Combination of Systemic and Lock-Therapies with Micafungin Eradicate Catheter-Based Biofilms and Infections Caused by Candida albicans and Candida parapsilosis in Neutropenic Rabbit Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Drugs
2.3. Organism, Inoculum, and Inoculation
2.3.1. Candida albicans
2.3.2. Candida parapsilosis
2.4. Immunosuppression of Rabbits and Maintenance of Neutropenia
2.5. Antifungal Therapy
2.5.1. Candida albicans
2.5.2. Candida parapsilosis
2.6. Outcome Variables/Rabbit Model of Catheter-Related Disseminated Candidemia
2.6.1. Quantitation of C. albicans or C. parapsilosis in the Blood
2.6.2. Quantitation of C. albicans or C. parapsilosis in the Tissues
2.6.3. (1→3)-β-D-Glucan Levels in Serum
2.7. Statistical Analysis
3. Results
3.1. Disseminated Candidiasis Caused by C. albicans in a Neutropenic Rabbit Model
3.1.1. Fungal Burden in Central Venous Catheter Blood
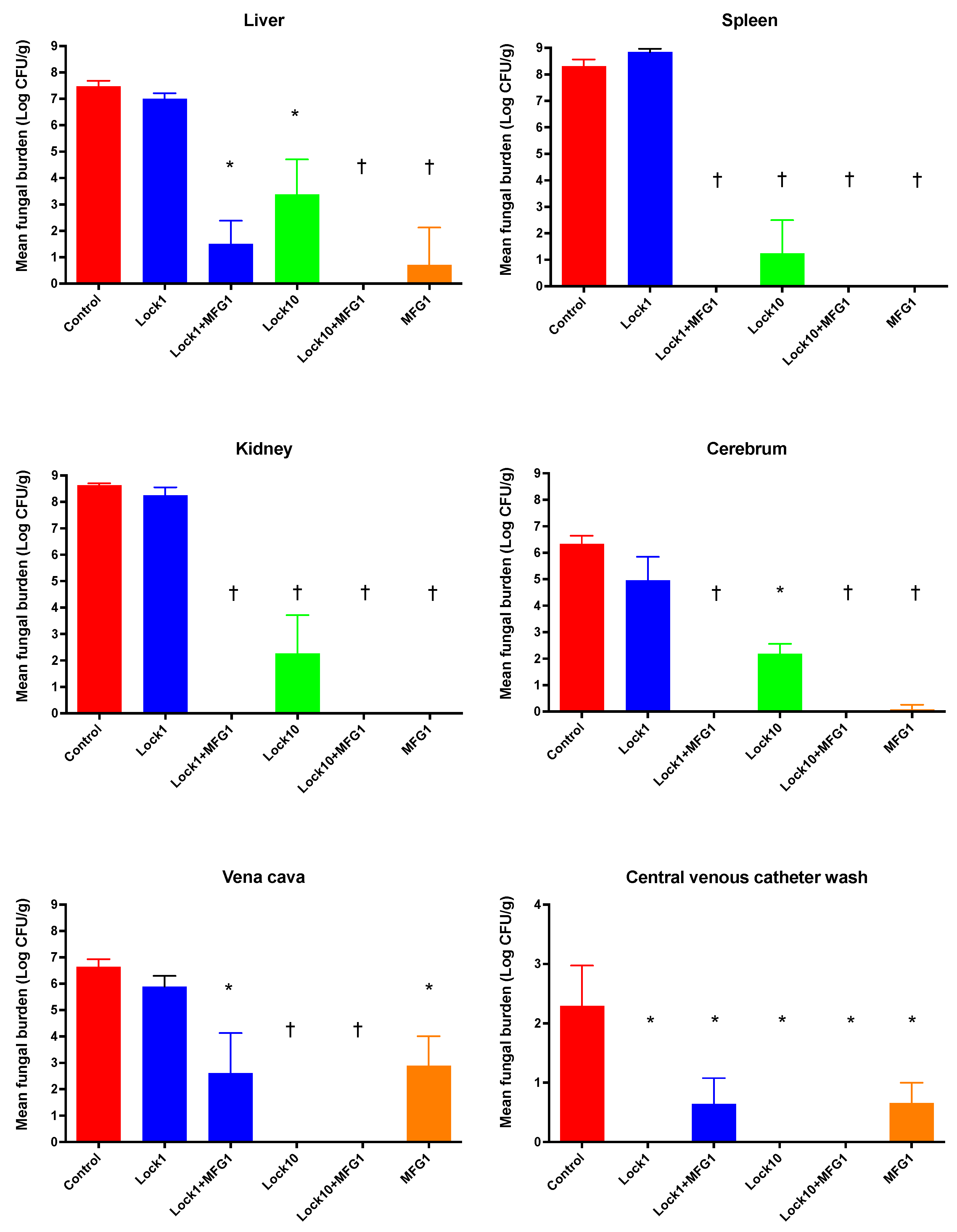
3.1.2. Efficacy of Lock Therapy and Systemic Therapy with Micafungin against C. albicans
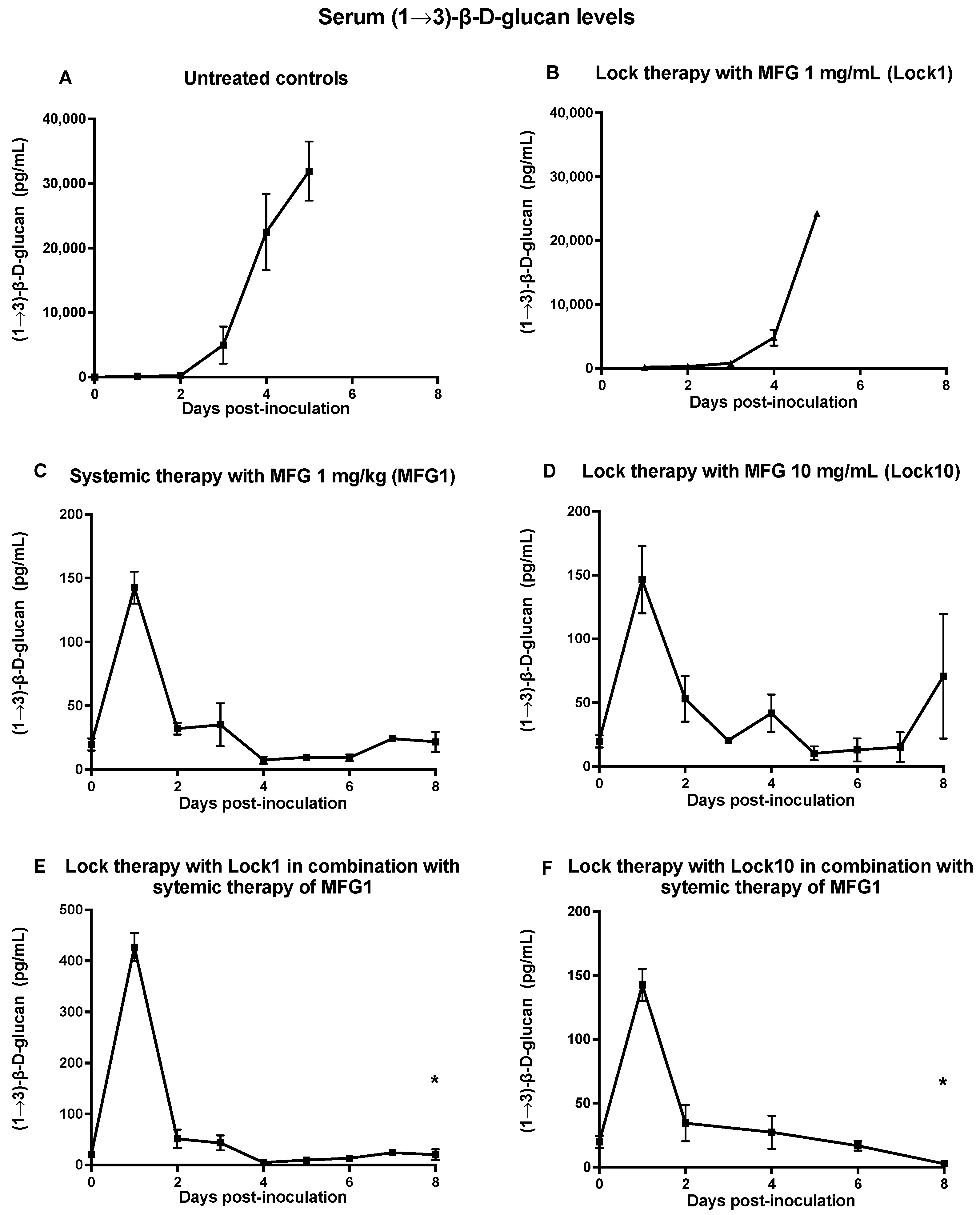
3.1.3. Assessment of (1→3)-β-D-Glucan Levels
3.2. Disseminated Candidiasis Caused by C. parapsilosis in a Neutropenic Rabbit Model
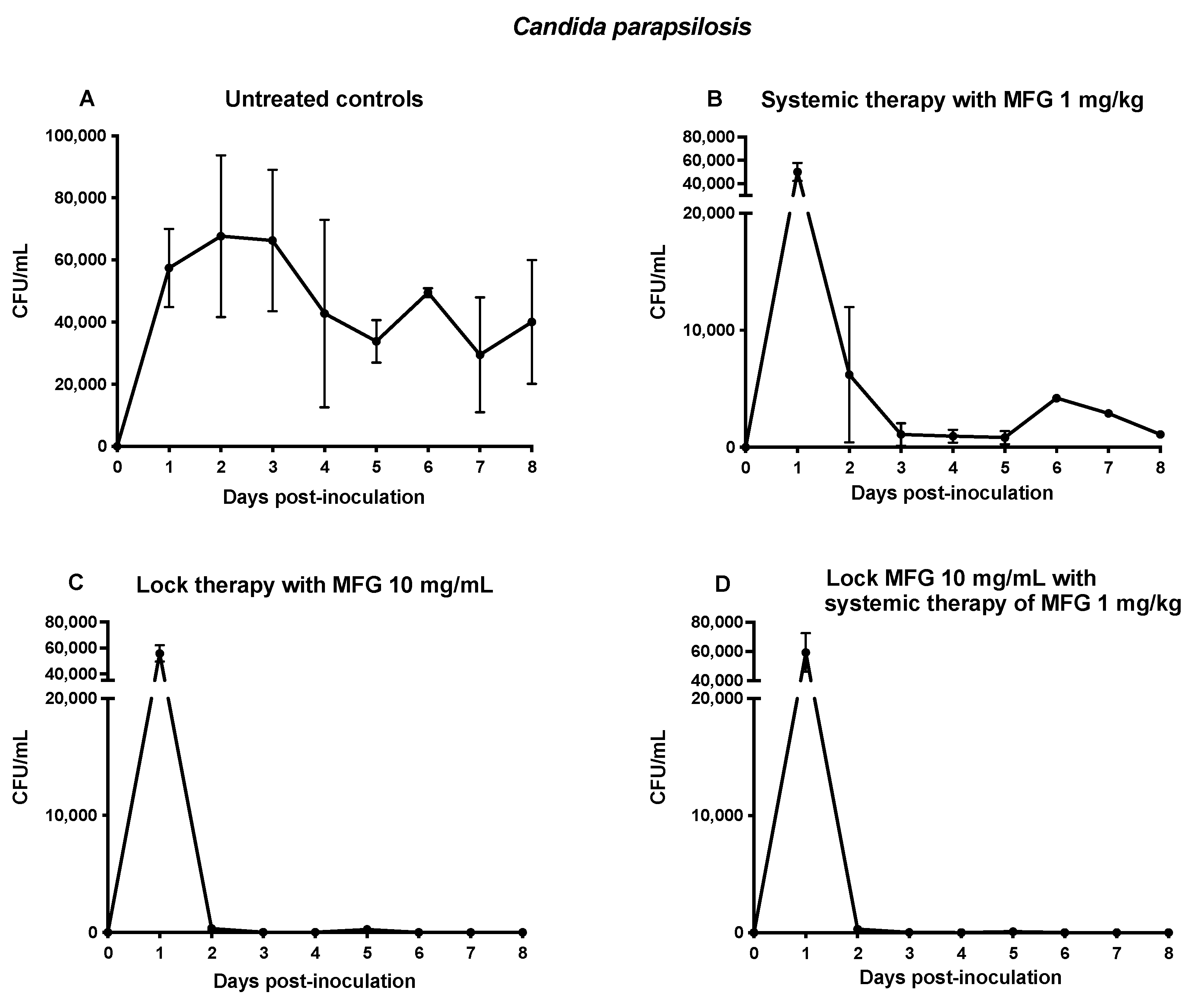
3.2.1. Fungal Burden in Central Venous Catheter Blood
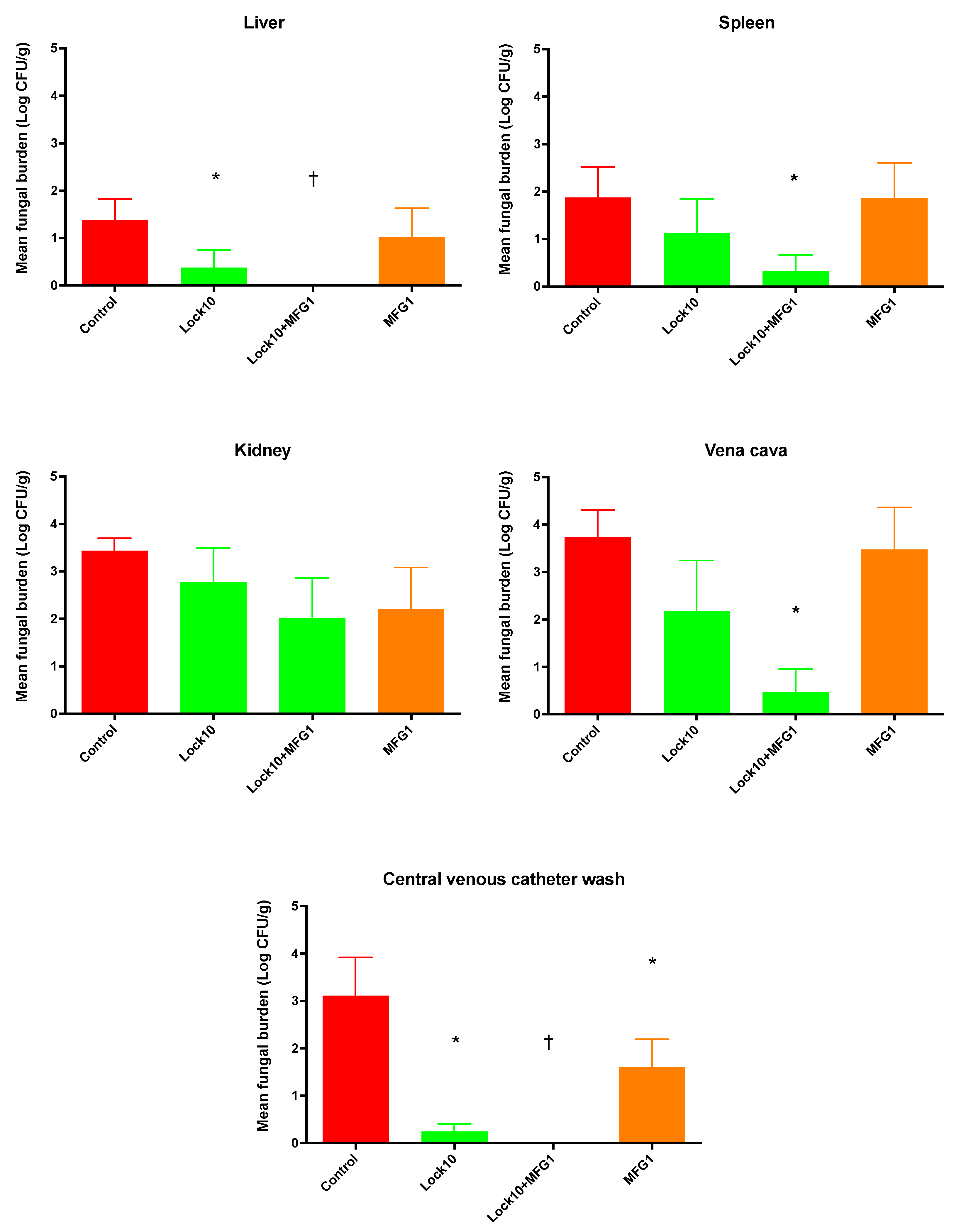
3.2.2. Efficacy of Lock Therapy and Systemic Therapy with Micafungin against C. parapsilosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl S7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lara, M.F.; Ostrosky-Zeichner, L. Invasive Candidiasis. Semin. Respir. Crit. Care Med. 2020, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, S.C.; Hoenigl, M.; Koehler, P.; Kumar, A.; Lagrou, K.; Lass-Florl, C.; Meis, J.F.; Menon, V.; Rautemaa-Richardson, R.; Cornely, O.A. EQUAL Candida Score: An ECMM score derived from current guidelines to measure Quality of Clinical