Abstract
The slow action of fungi is one of the biggest challenges in using entomopathogenic fungi. A promising alternative to reduce the time of action is to combine conidia with extracellular enzymes. This study aimed to characterize the production of Pr1 subtilisin protease and lipases by Beauveria bassiana and Metarhizium anisopliae in different culture media and to evaluate the efficiency of the enzymatic treatment against Aphis gossypii and Spodoptera frugiperda. The isolates were cultivated in five different liquid cultures, and, after 7 days, the culture was filtered and centrifuged, and the activity of the Pr1 and lipases was measured. The fungi cultured in a Luria–Bertani broth medium had the highest activity of proteases and lipases. The mortality of A. gossypii nymphs treated with conidia 7 days after the treatment was 39% (JEF-410), 76.5% (JEF-492), 74.8% (ERL-836), and 70.9% (JEF-214). The B. bassiana JEF-410 supernatant combined with conidia increased the fungal virulence at day 5 and day 6 after treatment. When S. frugiperda larvae were treated with B. bassiana JEF-492 conidia combined with its supernatant, the time of infection was shorter compared to the larvae treated with conidia only. Once the supernatant was incubated at 37 °C, the relative activity decreased from 100% to 80% after 2 h and to 45% after 24 h. The results suggest that the supernatant of entomopathogenic fungi may be formulated and used as a biopesticide in an efficient strategy for the biological control of pests.
1. Introduction
Studies have investigated the use of entomopathogenic fungi as biological agents to establish rational and effective strategies for arthropod control. Fungi such as Metarhizium anisopliae (Metschn.) Sorokin, 1883 and Beauveria bassiana (Bals.-Criv.) Vuill., 1912 complexes have proven highly effective against various arthropod species [1]. Entomopathogenic fungi, such as B. bassiana, are effective biocontrol agents against many agricultural pests [2], as well as baculoviruses and bacteria including Bacillus thuringiensis [3]. In Brazil, the most extensive microbiological control program utilizes M. anisopliae s.str. against spittlebugs in sugarcane crops [4]. Also, B. bassiana controls whiteflies, coffee berry borer, and other agricultural pests [5].
The primary mechanism of fungal infection against insects is through the cuticle, unlike other pathogens that enter the insect hosts by ingesting contaminated food [6]. The penetration process through integument occurs via the degradation of the cuticle due to the action of several enzymes—such as proteases (EC 3.4) and lipases (EC 3.1.1.3)—combined with the mechanical pressure of the hyphae [7]. The fungal penetration continues through all the integument layers until the hyphae reach the hemocoel [8].
The insect’s cuticle is the first barrier against microbial invasion. The external layer, the epicuticle, is hydrophobic and mainly composed of lipids, fatty acids, esters, and long-chain alkenes [1]. Therefore, lipases are produced during the initial infection process and are responsible for the hydrolysis of the ester bonds of fats, waxes, and lipoproteins from the epicuticle [9].
Subsequently, the fungus has to penetrate the host through the procuticle—a proteinaceous layer. The insect’s cuticle is mainly constituted of proteins (up to 70%), and, for this reason, proteases are one of the most vital enzymes related to the infection process [1]. Among the subtilisins, trypsins, metalloproteases, and exopeptidases, Pr1(subtilisin) is mainly responsible for the degree of pathogenicity of the isolate [1,9,10]. It can be found in 11 isoforms (Pr1A-K), each with a distinct participation in the infection process [11].
The slow action is one of the most significant challenges in using entomopathogenic fungi. Fungi, as biopesticides, usually take more days to eliminate the insects than chemical pesticides, which may represent economic losses to farmers [7]. One alternative to reduce the time of infection is to combine entomopathogenic fungi with chemical pesticides [12,13]. However, combining fungi with biochemical pesticides may be a more sustainable way to use them.
A new approach to exploring entomopathogenic fungi as biopesticides involves using their extracellular enzymes to accelerate the time of infection. Studies have tested fungal enzymes as biopesticides with promising results, as reviewed by [7]. As the enzymes act in the degradation of the arthropod cuticle, the time of infection can potentially be decreased if they are applied along with fungal conidia [14]. Bioproducts are promising alternatives to the exclusive use of synthetic pesticides because the overuse of these chemicals, including organophosphates, carbamates, and pyrethroids, may select resistant insect populations [15].
Aphis gossypii (Glover) (Hemiptera: Aphididae), also known as the cotton aphid, is a sucking pest that causes physical damage to the plant not only by feeding but also by transmitting viruses and producing honeydew excretion. A. gossypii is a polyphagous pest found worldwide that feeds on several plants, such as vegetables, fruits, and flowers [16]. The fall armyworm (FAW) or Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae) is a highly polyphagous insect that feeds on leaves during the larval stage [3]. It prefers graminaceous plants, such as maize, sorghum, barley, and wheat. It can also eat other plants, including cotton and soybean [17]. S. frugiperda is a destructive pest in the Americas and recently arrived in Africa (2016–2017), Asia (2018–2019), and Oceania (2020) [18]. It is estimated that the FAW causes losses of about USD 300 million in the USA and USD 13 billion across sub-Saharan Africa per year [17].
This research aimed to characterize the production of protease Pr1 and lipases from different isolates of entomopathogenic fungi in various culture media and to evaluate the effectiveness of enzymatic treatments associated with conidia against different insect species of agricultural relevance.
2. Materials and Methods
2.1. Fungal Isolates
Fungal isolates were provided by the Jeonbuk National University Entomopathogenic Fungal Platform (JEF-LIBRARY). Four isolates were selected: three B. bassiana (JEF-410, JEF-492, and ERL-836) and one M. anisopliae (JEF-214). The isolates were collected from soil using the Tenebrio molitor larva-based baiting method. The collection sites were as follows: JEF-410 (Jeongseon, Republic of Korea); JEF-492 (Jeongseon, Republic of Korea); ERL-836 [CA, USA (original isolation code: CA13)]; and JEF-214 (Pyeongchang, Republic of Korea).
The fungal isolates were cultivated on one-quarter of Sabouraud dextrose agar (SDA, BD Difco, Sparks, MD, USA) at 27 ± 1 °C in the dark for 14 days. Five culture media were prepared: a Luria–Bertani broth (LB), a potato dextrose broth (PDB), a Sabouraud dextrose broth (SDB), a yeast extract–peptone–glucose broth (YPG), and a soluble starch–yeast extract–peptone broth (SSYP). All culture media had the initial pH adjusted to 6.0 [19].
Conidial suspensions of each fungal isolate at 1 × 107 conidia mL−1 (1%, v/v) were inoculated in 100 mL liquid cultures. The cultures were incubated on an orbital shaker at 27 ± 1 °C and 170 rpm for 7 days. After this period, the liquid cultures were filtered through filter paper (No. 2 Ø110, ADVANTEC, Tokyo, Japan). The filtrate was then centrifuged at 10,000 rpm for 6 min at 4 °C, and the supernatants were stored at 4 ± 1 °C. The PDB medium was used as the control medium [19].
2.2. Enzyme Assay
The activity of the subtilisin-like Pr1 protease in the culture supernatants was measured by a method adapted from [19,20]. The substrate for this reaction was 2 mM N-Suc-(Ala)2-Pro-Phe-p-nitroanilide (Sigma-Aldrich, STO, St. Louis, MO, USA). The reaction mixture was prepared by mixing 180 µL of the substrate, 120 µL of the supernatant, and 900 µL of 50 mM TRIS-HCl buffer (pH 8.0). The mixture was incubated at 37 °C for 1 h, and then an absorbance reading was performed in a spectrophotometer SM 1100-UV-VIS (Azzota, Claymont, DE, USA) at 405 nm. Enzyme activity is expressed as nanomoles of nitroanilide (NA) released per milliliter per minute at 37 °C.
