Impact of Nitric Oxide-Release Kinetics on Antifungal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antifungals
2.3. Fungal Strains
2.4. Synthesis of Small-Molecule NO-Releasing Compounds
2.5. Characterization of NO Release
2.6. Susceptibility Assays
2.7. Checkerboard Assays
2.8. Passaging Assays
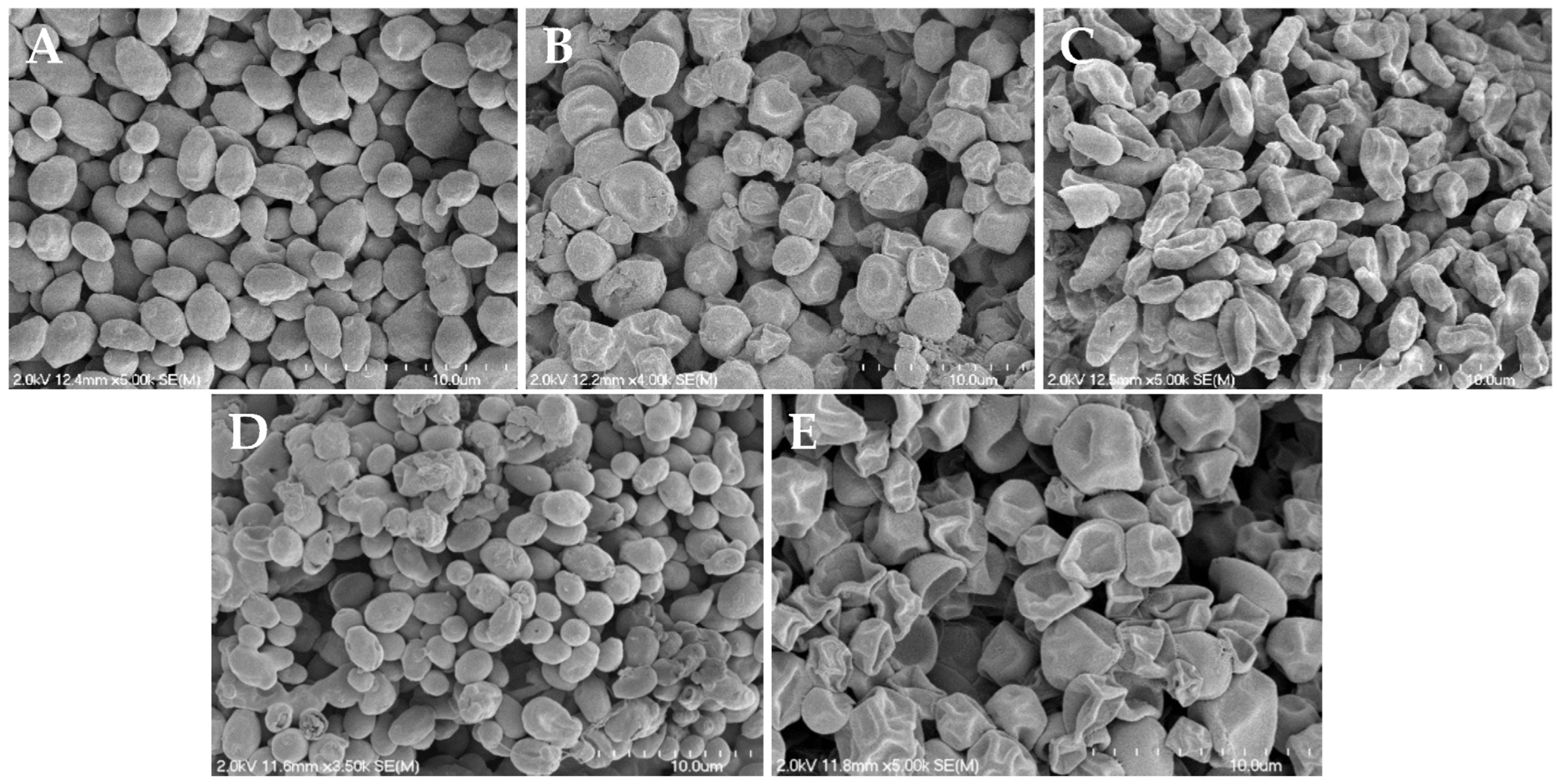
2.9. Scanning Electron Microscopy
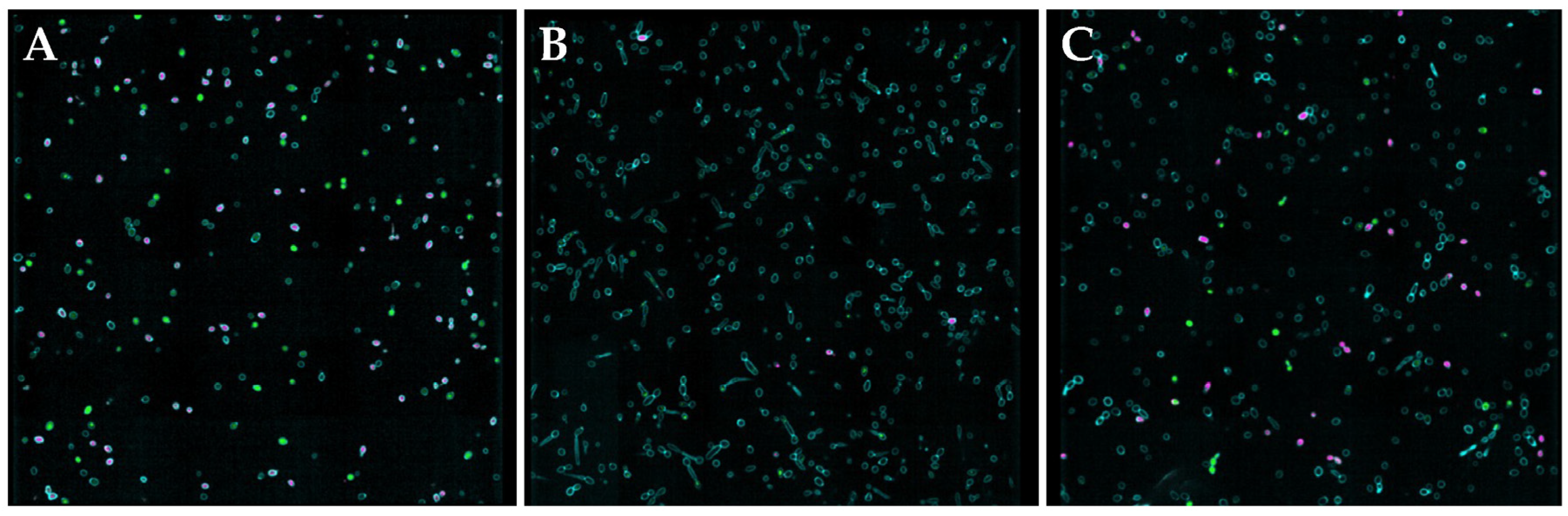
2.10. Confocal Microscopy
2.11. Tissue Viability Assays
2.12. Statistical Analysis
3. Results
3.1. Nitric Oxide-Releasing Small Molecules Exhibit Broad Spectrum Antifungal Activity
3.2. Resistance to NO Avoided at Repeated Sub-Lethal Dose Exposure
3.3. Nitric Oxide Treatment Is Compatible with Current Antifungals
3.4. Reduced Hyphae Formation and Surface Morphology Changes
3.5. Tissue Viability and Determination of Susceptibility Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasim, S.; Coleman, J.J. Targeting the Fungal Cell Wall: Current Therapies and Implications for Development of Alternative Antifungal Agents. Future Med. Chem. 2019, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Fausto, A.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar]
- Ksiezopolska, E.; Gabaldón, T. Evolutionary Emergence of Drug Resistance in Candida Opportunistic Pathogens. Genes 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal Infections in Humans: The Silent Crisis. Microb. Cell 2020, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical Implications of Globally Emerging Azole Resistance in Aspergillus Fumigatus. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150460. [Google Scholar] [CrossRef] [PubMed]
- Jeanvoine, A.; Rocchi, S.; Bellanger, A.P.; Reboux, G.; Millon, L. Azole-Resistant Aspergillus Fumigatus: A Global Phenomenon Originating in the Environment? Med. Mal. Infect. 2020, 50, 389–395. [Google Scholar] [CrossRef] [PubMed]
- C. neoformans Infection|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/index.html (accessed on 25 May 2023).