Candidaemia Management. Mycoses 2018, 61, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clark, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Rex, J.H. All catheter-related candidemia is not the same: Assessment of the balance between the risks and benefits of removal of vascular catheters. Clin. Infect. Dis. 2002, 34, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Almirante, B.; Rodríguez, D.; Cuenca-Estrella, M.; Almela, M.; Sanchez, F.; Ayats, J.; Alonso-Tarres, C.; Rodriguez-Tudela, J.L.; Pahissa, A. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: Case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2006, 44, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Marodi, L.; Johnston, R.B., Jr. Invasive Candida species disease in infants and children: Occurrence, risk factors, management, and innate host defense mechanisms. Curr. Opin. Pediatr. 2007, 19, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Yamin, D.H.; Husin, A.; Harun, A. Risk factors of Candida parapsilosis catheter-related bloodstream. Front. Public Health 2021, 9, 631865. [Google Scholar] [CrossRef]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Kriesche, H.U.; Quan, S.P.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004–2008. Diagn. Microbiol. Infect. Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef]
- Chitnis, A.S.; Magill, S.S.; Edwards, J.R.; Chiller, T.M.; Fridkin, S.K.; Lessa, F.C. Trends in Candida central line-associated bloodstream infections among NICUs, 1999–2009. Pediatrics 2012, 130, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 2002, 70, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Zhou, G.; Munyon, R.; Ghannoum, M.A. Candida biofilm: A well-designed protected environment. Med. Mycol. 2005, 43, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Andes, D.R. Contributions of the biofilm matrix to Candida pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Katragkou, A.; Chatzimoschou, A.; Simitsopoulou, M.; Dalakiouridou, M.; Diza-Mataftsi, E.; Tsantali, C.; Roilides, E. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob. Agents Chemother. 2008, 52, 357–360. [Google Scholar] [CrossRef]