The substrate used for this reaction was 28 mM pNPB. The reaction mixture consisted of 325 µL of the substrate, 200 µL of the supernatant, and 800 µL of the phosphate buffer (pH 7.25). Next, the mixture was incubated at 37 °C for 5 min, and exactly after that time, a kinetic assay was performed in the spectrophotometer at 405 nm [19,21]. Each enzyme assay was replicated three times using different samples of the four fungal isolates cultivated in different liquid cultures.
2.3. Thermostability
The supernatant was incubated at 37 ± 1 °C in a heating chamber for 2, 4, 12, and 24 h. Following the incubation, the supernatant was used for the enzyme assay (as previously described) of the Pr1 and the lipase assay.
2.4. Virulence Assay
2.4.1. Aphis gossypii
First, all the isolates were cultivated in one-quarter of SDA to produce conidia for the virulence assay, as mentioned previously. Fungal suspensions were prepared at 1 × 107 conidia mL−1. The colonies of cotton aphids were provided by the National Institute of Agricultural Sciences, Republic of Korea. They were maintained on three leaf-stage cucumber plants (Ilmi Samcheok, Green Heart Bio, Yeoju, Republic of Korea) grown under laboratory conditions at 26 ± 1 °C, 50 ± 5% relative humidity (RH), and 16:8 h (L:D) photoperiod. All experiments were performed on wingless aphids. Ten aphid nymphs were transferred to a cucumber leaf disc (110 mm diameter). A 1.0 mL aliquot of the fungal conidial suspension was sprayed onto the cucumber leaf discs with the aphids (110 mm diameter) from a distance of 15 cm for 10 sec. The discs were then dried for 10 min at room temperature. Then, the leaves were transferred to disposable plastic cups (50 mL) containing 5 mL of 1% agar (BD Difco, USA). All cups were kept at 26 ± 1 °C, 90 ± 5% relative humidity (RH), and 16L:8D (L:D) cycles. The number of dead aphids was evaluated daily for one week [22]. The mortality was calculated by the proportion of dead aphids compared to the total number of aphids, as demonstrated below:
A virulence assay to evaluate the action of the enzymes from the isolate JEF-410 was conducted by forming six groups. These groups included a negative control (Siloxane solution 0.03% (Silwet, FarmHannong Inc., Nonsan, Republic of Korea)), a positive control (Imidacloprid 10% suspension concentrate (SC) (2000×)), fungal conidia, conidia + supernatant, supernatant, and denatured supernatant (boiled for 1 h). The supernatant was produced in an LB broth medium, and aerial conidia were produced on solid one-quarter of SDA medium. The aphids were prepared, and the tests were conducted as mentioned above. The experiment was repeated on three different days.
2.4.2. Spodoptera frugiperda
The National Institute of Agricultural Sciences, Republic of Korea, provided the colonies of S. frugiperda. The larvae were maintained on a modified artificial diet [23,24], and the insects were maintained at 27 ± 1 °C with a RH of 50 ± 5% and a 14:10 (L:D) photoperiod.
The experiments were prepared as described for the A. gossypii, except for the positive control. The B. bassiana JEF-492 was tested against the S. frugiperda larvae with a negative control (Silwet 0.03%), fungal conidia, conidia + supernatant, supernatant, and denatured supernatant (boiled for 1 h). Five-second instar larvae were transferred to Petri dishes (35 × 10 mm), and a 1.0 mL aliquot of the treatment was sprayed over them from a distance of 15 cm for 10 sec. Next, the larvae were individually transferred to 1% agar cups, and mung bean leaves (Vigna radiate) (110 mm diameter) were used as nutritional sources. Mung beans at the three-leaf stage were used in laboratory conditions at 27 ± 1 °C, 50 ± 5% relative humidity (RH), and a 14:10 (L:D) photoperiod. New leaves were provided according to the larvae’s needs. The number of dead larvae was evaluated daily for one week [23]. The mortality was calculated by the proportion of dead larvae compared to the total number of S. frugiperda larvae, as demonstrated above with the aphids.
The experiments were repeated on three different days.
2.5. Statistical Analysis
All the data were subjected to analysis of variance (ANOVA) and Tukey’s test using BioEstat 5.0 [25]. The p values less than 0.05 were considered significant.
3. Results
3.1. Enzyme Activity
3.1.1. Protease
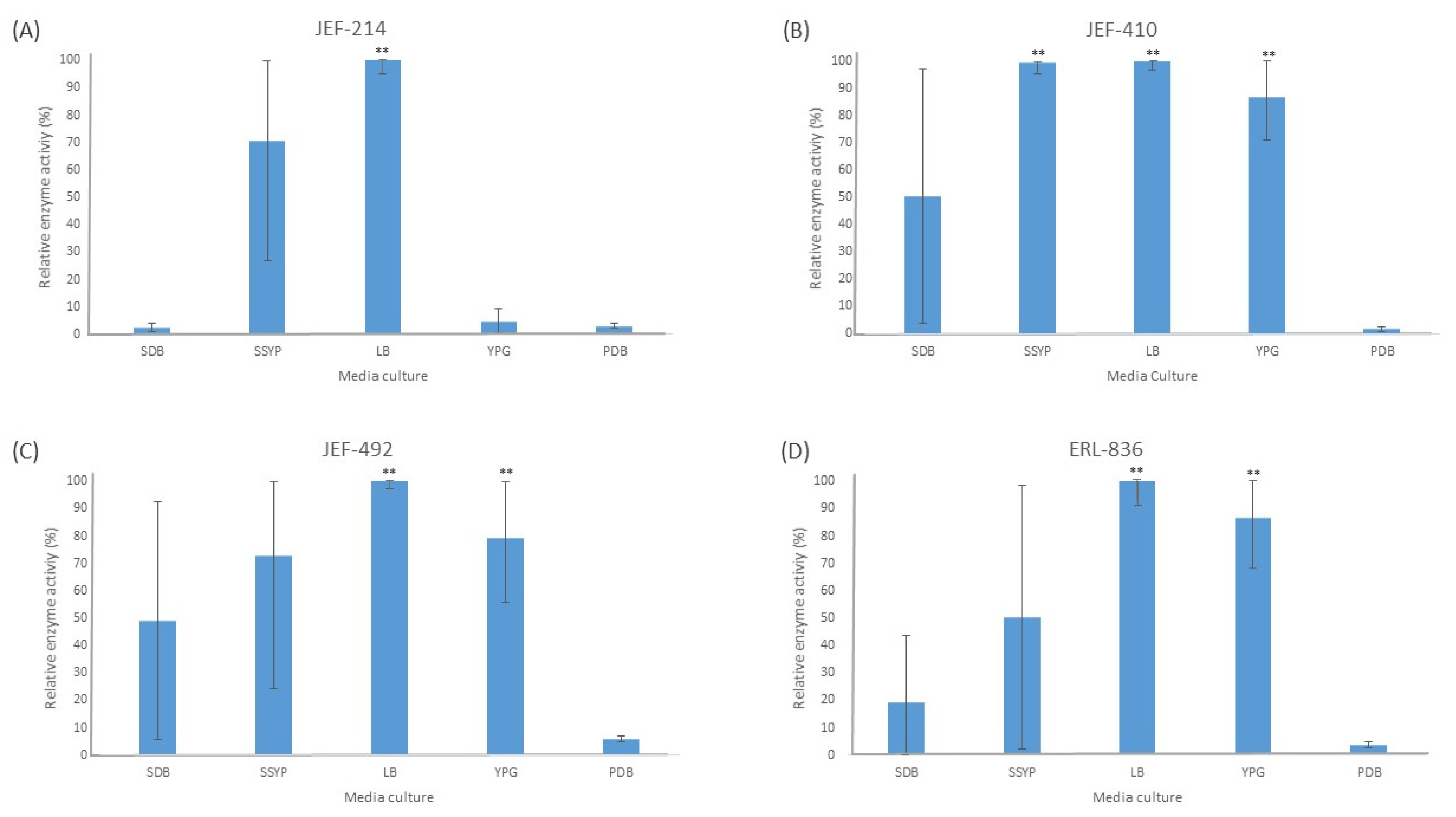
The liquid culture of all the fungal isolates in different cultured media demonstrated significant activity of the Pr1 protease (Figure 1). The PDB medium did not induce extracellular Pr1 protease significantly among the isolates tested. Furthermore, all the isolates cultured in the liquid LB medium showed a high Pr1 activity, which differed significantly (p < 0.01) from the activity in the PDB culture medium. The YPG medium, however, induced Pr1 in the B. bassiana isolates (p < 0.05) but not in the M. anisopliae JEF-214.