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 5 November 2022).
- Candida auris|Candida auris|Fungal Diseases| CDC. Available online: https://www.cdc.gov/fungal/candida-auris/index.html (accessed on 5 November 2022).
- Dixon, D.M.; Walsh, T.J. Antifungal Agents. Med. Microbiol. 1996, 8, 404. [Google Scholar]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, Mechanism of Action and Use in Antifungal Therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.J.; Dankert, J. Flucytosine: A Review of Its Pharmacology, Clinical Indications, Pharmacokinetics, Toxicity and Drug Interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Wall, G.; Lopez-Ribot, J.L. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin Primarily Kills Yeast by Simply Binding Ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, B.; Bonio, K.; Dabrowski, W.; Pietrzak, D.; Niewiadomy, A.; Olender, A.; Malodobry, K.; Gagoś, M. Synergistic Antifungal Interactions of Amphotericin B with 4-(5-Methyl-1,3,4-Thiadiazole-2-Yl) Benzene-1,3-Diol. Sci. Rep. 2019, 9, 12945. [Google Scholar] [CrossRef] [PubMed]
- Boyce, K.J.; Andrianopoulos, A. Fungal Dimorphism: The Switch from Hyphae to Yeast Is a Specialized Morphogenetic Adaptation Allowing Colonization of a Host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Overview of Fungal Infections—Infections—Merck Manuals Consumer Version. Available online: https://www.merckmanuals.com/home/infections/fungal-infections/overview-of-fungal-infections (accessed on 27 January 2022).
- Treatment and Management of Infections and Colonization|Candida auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html (accessed on 5 May 2022).
- Antifungal Susceptibility Testing and Interpretation Candida auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 10 May 2022).
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef] [PubMed]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Rouillard, K.R.; Novak, O.P.; Pistiolis, A.M.; Yang, L.; Ahonen, M.J.R.; McDonald, R.A.; Schoenfisch, M.H. Exogenous Nitric Oxide Improves Antibiotic Susceptibility in Resistant Bacteria. ACS Infect. Dis. 2021, 7, 23–33. [Google Scholar] [CrossRef]
- Maloney, S.E.; Mcgrath, K.V.; Ahonen, M.J.R.; Soliman, D.S.; Feura, E.S.; Hall, H.R.; Wallet, S.M.; Maile, R.; Schoenfisch, M.H. Nitric Oxide-Releasing Hyaluronic Acid as an Antibacterial Agent for Wound Therapy. Biomacromolecules 2020, 22, 867–879. [Google Scholar] [CrossRef]
- Stasko, N.; McHale, K.; Hollenbach, S.J.; Martin, M.; Doxey, R. Nitric Oxide-Releasing Macromolecule Exhibits Broad-Spectrum Antifungal Activity and Utility as a Topical Treatment for Superficial Fungal Infections. Antimicrob. Agents Chemother. 2018, 62, e01026-17. [Google Scholar] [CrossRef]
- Macherla, C.; Sanchez, D.A.; Ahmadi, M.S.; Vellozzi, E.M.; Friedman, A.J.; Nosanchuk, J.D.; Martinez, L.R. Nitric Oxide Releasing Nanoparticles for Treatment of Candida Albicans Burn Infections. Front. Microbiol. 2012, 3, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, X.; Liu, S.; Hu, Y.; Ma, D. Near-Infrared Light-Triggered Nitric Oxide Release Combined with Low-Temperature Photothermal Therapy for Synergetic Antibacterial and Antifungal. Smart Mater. Med. 2021, 2, 302–313. [Google Scholar] [CrossRef]