- Chatzimoschou, A.; Katragkou, A.; Simitsopoulou, M.; Antachopoulos, C.; Georgiadou, E.; Walsh, T.J.; Roilides, E. Activities of triazole-echinocandin combinations against Candida species in biofilms and as planktonic cells. Antimicrob. Agents Chemother. 2011, 55, 1968–1974. [Google Scholar] [CrossRef]
- Ghannoum, M.; Roilides, E.; Katragkou, A.; Petraitis, V.; Walsh, T.J. The role of echinocandins in Candida biofilm-related vascular catheter infections: In vitro and in vivo model systems. Clin. Infect. Dis. 2015, 61, S618–S621. [Google Scholar] [CrossRef]
- Lazzell, A.L.; Chaturvedi, A.K.; Pierce, C.G.; Prasad, D.; Uppuluri, P.; Lopez-Ribot, J.L. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J. Antimicrob. Chemother. 2009, 64, 567–570. [Google Scholar] [CrossRef]
- Bink, A.; Kucharíková, S.; Neirinck, B.; Vleugels, J.; Van Dijck, P.; Cammue, B.P.; Thevissen, K. The nonsteroidal antiinflammatory drug diclofenac potentiates the in vivo activity of caspofungin against Candida albicans biofilms. J. Infect. Dis. 2012, 206, 1790–1797. [Google Scholar] [CrossRef]
- Kucharíková, S.; Tournu, H.; Holtappels, M.; Van Dijck, P.; Lagrou, K. In vivo efficacy of anidulafungin against mature Candida albicans biofilms in a novel rat model of catheter-associated candidiasis. Antimicrob. Agents Chemother. 2010, 54, 4474–4475. [Google Scholar] [CrossRef] [PubMed]
- Kucharíková, S.; Sharma, N.; Spriet, I.; Maertens, J.; Van Dijck, P.; Lagrou, K. Activities of systemically administered echinocandins against in vivo mature Candida albicans biofilms developed in a rat subcutaneous model. Antimicrob. Agents Chemother. 2013, 57, 2365–2368. [Google Scholar] [CrossRef]