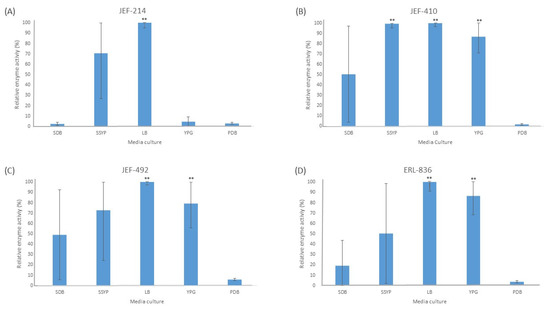
Figure 1.
Activity of extracellular Pr1 subtilisin protease. The isolates of Beauveria bassiana and Metarhizium anisopliae were cultured in SDB (Sabouraud dextrose broth), SSYP (soluble starch–yeast extract–peptone), LB (Luria–Bertani), YPG (yeast extract–peptone–glucose), and PDB (potato dextrose broth) by liquid culture. (A) M. anisopliae JEF-214; (B) B. bassiana JEF-410; (C) B. bassiana JEF-492; and (D) B. bassiana ERL-836. The cultures were cultivated for 7 days and then filtered and centrifuged to obtain a cell-free supernatant. The vertical bars correspond to the standard error. ** p < 0.01 according to the Tukey’s Test.
3.1.2. Lipase
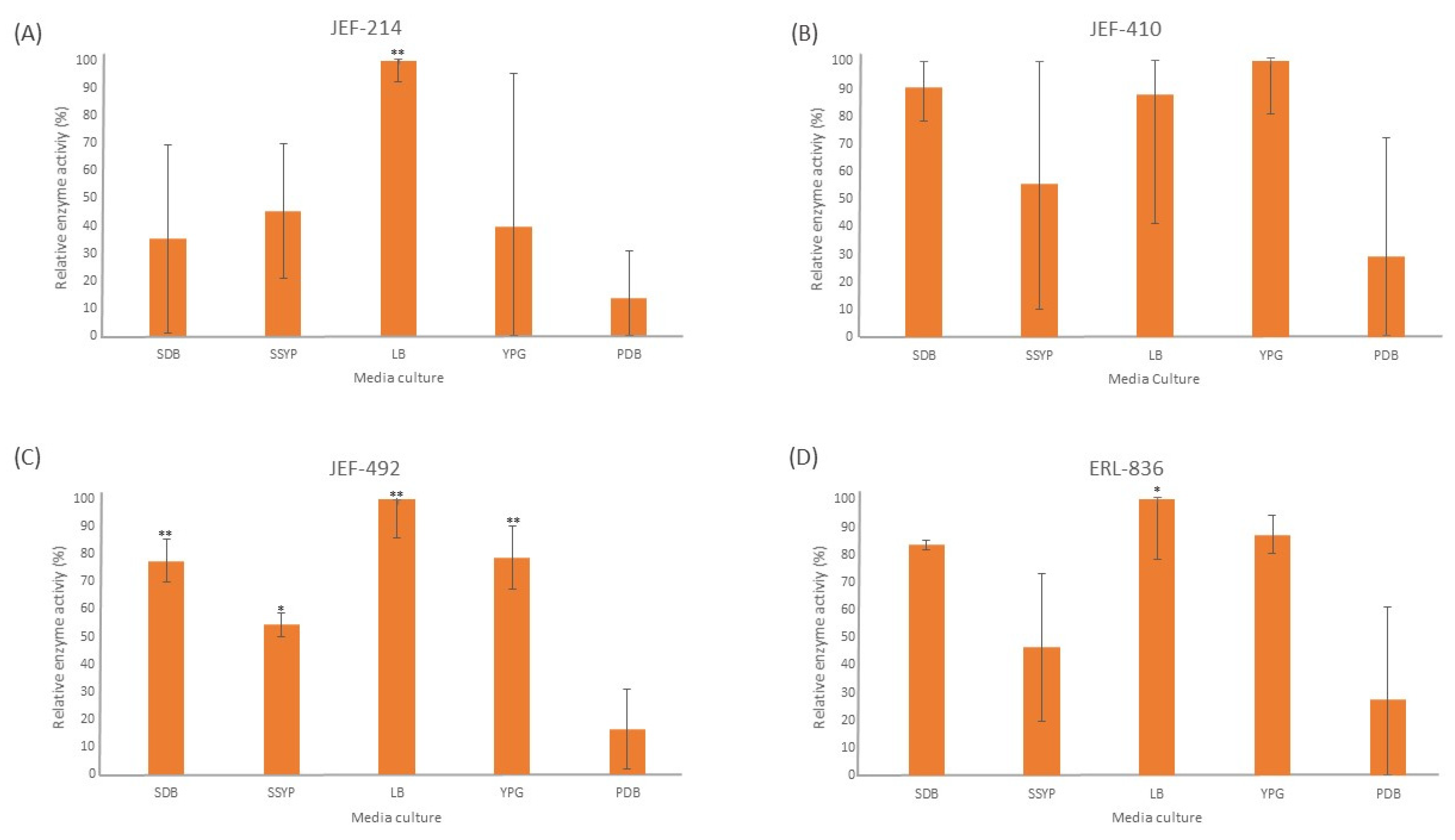
The liquid culture of all the fungal isolates demonstrated significant lipase activity in different cultured media (Figure 2). The LB medium induced a high lipase activity in all the isolates tested, and four out of five isolates differed significantly (p < 0.05) from the PDB medium. The isolate JEF-410 was the only isolate with a similar lipase activity in different culture media. The isolate JEF-492 was the only one with a significantly different lipase activity in the SDB, LB, YPG (p < 0.01), and SSYP (p < 0.05) culture media compared to the activity in the PDB medium.
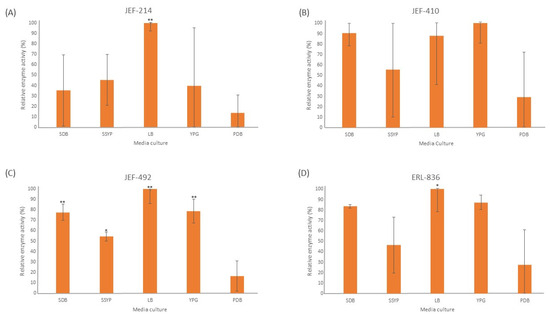
Figure 2.
Activity of extracellular lipases. The isolates of Beauveria bassiana and Metarhizium anisopliae were cultured in SDB (Sabouraud dextrose broth), SSYP (soluble starch–yeast extract–peptone), LB (Luria–Bertani), YPG (yeast extract–peptone–glucose), and PDB (potato dextrose broth) by liquid culture. (A) M. anisopliae JEF-214; (B) B. bassiana JEF-410; (C) B. bassiana JEF-492; and (D) B. bassiana ERL-836. The cultures were cultivated for 7 days and then filtered and centrifuged to obtain a cell-free supernatant. The vertical bars correspond to the standard error. * p < 0.05 and ** p < 0.01 according to the Tukey’s Test.