- Madariaga-Venegas, F.; Fernández-Soto, R.; Duarte, L.F.; Suarez, N.; Delgadillo, D.; Jara, J.A.; Fernández-Ramires, R.; Urzia, B.; Molina-Berríos, A. Characterization of a Novel Antibiofilm Effect of Nitric Oxide-Releasing Aspirin (NCX-4040) on Candida Albicans Isolates from Denture Stomatitis Patients. PLoS ONE 2017, 12, e0176755. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Cruz, N.; Reitzel, R.A.; Rosenblatt, J.; Chaftari, A.M.; Dib, R.W.; Hachem, R.; Kontoyiannis, D.P.; Raad, I.I. Nitroglycerin-Citrate-Ethanol Catheter Lock Solution Is Highly Effective for In Vitro Eradication of Candida Auris Biofilm. Antimicrob. Agents Chemother. 2019, 63, e00299-19. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Shin, J.H.; Stasko, N.A.; Johnson, C.B.; Wespe, D.A.; Holmuhamedov, E.; Schoenfisch, M.H. Bactericidal Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano 2008, 2, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Dual Action Antimicrobials: Nitric Oxide Release from Quaternary Ammonium-Functionalized Silica Nanoparticles. Biomacromolecules 2012, 13, 3334–3342. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Reighard, K.P.; Saavedra, J.E.; Schoenfisch, M.H. O2-Protected Diazeniumdiolate-Modified Silica Nanoparticles for Extended Nitric Oxide Release from Dental Composites. Biomater. Sci. 2013, 1, 456–459. [Google Scholar] [CrossRef] [PubMed]
- McElhaney-Feser, G.E.; Raulli, R.E.; Cihlar, R.L. Synergy of Nitric Oxide and Azoles against Candida Species In Vitro. Antimicrob. Agents Chemother. 1998, 42, 2342. [Google Scholar] [CrossRef] [PubMed]
- CLSI M27; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. CLSI: Wayne, PA, USA, 2015.
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs for Antifungal Agents. Available online: https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals (accessed on 25 May 2023).
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and Simplified Method for Drug Combination Studies by Checkerboard Assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Salama, A.H. Study the Activity of Conjugated Antimicrobial Peptide WW-185 Against Clinically Important Bacteria. Pharmacia 2023, 70, 331–336. [Google Scholar] [CrossRef]
- Yang, L.; Schoenfisch, M.H. Nitric Oxide-Releasing Hyperbranched Polyaminoglycosides for Antibacterial Therapy. ACS Appl. Bio Mater. 2018, 1, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Storm, W.L.; Schoenfisch, M.H. Nitric Oxide-Releasing Xerogels Synthesized from N-Diazeniumdiolate-Modified Silane Precursors. ACS Appl. Mater. Interfaces 2013, 5, 4904–4912. [Google Scholar] [CrossRef]
- Reighard, K.P.; Schoenfisch, M.H. Antibacterial Action of Nitric Oxide-Releasing Chitosan Oligosaccharides against Pseudomonas Aeruginosa under Aerobic and Anaerobic Conditions. Antimicrob. Agents Chemother. 2015, 59, 6506–6513. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, K.R.; Markovetz, M.R.; Bacudio, L.G.; Hill, D.B.; Schoenfisch, M.H. Pseudomonas Aeruginosa Biofilm Eradication via Nitric Oxide-Releasing Cyclodextrins. ACS Infect. Dis. 2020, 6, 1940–1950. [Google Scholar] [CrossRef]
- Hrabie, J.A.; Keefer, L.K. Chemistry of the Nitric Oxide-Releasing Diazeniumdiolate (“nitrosohydroxylamine”) Functional Group and Its Oxygen-Substituted Derivatives. Chem. Rev. 2002, 102, 1135–1154. [Google Scholar] [CrossRef]
- Folk, D; Anderson, R.G.; Simons, J.K.; Ahonen, M.J.R.; McDonald, R.A. Nitric Oxide-Releasing Antibacterial Compounds, Formulations, and Methods Pertaining Thereto. International Patent WO 2021/158954 A1, 5 February 2021.