- Fujimoto, K.; Takemoto, K. Efficacy of liposomal amphotericin B against four species of Candida biofilms in an experimental mouse model of intravascular catheter infection. J. Infect. Chemother. 2018, 24, 958–964. [Google Scholar] [CrossRef]
- Salinas, B.; Guembe, M.; Cussó, L.; Kestler, M.; Guinea, J.; Desco, M.; Muñoz, P.; Bouza, E. Assessment of the anti-biofilm effect of micafungin in an animal model of catheter-related candidemia. Med. Mycol. 2019, 57, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Shuford, J.A.; Rouse, M.S.; Piper, K.E.; Steckelberg, J.M.; Patel, R. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J. Infect. Dis. 2006, 194, 710–713. [Google Scholar] [CrossRef]
- Basas, J.; Morer, A.; Ratia, C.; Martín, M.T.; Del Pozo, J.L.; Gomis, X.; Rojo-Molinero, E.; Torrents, E.; Almirante, B.; Gavaldà, J. Efficacy of anidulafungin in the treatment of experimental Candida parapsilosis catheter infection using an antifungal-lock technique. J. Antimicrob. Chemother. 2016, 71, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Basas, J.; Palau, M.; Gomis, X.; Almirante, B.; Gavaldà, J. Efficacy of liposomal amphotericin B and anidulafungin using an antifungal lock technique (ALT) for catheter-related Candida albicans and Candida glabrata infections in an experimental model. PLoS ONE 2019, 14, e0212426. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Groll, A.H.; Roussillon, K.; Hemmings, M.; Lyman, C.A.; Sein, T.; Bacher, J.; Bekersky, I.; Walsh, T.J. Comparative antifungal activity and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 2002, 46, 1857–1869. [Google Scholar] [CrossRef]
- Hope, W.W.; Mickiene, D.; Petraitis, V.; Petraitiene, R.; Kelaher, A.M.; Hughes, J.E.; Cotton, M.P.; Bacher, J.; Keirns, J.J.; Buell, D.; et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: Implications for echinocandin therapy in neonates. J. Infect. Dis. 2008, 197, 163–171. [Google Scholar] [CrossRef]