3.2. Thermostability of Supernatant
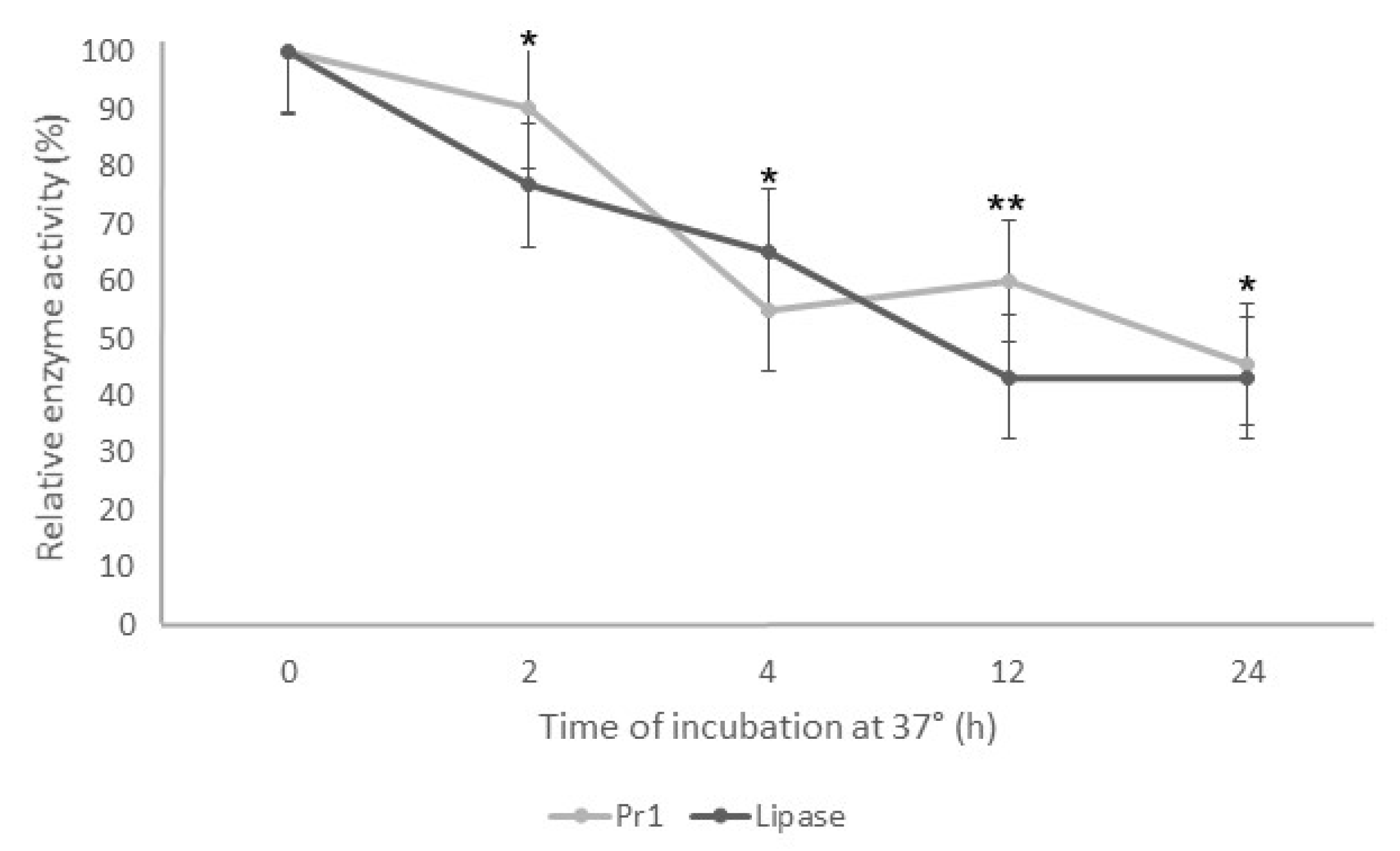
The enzymatic assays decreased the Pr1 and lipase activity when the supernatant was incubated at 37 °C. As the incubation time increased, the enzymatic activity decreased for both enzymes (Figure 3). After 2 h of incubation, both enzymes showed relative activity around 80% and 50% after 4 h. Despite the fast reduction in the protease and lipase activity in the first 4 h incubation, there was a slight difference from 4 to 24 h, with approximately 45% relative activity for both enzymes.
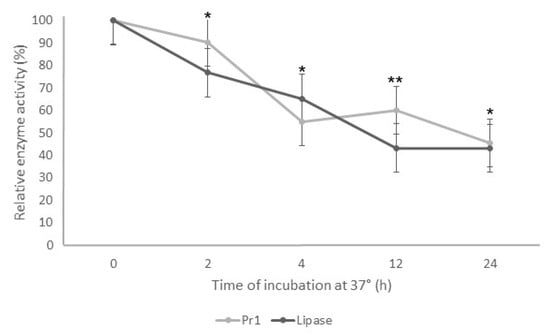
Figure 3.
Thermostability of Pr1 and lipases produced by Beauveria bassiana JEF-492 after incubation at 37 °C. The isolate was grown in a Luria–Bertani broth medium by liquid culture for 7 days. The supernatant was incubated at 37 °C for 2, 4, 12, or 24 h, and then the protease and lipase activity was evaluated. * p < 0.05 and ** p < 0.01 according to the Tukey’s Test.
3.3. Virulence
3.3.1. Aphis gossypii
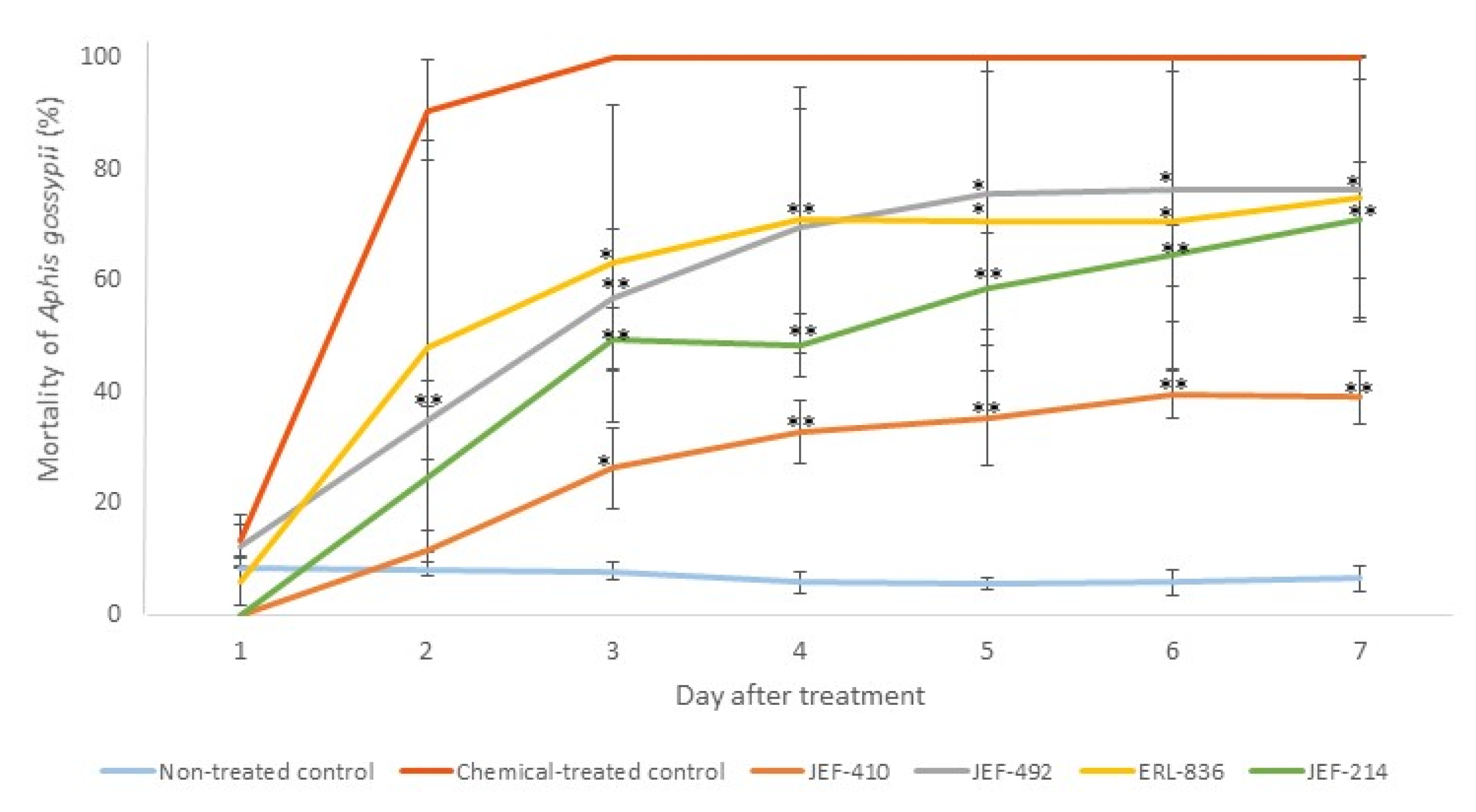
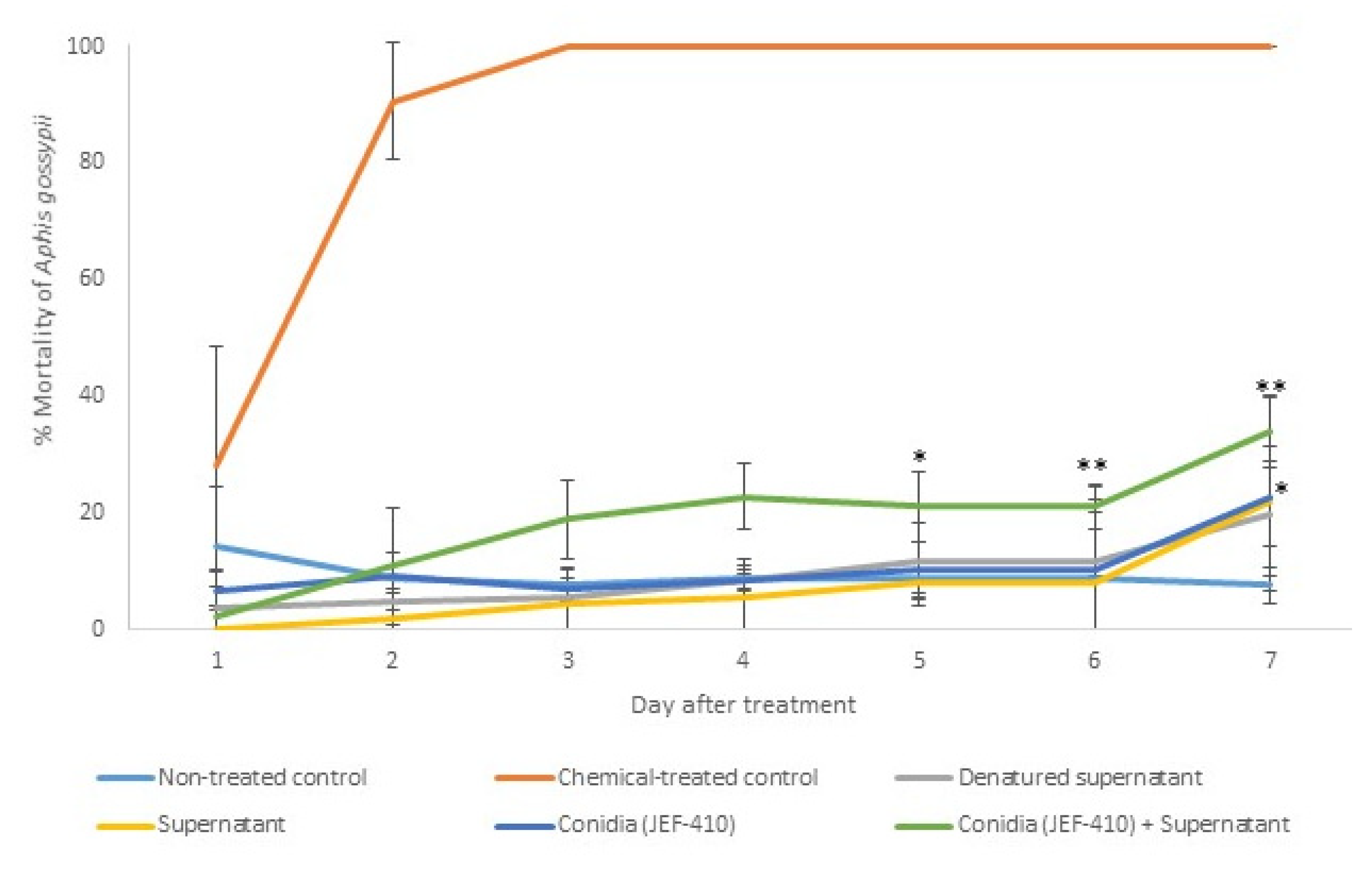
None of the five isolates caused 100% mortality of the A. gossypii. The isolate JEF-410, however, demonstrated the lowest virulence against aphids (Figure 4).
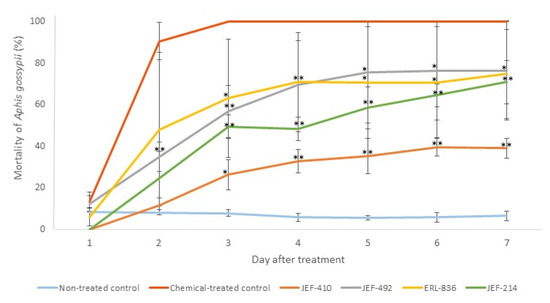
Figure 4.
Mortality of Aphis gossypii treated with Beauveria bassiana and Metarhizium anisopliae. The nymphs of the aphids were treated with 1 mL of 1 × 107 conidia mL−1 from the B. bassiana isolates JEF-410, JEF-492, and ERL-836 and the M. anisopliae isolate JEF-214. The isolates were inoculated in one-quarter of SDA for the conidial production. The negative control was 0.03% Silwet (the non-treated control), and the positive control was Imidacloprid (SC) (2000×) (the chemical-treated control). The fungi treatments were only compared to the non-treated control. The analysis was conducted each day after the treatment. * p < 0.05 and ** p < 0.01 according to the Tukey’s Test.
