellia tenuiflora inoculated with arbuscular mycorrhizal fungi reveal stress response mechanisms in alkali-degraded soil. Land Degrad. Dev. 2019, 30, 1584–1598. [Google Scholar] [CrossRef]
- Liu, W.Y.; Yu, K.M.; He, T.F.; Li, F.F.; Zhang, D.X.; Liu, J.X. The low temperature induced physiological responses of Avena nuda L., a cold-tolerant plant species. Sci. World J. 2013, 2013, 658793. [Google Scholar]
- Zhang, Z.J.; Li, H.Z.; Zhou, W.; Takeuchi, Y.; Yoneyama, K. Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2011, 16, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, X.B.; Tian, D.Q.; Fang, X.P.; Yu, Y.M.; Zhu, H.Y.; Ge, Y.Y.; Ma, G.Y.; Wang, W.Y.; Xiao, W.F.; et al. Antioxidant properties and color parameters of herbal teas in China. Ind. Crop. Prod. 2016, 87, 198–209. [Google Scholar]
- Zhang, H.X.; Birch, J.; Ma, Z.F.; Xie, C.N.; Yang, H.Y.; Bekhit, A.E.D.A.; Dias, G. Optimization of microwave-assisted extraction of bioactive compounds from new zealand and chinese Asparagus officinalis L. Roots. J. Food Sci. Technol. 2019, 56, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-assisted extraction of cannabinoids from Cannabis sativa L. optimized by response surface methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L.X. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.L.; Yang, M.K.; Han, H.W.; Fazal, A.; Liao, Y.H.; Ren, R.; Yin, T.M.; Qi, J.L.; Sun, S.C.; Lu, G.H.; et al. Mycorrhizae enhance soybean plant growth and aluminum stress tolerance by shaping the microbiome assembly in an acidic soil. Microbiol. Spectr. 2023, 11, 2. [Google Scholar] [CrossRef]
- Zong, J.W.; Zhang, Z.L.; Huang, P.L.; Yang, Y.H. Arbuscular mycorrhizal fungi alleviates salt stress in Xanthoceras sorbifolium through improved osmotic tolerance, antioxidant activity, and photosynthesis. Front. Microbiol. 2023, 14, 1138771. [Google Scholar] [CrossRef]
- Chen, S.C.; Jin, W.J.; Liu, A.R.; Zhang, S.J.; Liu, D.L.; Wang, F.H.; Lin, X.M.; He, C.X. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Elsevier: New York, NY, USA, 2008; pp. 1–800. [Google Scholar]
- Johnson, N.C.; Rowland, D.L.; Corkidi, L.; Egerton-Warburton, L.M.; Allen, E.B. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 2003, 84, 1895–1908. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D.; Reinhardt, D.R. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 1995, 103, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.N.; Aili, Y.; Ma, X.D.; Wang, H.O.; Dawuti, M. Mycorrhizal fungal colonization promotes apparent growth and physiology of Alhagi sparsifolia seedlings under salt or drought stress at vulnerable developmental stage. Plant Growth Regul. 2024, 102, 267–278. [Google Scholar] [CrossRef]
- Citterio, S.; Prato, N.; Fumagalli, P.; Aina, R.; Massa, N.; Santagostino, A.; Sgorbati, S.; Berta, G. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 2005, 59, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.G.; Shi, Z.Y.; Lu, S.C.; Wang, F.Y. AMF inoculation alleviates molybdenum toxicity to maize by protecting leaf performance. J. Fungi 2023, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Lin, J.X.; Yang, F.; Tao, S.; Yan, X.F.; Zhou, Z.Q.; Zhang, Y.H. Arbuscular mycorrhizal fungi improve the growth and performance in the seedlings of Leymus chinensis under alkali and drought stresses. PeerJ 2022, 10, e12890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wang, J.H.; Yan, X.F.; Sun, S.N.; Lin, J.X. The effect of arbuscular mycorrhizal fungi on photosystem II of the host plant under salt stress: A meta-analysis. Agronomy 2019, 9, 806. [Google Scholar] [CrossRef]