- Petraitiene, R.; Petraitis, V.; Hope, W.W.; Walsh, T.J. Intermittent dosing of micafungin is effective for treatment of experimental disseminated candidiasis in persistently neutropenic rabbits. Clin. Infect. Dis. 2015, 61, S643–S651. [Google Scholar] [CrossRef] [PubMed]
- Simitsopoulou, M.; Chlichlia, K.; Kyrpitzi, D.; Walsh, T.J.; Roilides, E. Pharmacodynamic and immunomodulatory effects of micafungin on host responses against biofilms of Candida parapsilosis in comparison to those of Candida albicans. Antimicrob. Agents Chemother. 2018, 62, e00478-18. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Goutelle, S.; Jelliffe, R.; Golden, J.A.; Little, E.; DeVoe, C.; Mickiene, D.; Hayes, M.; Conte, J.E., Jr. Intrapulmonary pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant patients. Antimicrob. Agents Chemother. 2010, 54, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.K., Jr.; Kaufman, D.; Hope, W.W.; Smith, P.B.; Arrieta, A.; Manzoni, P.; Kovanda, L.L.; Lademacher, C.; Isaacson, B.; Jednachowski, D.; et al. A Phase 3 Study of micafungin versus amphotericin B deoxycholate in infants with invasive candidiasis. Pediatr. Infect. Dis. J. 2018, 37, 992–998. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Walsh, T.J.; Bacher, J.; Pizzo, P.A. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab. Anim. Sci. 1988, 38, 467–471. [Google Scholar] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; fourth informational supplement, M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Ernst, E.J.; Klepser, M.E.; Pfaller, M.A. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 2000, 44, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Mickiene, D.; Petraitis, V.; Petraitiene, R.; Ibrahim, K.H.; Piscitelli, S.C.; Bekersky, I.; Walsh, T.J. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob. Agents Chemother. 2001, 45, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.J.; Roling, E.E.; Petzold, C.R.; Keele, D.J.; Klepser, M.E. In vitro activity of micafungin (FK-463) against Candida spp.: Microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 2002, 46, 3846–3853. [Google Scholar] [CrossRef]