The isolate JEF-410 was selected to investigate the supernatant’s potential to improve fungal virulence and to evaluate whether the supernatant could reduce the mortality rate. The treatment with combined conidia and active supernatant was the only treatment that significantly differed from the negative control 5 and 6 days after the treatment (p < 0.05 and p < 0.01, respectively). Seven days after the treatment, the groups treated with conidia and conidia + supernatant had a higher mortality than the negative control (p < 0.05). The treatments with supernatant combined with fungal conidia caused mortality rates below 50%. The differences between the negative and positive control and the infection caused by JEF-410 are shown in Figure 5.
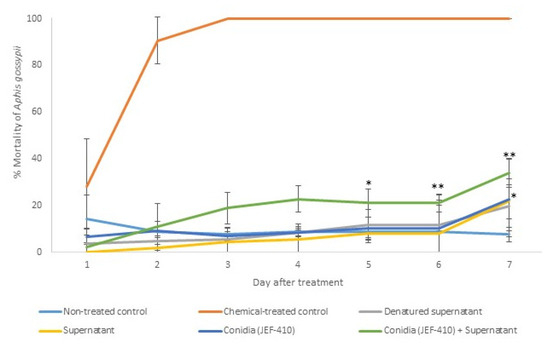
Figure 5.
Mortality of Aphis gossypii nymphs treated with Beauveria bassiana JEF-410. The B. bassiana JEF-410 was inoculated in one-quarter of SDA culture medium for the conidial production and the LB broth for the supernatant production. Six treatments were prepared: negative control—0.03% Silwet (non-treated control); positive control—Imidacloprid (SC) (2000×) (chemical-treated control); denatured supernatant (DS); supernatant (S); conidia (C); and conidia + supernatant (CS). One milliliter of the treatment was sprayed over ten nymphs of the aphids, and the number of dead aphids was evaluated daily. The mortality was calculated by the proportion of dead aphids compared to the total number of aphids. The treatments were only compared to the non-treated control. The analysis was conducted each day after the treatment. * p < 0.05 and ** p < 0.01 according to the Tukey’s Test.