- Różalska, B.; Sadowska, B.; Budzyńska, A.; Bernat, P.; Różalska, S. Biogenic Nanosilver Synthesized in Metarhizium Robertsii Waste Mycelium Extract—As a Modulator of Candida Albicans Morphogenesis, Membrane Lipidome and Biofilm. PLoS ONE 2018, 13, e0194254. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Zhuge, Y.; Zhang, J.; Xu, K.; Zhang, Q.; Zhang, H.; Chen, H.; Chu, M.; Jia, C. Photodynamic Antifungal Activity of Hypocrellin a against Candida Albicans. Front. Microbiol. 2019, 10, 1810. [Google Scholar] [CrossRef]
- Zhou, X.; He, P. Improved Measurements of Intracellular Nitric Oxide in Intact Microvessels Using 4,5-Diaminofluorescein Diacetate. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H108. [Google Scholar] [CrossRef]
- Neilson, L.; Mankus, C.; Thorne, D.; Jackson, G.; DeBay, J.; Meredith, C. Development of an in Vitro Cytotoxicity Model for Aerosol Exposure Using 3D Reconstructed Human Airway Tissue; Application for Assessment of e-Cigarette Aerosol. Toxicol. In Vitro 2015, 29, 1952–1962. [Google Scholar] [CrossRef]
- Li, Z.; Lu, G.; Meng, G. Pathogenic Fungal Infection in the Lung. Front. Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef]
- Mota Fernandes, C.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Del Poeta, M. The Future of Antifungal Drug Therapy: Novel Compounds and Targets. Antimicrob. Agents Chemother. 2021, 65, e01719-20. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, D.; Marcos, J.F.; Marcos, A.T.; Strauss, J. Nitric Oxide in Fungi: Is There NO Light at the End of the Tunnel? In Current Genetics; Springer: Berlin/Heidelberg, Germany, 2016; pp. 513–518. [Google Scholar]
- Privett, B.J.; Nutz, S.T.; Schoenfisch, M.H. Efficacy of Surface-Generated Nitric Oxide against Candida Albicans Adhesion and Biofilm Formation. Biofouling 2010, 26, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The Toxicology of Inhaled Nitric Oxide. Toxicol. Sci. 2001, 59, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.S.; Lee, H.H.; Sanchez, D.A.; Friedman, A.J.; Tar, M.T.; Davies, K.P.; Nosanchuk, J.D.; Martinez, L.R. Sustained Nitric Oxide-Releasing Nanoparticles Induce Cell Death in Candida Albicans Yeast and Hyphal Cells, Preventing Biofilm Formation In Vitro and in a Rodent Central Venous Catheter Model. Antimicrob. Agents Chemother. 2016, 60, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Hrabie, J.A.; Klose, J.R.; Wink, D.A.; Keefer, L.K. New Nitric Oxide-Releasing Zwitterions Derived from Polyamines. J. Org. Chem. 1993, 58, 1472–1476. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.; Park, K.; Kim, M.; Hong, J. Sustained Nitric Oxide-Providing Small Molecule and Precise Release Behavior Study for Glaucoma Treatment. Mol. Pharm. 2020, 17, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.; Burmester, A.; Hipler, U.C.; Neumeister, C.; Götz, M.R.; Wiegand, C. Efficacy of Antifungal Agents against Fungal Spores: An In Vitro Study Using Microplate Laser Nephelometry and an Artificially Infected 3D Skin Model. Microbiologyopen 2022, 11, e1257. [Google Scholar] [CrossRef] [PubMed]
- Seidl, H.P.; Jäckel, A.; Müller, J.; Schaller, M.; Borelli, C.; Polak, A. Sporicidal Effect of Amorolfine and Other Antimycotics Used in the Therapy of Fungal Nail Infections. Mycoses 2015, 58, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, A.; Gow, N.A.R.; Brown, A.J.P. Nitric Oxide and Nitrosative Stress Tolerance in Yeast. Biochem. Soc. Trans. 2011, 39, 219–223. [Google Scholar] [CrossRef]
- Farrugia, G.; Balzan, R. Oxidative Stress and Programmed Cell Death in Yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef]
- Ullmann, B.D.; Myers, H.; Chiranand, W.; Lazzell, A.L.; Zhao, Q.; Vega, L.A.; Lopez-Ribot, J.L.; Gardner, P.R.; Gustin, M.C. Inducible Defense Mechanism against Nitric Oxide in Candida Albicans. Eukaryot. Cell 2004, 3, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The Regulation of Hyphae Growth in Candidaï¿¿albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Barugahare, A.A.; Lo, T.L.; Huang, C.; Schittenhelm, R.B.; Powell, D.R.; Beilharz, T.H.; Traven, A. A Metabolic Checkpoint for the Yeast-to-Hyphae Developmental Switch Regulated by Endogenous Nitric Oxide Signaling. Cell Rep. 2018, 25, 2244–2258.e7. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, P.; Sjollema, J.; Cammue, B.P.A.; Lagrou, K.; Berman, J.; d’Enfert, C.; Andes, D.R.; Arendrup, M.C.; Brakhage, A.A.; Calderone, R.; et al. Methodologies for In Vitro and In Vivo Evaluation of Efficacy of Antifungal and Antibiofilm Agents and Surface Coatings against Fungal Biofilms. Microb. Cell 2018, 5, 300. [Google Scholar] [CrossRef]
- Sadozai, S.K.; Khan, S.A.; Baseer, A.; Ullah, R.; Zeb, A.; Schneider, M. In Vitro, Ex Vivo, and In Vivo Evaluation of Nanoparticle-Based Topical Formulation against Candida Albicans Infection. Front. Pharmacol. 2022, 13, 1. [Google Scholar] [CrossRef]
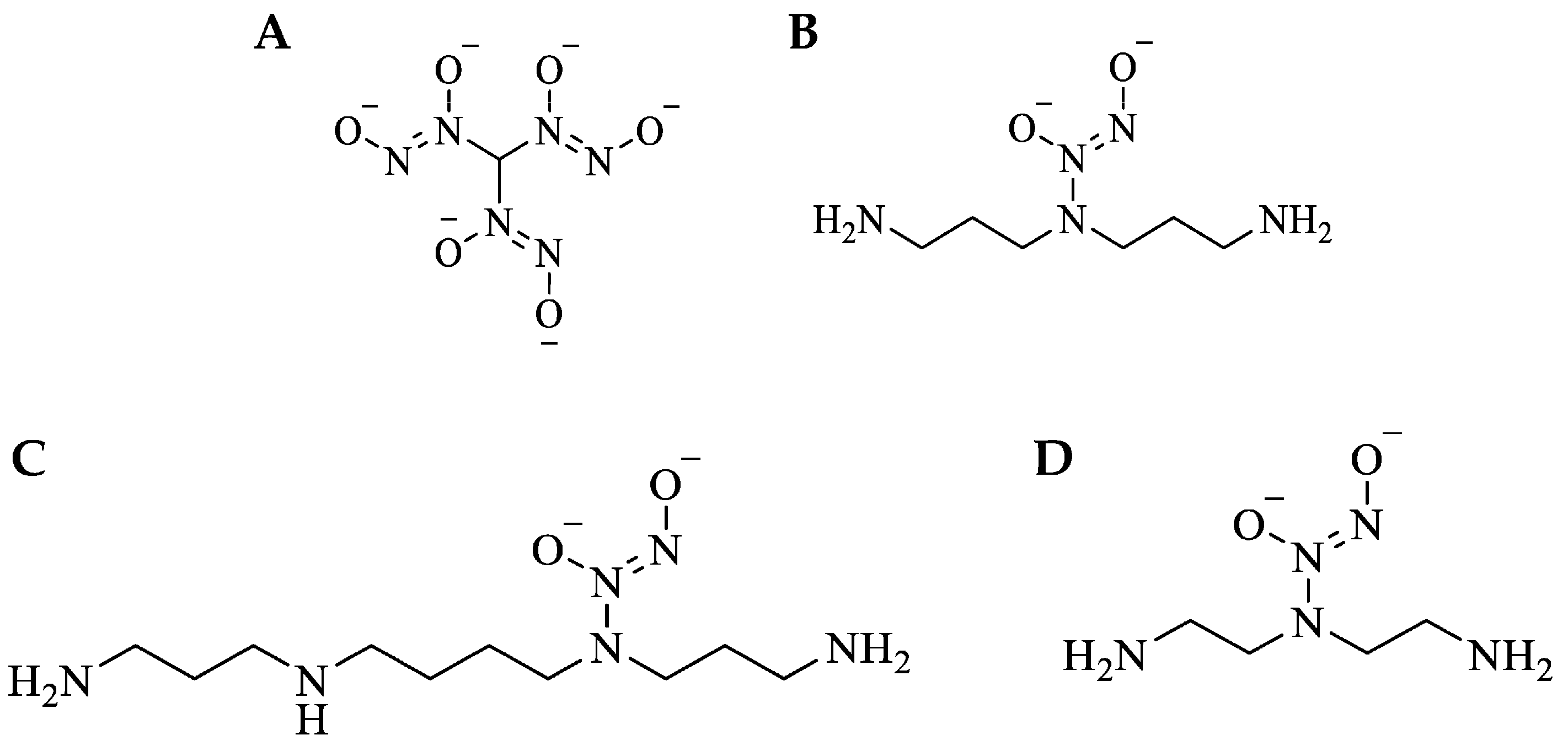