- Roling, E.E.; Klepser, M.E.; Wasson, A.; Lewis, R.E.; Ernst, E.J.; Pfaller, M.A. Antifungal activities of fluconazole, caspofungin (MK0991), and anidulafungin (LY 303366) alone and in combination against Candida spp. and Cryptococcus neoformans via time-kill methods. Diagn. Microbiol. Infect. Dis. 2002, 43, 13–17. [Google Scholar] [CrossRef]
- Gumbo, T.; Drusano, G.L.; Liu, W.; Ma, L.; Deziel, M.R.; Drusano, M.F.; Louie, A. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob. Agents Chemother. 2006, 50, 3695–3700. [Google Scholar] [CrossRef]
- Gumbo, T.; Drusano, G.L.; Liu, W.; Kulawy, R.W.; Fregeau, C.; Hsu, V.; Louie, A. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob. Agents Chemother. 2007, 51, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Diekema, D.J.; Pfaller, M.A.; Marchillo, K.; Bohrmueller, J. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 2008, 52, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Novy, E.; Roger, C.; Roberts, J.A.; Cotta, M.O. Pharmacokinetic and pharmacodynamic considerations for antifungal therapy optimisation in the treatment of intra-abdominal candidiasis. Crit. Care 2023, 27, 449. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Andes, D.R. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb. Perspect. Med. 2014, 5, a019653. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, C.G.; Rhomberg, P.R.; Pfaller, M.A.; Locke, J.B.; Castanheira, M. Evaluation of the post-antifungal effect of rezafungin and micafungin against Candida albicans, Candida parapsilosis and Candida glabrata. Mycoses 2022, 65, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Piersigilli, F.; Auriti, C.; Bersani, I.; Goffredo, B.; Bianco, G.; Savarese, I.; Dottam, A. Antifungal