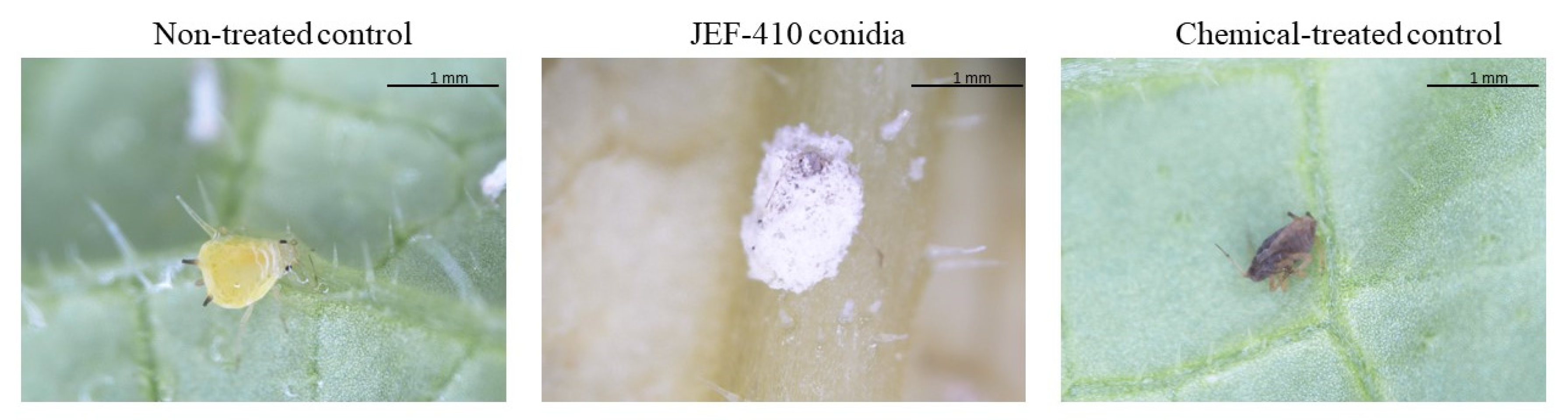
The difference between the treatment with conidia and the synthetic pesticide is demonstrated in Figure 6.
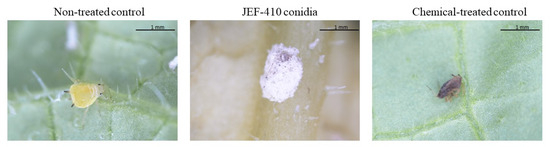
Figure 6.
Aphis gossypii 7 days after treatment with Beauveria bassiana JEF-410. The aphid nymphs were treated with 1 mL of different suspensions: the non-treated control (0.03% Silwet), chemical-treated control (Imidacloprid 10% suspension concentrate (SC) (2000×), and conidia (1 × 107 conidia mL−1). The B. bassiana JEF-410 was cultured on one-quarter of SDA medium for conidial production.
3.3.2. Spodoptera frugiperda
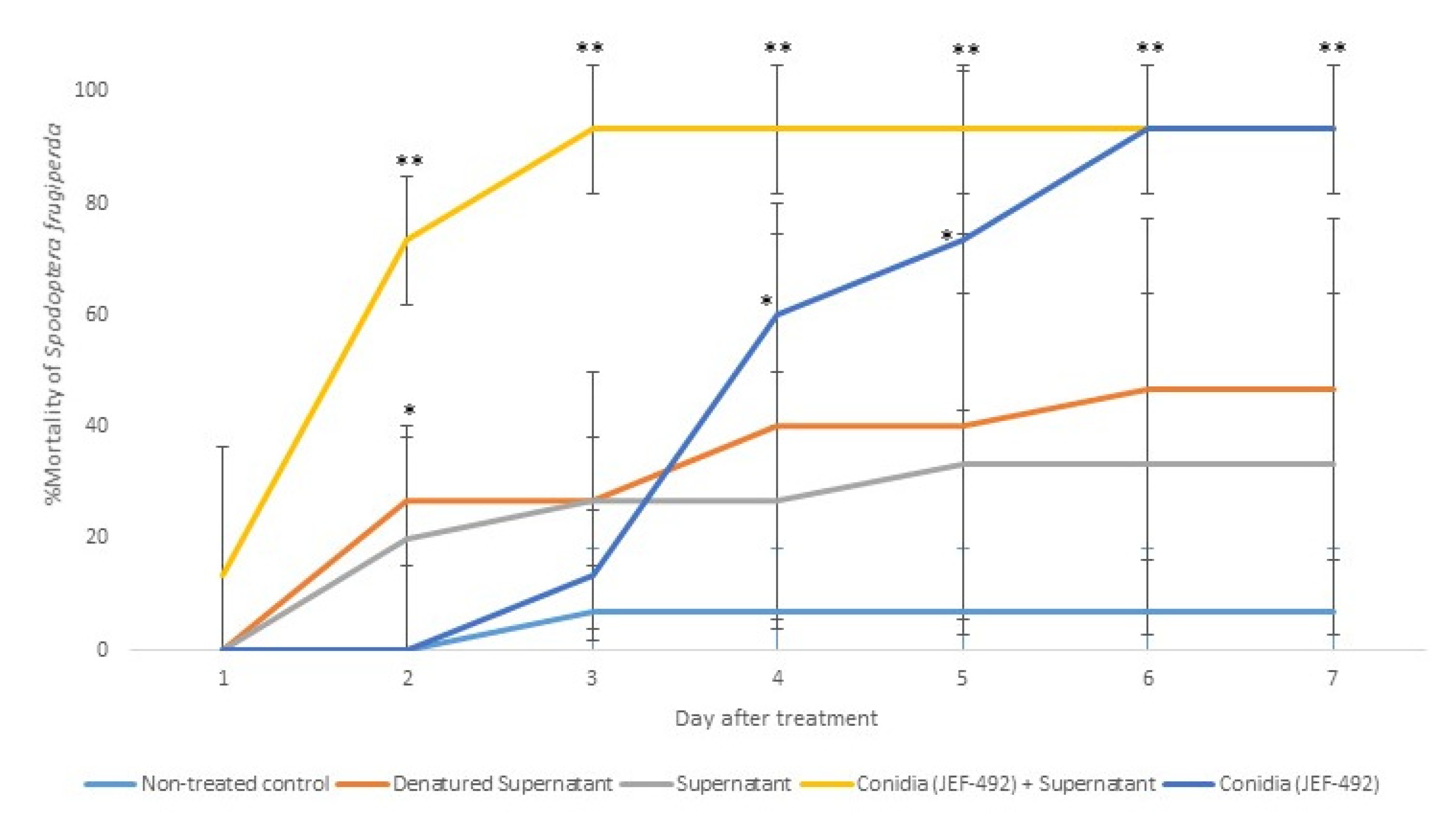
Previous laboratory studies demonstrated that the isolate JEF-492 is virulent against S. frugiperda [23]. Therefore, we evaluated the supernatant’s potential to increase the fungal virulence. The treatment with conidia + supernatant resulted in higher and faster mortality than using only conidia (Figure 7).
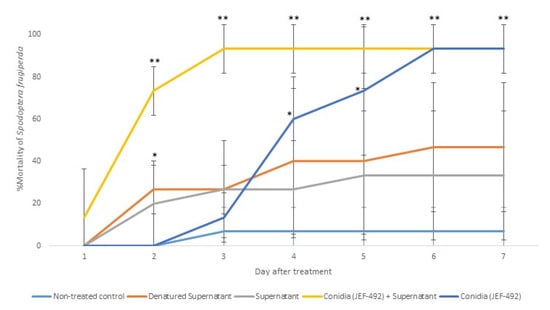
Figure 7.