- Liu, K.L. Crop Cultivation; China Agriculture Press: Beijing, China, 2008; pp. 475–476. [Google Scholar]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Mohasseli, V.; Farbood, F.; Moradi, A. Antioxidant defense and metabolic responses of lemon balm Melissa officinalis L. to fe-nano-particles under reduced irrigation regimes. Ind. Crop. Prod. 2020, 149, 112338. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Jin, Y.F.; Traore, I.; Dong, L.J.; Yang, G.; Wang, Y.B. Effects of arbuscular mycorrhizal fungi Glomus mosseae on the growth and medicinal components of Dysosma versipellis under copper stress. Bull. Environ. Contam. Toxicol. 2021, 107, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Chen, K.; Li, Q.; Tang, Y.L.; Jiang, Y.Y.; Su, Y. Effects of arbuscular mycorrhizal fungi on alleviating cadmium stress in Medicago truncatula gaertn. Plants 2023, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between specific leaf area, leaf thickness, leaf water content and spad-502 readings in six amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Mathur, S.; Agnihotri, R.; Sharma, M.P.; Reddy, V.R.; Jajoo, A. Effect of high-temperature stress on plant physiological traits and mycorrhizal symbiosis in maize plants. J. Fungi 2021, 7, 867. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Kataria, S.; Alamri, S.A.; Siddiqui, M.H.; Rastogi, A. Inoculation with arbuscular mycorrhizal fungi alleviates the adverse effects of high temperature in soybean. Plants 2022, 11, 2210. [Google Scholar] [CrossRef] [PubMed]
- Frosi, G.; Barros, V.; Oliveira, M.T.d.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G. Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 2018, 38, 25–36. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll α fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Rastogi, A.; Stróżecki, M.; Kalaji, H.M.; Łuców, D.; Lamentowicz, M.; Juszczak, R. Impact of warming and reduced precipitation on photosynthetic and remote sensing properties of peatland vegetation. Environ. Exp. Bot. 2019, 160, 71–80. [Google Scholar] [CrossRef]
- Rastogi, A.; Živčák, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovár, M.; He, X.; Živčák, M.; Kataria, S.; Kalaji, H.M.; Skalický, M.; Ibrahimova, U.; Hussain, S.; Mbarki, S.; et al. Special issue in honour of Prof. Reto J. Strasser-JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 2020, 58, 518–528. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Borkowska, B. Chlorophyll a fluorescence method as a physiological marker of plant response to light stress and endo-mycorrhiza (AMF). Acta Hortic. 2006, 711, 177–182. [Google Scholar] [CrossRef]
- Li, W.; Zhai, Y.L.; Xing, H.S.; Xing, L.J.; Guo, S.X. Arbuscular mycorrhizal fungi promote photosynthesis in Antirrhinum majus L. under low-temperature and weak-light conditions. Not. Bot. Horti Agrobot. Cluj-Na. 2023, 51, 13012. [Google Scholar] [CrossRef]
- Yang, M.; Wei, L.; Zhuang, W.F.; Yuan, H.Y.; Zhao, D.X.; Sun, J.; Wei, S. Effects of low-temperature stress on electric conductivity and fluorescence parameters of maize seedling. Maize Sci. 2012, 20, 90–94. [Google Scholar]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R.; Gómez, M.R. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Azcón, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Shao, H.B.; Shao, C.Y.; Chen, P.; Zhao, S.J.; Brestič, M.; Chen, X.B. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- Hammer, E.C.; Nasr, H.; Pallon, J.; Olsson, P.A.; Wallander, H. Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 2011, 21, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Faizal, A.; Geelen, D. Saponins and their role in biological processes in plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Q.; Zhang, Z.W.; Guo, A.Q.; Luan, L.Y. Progress on the stress-resistant ecological function of plant polyphenols. Acta Bot. Boreali-Occident. Sin. 2011, 31, 423–430. [Google Scholar]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martinez, D.; Garcia-Zara, S. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Espinosa-Leal, C.A.; García-Lara, S. Effects of saline elicitors on saponin production in Agave salmiana plants grown in vitro. Plant Physiol. Biochem. 2021, 162, 476–482. [Google Scholar] [CrossRef]
- Yuan, G.F.; Wang, X.P.; Guo, R.F.; Wang, Q.M. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Leelapriya, T.; Kumari, B.D.R. Effects of pulsed magnetic field treatment of soybean seeds on calli growth, cell damage, and biochemical changes under salt stress. Bioelectromagnetics 2012, 33, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Palhares Neto, L.; Silva-Santos, L.; De Souza, L.M.; De Morais, M.B.; Corte-Real, N.; Monte Junior, I.P.; Gomes Camara, C.A.; Moraes, M.M.; Ulisses, C. Influence of arbuscular mycorrhizal fungi on morphophysiological responses and secondary metabolism in Lippia alba (Verbenaceae) under different water regimes. J. Plant Growth Regul. 2023, 42, 827–841. [Google Scholar] [CrossRef]