lock therapy with combined 70% ethanol and micafungin in a critically ill infant. Pediatr. Infect. Dis. J. 2014, 33, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Auriti, C.; Piersigilli, F.; Ronchetti, M.P.; Campi, F.; Amante, P.G.; Falcone, M.; Goffredo, B.M. Shunt lock therapy with micafungin to treat shunt-associated Candida albicans meningitis in an infant. J. Antimicrob. Chemother. 2016, 71, 2060–2061. [Google Scholar] [CrossRef]
- Ozdemir, H.; Karbuz, A.; Ciftçi, E.; Dinçaslan, H.U.; Ince, E.; Aysev, D.; Yavuz, G.; Doğru, U. Successful treatment of central venous catheter infection due to Candida lipolytica by caspofungin-lock therapy. Mycoses 2011, 54, e647–e649. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petraitiene, R.; Petraitis, V.; Zaw, M.H.; Hussain, K.; Ricart Arbona, R.J.; Roilides, E.; Walsh, T.J. Combination of Systemic and Lock-Therapies with Micafungin Eradicate Catheter-Based Biofilms and Infections Caused by Candida albicans and Candida parapsilosis in Neutropenic Rabbit Models. J. Fungi 2024, 10, 293. https://doi.org/10.3390/jof10040293
Petraitiene R, Petraitis V, Zaw MH, Hussain K, Ricart Arbona RJ, Roilides E, Walsh TJ. Combination of Systemic and Lock-Therapies with Micafungin Eradicate Catheter-Based Biofilms and Infections Caused by Candida albicans and Candida parapsilosis in Neutropenic Rabbit Models. Journal of Fungi. 2024; 10(4):293. https://doi.org/10.3390/jof10040293
Chicago/Turabian StylePetraitiene, Ruta, Vidmantas Petraitis, Myo H. Zaw, Kaiser Hussain, Rodolfo J. Ricart Arbona, Emanuel Roilides, and Thomas J. Walsh. 2024. "Combination of Systemic and Lock-Therapies with Micafungin Eradicate Catheter-Based Biofilms and Infections Caused by Candida albicans and Candida parapsilosis in Neutropenic Rabbit Models" Journal of Fungi 10, no. 4: 293. https://doi.org/10.3390/jof10040293