Mortality of Spodoptera frugiperda larvae treated with Beauveria bassiana JEF-492. The B. bassiana JEF-492 was inoculated on one-quarter of SDA medium for the conidial production and the LB broth for the supernatant production. Five treatments were prepared: non-treated control (0.03% Silwet); denatured supernatant (DS); supernatant (S); conidia (C); conidia + supernatant (CS). One milliliter of the treatment was sprayed over five-second instar larvae, and the number of dead larvae was evaluated daily. The mortality was calculated by the proportion of dead larvae compared to the total number of larvae. The conidia and supernatant treatments were only compared to the non-treated control. The analysis was conducted each day after the treatment. * p < 0.05 and ** p < 0.01 according to the Tukey’s Test.
The group treated with conidia and supernatant showed fungal sporulation in a shorter period than those treated only with conidia (Figure 8). Although the denatured supernatant and supernatant had significant mortality only on day 2 after the treatment, the dead larvae showed a dark brown color. This suggests a possible secondary metabolite toxicity causing larval mortality in both treatments. The treatments with conidia + supernatant caused significant mortality starting on day 2 and sporulation on day 4. By day 6 after treatment, the fungal mycelium covered the cadaver completely.

Figure 8.
Spodoptera frugiperda larvae after treatment with Beauveria bassiana JEF-492. Five second instar larvae were treated by spraying with 1 mL of different suspensions: the non-treated control (0.03% Silwet); supernatant; conidia (1 × 107 conidia mL−1), or the conidia suspended in supernatant (1 × 107 conidia mL−1). Four days after the treatment, the larvae treated with the supernatant showed a dark brown coloration. The conidia treatment showed dead larvae but no mycelium growing over the insect cadaver. The treatment with conidia + supernatant showed dead larvae and mycelium growing over the insect cadaver. The insect treated with conidia or conidia + supernatant showed growing fungal mycelium on day 6 after treatment.
4. Discussion
The culture media impact fungal growth differently. The Luria–Bertani broth medium induced a high quantity of extracellular enzymes, such as Pr1 protease and lipases, for Beauveria bassiana and Metarhizium anisopliae isolates [19]. Different fungal isolates produce different quantities of enzymes in the same liquid culture media, so it is crucial to examine the impact of the culture media on each fungal isolate to optimize enzyme production.
When the fungus is cultivated in liquid culture media containing simple compounds, such as carbohydrates, it does not induce the production of enzymes involved in the infection process. In contrast, a medium that does not offer simple carbohydrates induces the production of extracellular enzymes [26]. Starvation conditions and complex compounds in the culture medium enhance the virulence of entomopathogenic fungi and induce the production of enzymes, such as chitinases, proteases, and lipases [26,27].
Fungal isolates may exhibit a distinct virulence towards specific insect species. The insect cuticle serves as the primary barrier against the penetration of external compounds. It consists of different layers of lipids, proteins, hydrocarbons, and chitin fibers. The cuticle composition, however, varies according to age, sex, insect order, and environmental conditions [28]. The virulence of the fungal isolates depends on the ability to produce different isoforms capable of degrading the host cuticle, especially the protease Pr1, which contains 11 isoforms [10,29]. In this study, a screening was performed to evaluate the virulence of different fungal isolates against A. gossypii nymphs. The least virulent isolate, JEF-410, was selected to assess whether the treatment with conidia associated with their supernatant-containing enzymes would increase insect mortality; however, the highest mortality did not reach 50% after 7 days. Regardless of the number of enzymes applied in combined treatments with conidia, no significant increased mortality of A. gossypii nymphs was detected.
The opposite strategy was applied to treat S. frugiperda. B. bassiana JEF-492, a previously known virulent isolate, was tested in this study. In this case, when the conidia were associated with the supernatant, there was a significant reduction in the host infection time compared to the conidia treatment. This result reinforces the effectiveness of the enzyme isoforms in degrading the hosts’ cuticle. An increase in the quantity of these isoforms facilitated the infection process of the B. bassiana JEF-492 against the S. frugiperda larva.
The supernatant (i.e., crude extract or crude enzymes) from the fungal fermentation has the potential to be used as biopesticides [7]. The supernatant contains several molecules, including enzymes, other proteins, and secondary metabolites. All these components can contribute to the toxicity of the supernatant against insects. The insecticidal activity against S. litura larvae of the supernatant from the B. bassiana fermentation has already been demonstrated. The toxicity of the supernatant from the fungal fermentation was possibly due to the enzymes and metabolites [30]. This proposition is supported by studies that showed that the protein extract from the supernatant of entomopathogenic fungi causes lysis in the midgut epithelium and deterioration of the microvilli in S. littoralis larvae [31]. All these data are consistent with the results obtained in this current study, which demonstrated a decreased time of infection of the S. frugiperda larvae treated with the B. bassiana conidia combined with the supernatant from the fungal fermentation than the larvae treated with conidia only. This reinforces that the supernatant from the fungal fermentation can be used for the biological control of pests without requiring enzyme purification.
One of the biggest challenges of applying fungal enzymes for pest control is their low thermostability. This present study demonstrated that Pr1 and lipases still have high activity—around 80%—after 2 h at 37 °C. This is compatible with the results obtained from a previous study [32], which demonstrated that fungal alkaline protease had more than 80% residual activity after exposure at 40 °C for a 1 h incubation. Nonetheless, the residual activity of alkaline proteases decreased to 40% at 50 °C. Entomopathogenic fungi, in general, have an optimum radial growth between 25 °C and 30 °C—with a maximum thermal threshold at 37 °C for B. bassiana [33,34]. Considering that conidia are successfully applied in the field, the temperature in the application area is below 40 °C, indicating that the enzymes would not be denatured immediately. These findings suggest that the enzymes Pr1 and lipases exhibit substantial activity at 37 °C after 2 h and maintain significant activity after 24 h. This may be sufficient time to cause damage to the cuticle of immature insects, as observed for the second instar larvae of S. frugiperda. Additionally, a proper formulation can stabilize the enzyme activity at high temperatures for extended periods. This can be achieved, for example, using calcium chloride and glycine [35] or deep eutectic solvents [36].
5. Conclusions
The results of this study demonstrated that the extracellular enzymes produced by entomopathogenic fungi can be combined with conidia to reduce the time of infection, as demonstrated for S. frugiperda larvae. Moreover, our results indicate that the supernatant from the fermentation of entomopathogenic fungi has the potential to be used in new biopesticide formulations, providing efficient strategies for the biological control of insect pests.
6. Patents
All the isolates evaluated in this work are patented in Republic of Korea. The patent registration numbers are JEF-410: KR 10-2105252; ERL-836: KR 10-1626801; and JEF-214: KR 10-2054577; and the patent application number is JEF-492: KR 10-2022-0074643.