- Alizadeh, S.; Gharagoz, S.F.; Pourakbar, L.; Moghaddam, S.S.; Jamalomidi, M. Arbuscular mycorrhizal fungi alleviate salinity stress and alter phenolic compounds of Moldavian balm. Rhizosphere 2021, 19, 100417. [Google Scholar] [CrossRef]
- Si, L.B.; Li, J.M.; Li, G.Y.; Jiang, X.Y.; Lu, L.R.; Yang, Y.L. Effects of tea polyphenols on physiological characteristics in leaves of wheat seedlings under salt stress. Acta Ecol. Sin. 2020, 40, 3747–3755. [Google Scholar]
- Cheng, Y.W.; Kong, X.W.; Wang, N.; Wang, T.T.; Chen, J.; Shi, Z.Q. Thymol confers tolerance to salt stress by activating anti-oxidative defense and modulating Na+ homeostasis in rice root. Ecotoxicol. Environ. Saf. 2019, 188, 109894. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.F.; Ling, T.X.; Xue, Y.F.; Xu, C.F.; Zhou, W.; Hu, L.B.; Chen, J.; Shi, Z.Q. Thymol mitigates cadmium stress by regulating glutathione levels and reactive oxygen species homeostasis in tobacco seedlings. Molecules 2016, 21, 1339. [Google Scholar] [CrossRef] [PubMed]
- Pietrafusa, N.; Ferretti, A.; Trivisano, M.; de Palma, L.; Calabrese, C.; Pavia, G.C.; Tondo, I.; Cappelletti, S.; Vigevano, F.; Specchio, N. Purified cannabidiol for treatment of refractory epilepsies in pediatric patients with developmental and epileptic encephalopathy. Pediatr. Drugs 2019, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: A prospective cohort study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef]
- Seemakram, W.; Paluka, J.; Suebrasri, T.; Lapjit, C.; Kanokmedhakul, S.; Kuyper, T.W.W.; Ekprasert, J.; Boonlue, S. Enhancement of growth and cannabinoids content of hemp (Cannabis sativa) using arbuscular mycorrhizal fungi. Front. Plant Sci. 2022, 13, 845794. [Google Scholar] [CrossRef]



| Groups | Treatment Group | Methods |
|---|---|---|
| Control group | CK | 45% soil moisture content |
| Salt stress group | S1 | 100 mM NaCl treatment |
| S2 | 200 mM NaCl treatment | |
| Drought stress group | D1 | 25% soil moisture content |
| D2 | 15% soil moisture content | |
| Salt–drought interaction stress group | S1D1 | 100 mM NaCl and 25% soil moisture |
| S1D2 | content co-treatment | |
| Inoculation group | AM | Inoculation with AMF |
| Non-inoculated group | NM | No AMF inoculation |
| Treatment Group | CRAS | MD | |
|---|---|---|---|
| NM | AM | ||
| CK | 0 ± 0 g | 89.99 ± 1.67 a | 6.1 ± 1.04 c |
| S1 | 0 ± 0 g | 82.04 ± 1.81 c | 31.53 ± 3.77 b |
| S2 | 0 ± 0 g | 72.69 ± 0.88 f | 57.92 ± 2.17 a |
| D1 | 0 ± 0 g | 84.57 ± 1.28 b | 34.49 ± 1.87 b |
| D2 | 0 ± 0 g | 75.81 ± 1.27 e | 55.24 ± 2.16 a |
| S1D1 | 0 ± 0 g | 79.4 ± 1.14 d | 33.47 ± 2.84 b |
| S1D2 | 0 ± 0 g | 75.32 ± 1.04 e | 57.49 ± 2.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Si, H.; Ye, Y.; Ji, Q.; Wang, H.; Zhang, Y. Arbuscular Mycorrhizal Fungi-Mediated Modulation of Physiological, Biochemical, and Secondary Metabolite Responses in Hemp (Cannabis sativa L.) under Salt and Drought Stress. J. Fungi 2024, 10, 283. https://doi.org/10.3390/jof10040283
Yuan H, Si H, Ye Y, Ji Q, Wang H, Zhang Y. Arbuscular Mycorrhizal Fungi-Mediated Modulation of Physiological, Biochemical, and Secondary Metabolite Responses in Hemp (Cannabis sativa L.) under Salt and Drought Stress. Journal of Fungi. 2024; 10(4):283. https://doi.org/10.3390/jof10040283
Chicago/Turabian StyleYuan, Haipeng, Hao Si, Yunshu Ye, Qiuyan Ji, Haoyu Wang, and Yuhong Zhang. 2024. "Arbuscular Mycorrhizal Fungi-Mediated Modulation of Physiological, Biochemical, and Secondary Metabolite Responses in Hemp (Cannabis sativa L.) under Salt and Drought Stress" Journal of Fungi 10, no. 4: 283. https://doi.org/10.3390/jof10040283
APA StyleYuan, H., Si, H., Ye, Y., Ji, Q., Wang, H., & Zhang, Y. (2024). Arbuscular Mycorrhizal Fungi-Mediated Modulation of Physiological, Biochemical, and Secondary Metabolite Responses in Hemp (Cannabis sativa L.) under Salt and Drought Stress. Journal of Fungi, 10(4), 283. https://doi.org/10.3390/jof10040283