Author Contributions
Conceptualization, J.M.F., F.E.F.S. and J.S.K.; methodology, J.M.F.; validation, F.E.F.S., É.K.K.F. and J.S.K.; writing—original draft preparation, J.M.F.; writing—review and editing, J.M.F., F.E.F.S., É.K.K.F. and J.S.K.; and supervision, F.E.F.S., É.K.K.F. and J.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through Coordination for the Improvement of Higher Education Personnel (CAPES), which supported the exchange doctoral program (#10/2022) of J.M. Ferreira. CAPES also provided a PhD scholarship for J.M. Ferreira (Finance code 001). The National Council for Scientific and Technological Development (CNPq) of Brazil provided grant PQ 308375/2022-0 for É.K.K. Fernandes, and the Minas Gerais Research Funding Foundation (FAPEMIG) provided grant APQ-02466–21 for F.E.F. Soares. Furthermore, this work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ015927)”, Rural Development Administration, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank our colleagues from the Insect Microbiology & Biotechnology Laboratory (JBNU), especially the graduate students, for their assistance with insect rearing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef] [PubMed]
- Mseddi, J.; Ben Farhat-Touzri, D.; Azzouz, H. Selection and characterization of thermotolerant Beauveria bassiana isolates and with insecticidal activity against the cotton-melon aphid Aphis gossypii (Glover) (Hemiptera: Aphididae). Pest Manag. Sci. 2022, 78, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in Control Strategies against Spodoptera frugiperda. A Review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Lopes, R.B.; Delalibera Junior, I.; Fernandes, E.K.K.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrate and microorganisms: Plenty of new opportunities. BioControl 2017, 63, 39–59. [Google Scholar] [CrossRef]
- Alves, S.B.; Almeida, J.E.M.; Moino, A., Jr.; Alves, L.F.A. Técnicas de laboratório 637-771. In Controle Microbiano de Insetos; Alves, S.B., Ed.; Fealq: Piracicaba, Brazil, 1998; p. 1163. [Google Scholar]
- Ferreira, J.M.; Soares, F.E.F. Entomopathogenic fungi hydrolytic enzymes: A new approach to biocontrol? J. Nat. Pestic. Res. 2023, 3, 100020. [Google Scholar] [CrossRef]
- Pedrini, N. The Entomopathogenic Fungus Beauveria bassiana Shows Its Toxic Side within Insects: Expression of Genes Encoding Secondary Metabolites during Pathogenesis. J. Fungi 2022, 8, 488. [Google Scholar] [CrossRef]
- Mondal, S.; Baksi, S.; Koris, A.; Vatai, G. Journey of enzymes in entomopathogeni fungi. Pac. Sci. 2016, 18, 85–99. [Google Scholar] [CrossRef]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae enzymes and toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef]
- Rosa-Garcia, N.M.; López-Barrera, G.L.; Mireles-Martínez, M.; Villegas-Mendoza, J.M. Metarhizium anisopliae Pr1 Isoform Activation during Pathogenesis in Diaphorina citri. Southwest. Entomol. 2018, 43, 199–207. [Google Scholar] [CrossRef]
- Khun, K.K.; Wilson, B.A.L.; Stevens, M.M.; Huwer, R.K.; Ash, G.J. Integration of entomopathogenic fungi into IPM programs: Studies involving Weevils (Coleoptera: Curculionoidea) affecting horticultural crops. Insects 2020, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.H.A.; Mohammed, S.H.; Eltaly, R.I.; Moustafa, M.A.M.; Fónagy, A.; Farag, S.M. Co-application of entomopathogenic fungi with chemical insecticides against culex pipiens. J. Invertebr. Pathol. 2023, 198, 107916. [Google Scholar] [CrossRef] [PubMed]
- Vidhate, R.; Singh, J.; Ghormade, V.; Chavan, S.B.; Patil, A.; Deshpande, M.V. Use of hydrolytic enzymes of Myrothecium verrucaria and conidia of Metarhizium anisopliae, singly and sequentially to control pest and pathogens in grapes and their compatibility with pesticides used in the field. Biopestic. Int. 2015, 11, 48–60. [Google Scholar]
- Zhang, P.; Zhang, X.; Zhao, Y.; Ren, Y.; Mu, W.; Liu, F. Efficacy of granular applications of clothianidin and nitenpyram against Aphis gossypii (Glover) and Apolygus lucorum (Meyer-Dür) in cotton fields in China. Crop Prot. 2015, 78, 27–34. [Google Scholar] [CrossRef]
- Ma, K.S.; Tang, Q.L.; Liang, P.Z.; Li, J.H.; Gao, X.W. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 2022, 21, 2055–2064. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- FAO. The Global Action for Fall Armyworm Control: Action Framework 2020–2022. Working Together to Tame the Global Threat; Food and Agriculture Organization of United Nations: Rome, Italy, 2020.
- Kim, J.C.; Lee, M.R.; Kim, S.; Lee, S.J.; Park, S.E.; Nai, Y.S.; Lee, G.S.; Shin, T.Y.; Kim, J.S. Tenebrio molitor-mediated entomopathogenic fungal library construction for pest management. J. Asia-Pac. Entomol. 2018, 21, 196–204. [Google Scholar] [CrossRef]
- Campos, R.A.; Arruda, W.; Boldo, J.T.; Silva, M.V.; Barros, N.M.; Azevedo, J.L.; Schrank, A.; Vainstein, M.H. Boophilus microplus infection by Beauveria amorpha and Beauveria bassiana: SEM analysis and regulation of subtilisin-like proteases and chitinases. Curr. Microbiol. 2005, 50, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, T.; Ninomiya, H.; Okuda, H. P-nitrophenyl butyrate hydrolyzing activity of hormone-sensitive lipase from bovine adipose tissue. J. Lipid. Res. 1989, 30, 997–1004. [Google Scholar] [CrossRef]
- Im, Y.; Park, S.-E.; Lee, S.Y.; Kim, J.-C.; Kim, J.S. Early-Stage Defense Mechanism of Cotton Aphid Aphis gossypii against infection with the insect-killing fungus Beauveria bassiana JEF-544. Front. Immunol. 2022, 13, 907088. [Google Scholar] [CrossRef]
- Jeon, I.; Kim, J.S. Soil treatment with Beauveria and Metarhizium to control fall armyworm, Spodoptera frugiperda, during the soil-dwelling stage. J. Asia-Pac. Entomol. 2024, 27, 102193. [Google Scholar] [CrossRef]
- Jung, Y.; Yang, H.; Lee, I.-Y.; Yong, T.-S.; Lee, S. Core/Sheath-Structured Composite Nanofibers Containing Cinnamon Oil: Their Antibacterial and Antifungal Properties and Acaricidal Effect against House Dust Mites. Polymers 2020, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Ayres Júnior, M.; Ayres, D.L.; Santos, A.A. Bioestat—Aplicações Estatísticas nas Áreas das Ciências Bio-Médicas; Ong Mamirauá: Belém, PA, Brazil, 2007. [Google Scholar]
- Montesinos-Matías, R.; Ordaz-Hernández, A.; Angel-Cuapio, A.; Colin-Bonifacio, Y.; Garcia-Garcia, R.E.; Ángel-Sahagún, C.A.; Arredondo-Bernal, H.C. Principal component analysis of the biological characteristics of entomopathogenic fungi in nutrient-limited and cuticle-based media. J. Basic Microbiol. 2021, 61, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Wang, C.S.; Butt, T.M. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Rev. 2005, 251, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Andreis, F.C.; Schrank, A.; Thompson, C.E. Molecular evolution of Pr1 proteases depicts ongoing diversification in Metarhizium spp. Mol. Genet. Genom. 2019, 294, 901–917. [Google Scholar] [CrossRef] [PubMed]
- Ayudya, D.R.; Herlinda, S.; Suwandi, S. Insecticidal activity of culture filtrates from liquid medium of Beauveria bassiana isolates from South Sumatra (Indonesia) wetland soil against larvae of Spodoptera litura. Biodivers. J. 2019, 20, 8. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Carrasco-Díaz, J.-A.; Santiago-Álvarez, C. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae). J. Appl. Entomol. 2006, 130, 442–452. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Laxman, R.S. Enhanced thermostability of a fungal alkaline protease by different additives. Enzyme Res. 2014, 2014, 109303. [Google Scholar] [CrossRef]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Rougier, M. Effect of temperature on vegetative growth of Beauveria bassiana isolates from different origins. Mycologia 1997, 89, 389–392. [Google Scholar] [CrossRef]
- Fargues, J.; Maniania, N.K.; Delmas, J.C.; Smits, N. Influence de la température sur la croissance in vitro d’hyphomycètes entomopathogènes. Agronomie 1992, 12, 557–564. [Google Scholar] [CrossRef]
- Bhosale, S.H.; Rao, M.B.; Deshpande, V.V.; Srinivasan, M.C. Thermostability of high-activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20). Enzyme Microb. Technol. 1995, 17, 136–139. [Google Scholar] [CrossRef]
- Lin, K.P.; Feng, G.J.; Pu, F.L.; Hou, X.D.; Cao, S.L. Enhancing the Thermostability of Papain by Immobilizing on Deep Eutectic Solvents-Treated Chitosan with Optimal Microporous Structure and Catalytic Microenvironment. Front. Bioeng. Biotechnol. 2020, 8, 576266. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).