| Material | [NO]t (μmol mg−1) b | [NO]max (ppb mg−1) c | t1/2 (h) d | td (h) e |
|---|---|---|---|---|
| SPER/NO | 5.1 ± 0.2 | 7535 ± 222 | 1.1 ± 0.2 | 14.8 ± 2.9 |
| MD3 | 5.0 ± 0.7 | 2800 ± 170 | 3.6 ± 0.8 | 42.8 ± 12.5 |
| DPTA/NO | 7.0 ± 1.1 | 2867 ± 211 | 3.7 ± 0.3 | 31.0 ± 3.3 |
| DETA/NO | 6.3 ± 0.6 | 735 ± 94 | 22.5 ± 3.3 | 107.6 ± 19.9 |
| Strains | MIC (µg mL−1) (MIC NO Dose (µg mL−1)) | |||
|---|---|---|---|---|
| MD3 | SPER/NO | DPTA/NO | DETA/NO | |
| Candida albicans | ||||
| ATCC MYA-2876 | 60 (8.98) | 125 (19.2) | 500 (105) | 125 (23.5) |
| ATCC 18804 | 40 (5.98) | 150 (23.1) | 625 (131) | 625 (117) |
| ATCC 14053 | 150 (22.4) | 250 (38.6) | 625 (131) | 625 (117) |
| Candida auris | ||||
| ATCC MYA-5000 | 30 (4.49) | 125 (19.2) | 625 (131) | 625 (117) |
| ATCC MYA-5001 | 20 (2.25) | 250 (38.6) | 500 (105) | 30 (5.65) |
| ATCC MYA-5003 | 40 (5.98) | 125 (19.2) | 310 (65.3) | 310 (58.4) |
| Cryptococcus neoformans | ||||
| ATCC 208821 | 30 (4.50) | 500 (76.8) | 1000 (210) | 250 (47.0) |
| ATCC MYA-4566 | 19 (2.99) | 19 (3.08) | 625 (131) | 78 (14.7) |
| ATCC MYA-4567 | 20 (2.99) | 78 (12.0) | 625 (131) | 310 (58.4) |
| Aspergillus fumigatus | ||||
| ATCC 1022 | 70 (10.5) | 70 (10.7) | 1250 (262) | 320 (60.5) |
| Antifungal | NO Donor | C. albicans | C. auris | C. neoformans | A. fumigatus |
|---|---|---|---|---|---|
| ∑FIC | ∑FIC | ∑FIC | ∑FIC | ||
| Caspofungin | MD3 | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) |
| SPER/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DPTA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DETA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| Fluconazole | MD3 | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) |
| SPER/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DPTA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DETA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| 5-Fluorocytosine | MD3 | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) |
| SPER/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DPTA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DETA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| Amphotericin B | MD3 | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) |
| SPER/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DPTA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DETA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| Butenafine | MD3 | ≥1 (I) | ≥1 (I) | 0.500 (A) | ≥1 (I) |
| SPER/NO | ≥1 (I) | ≥1 (I) | 0.562 (A) | ≥1 (I) | |
| DPTA/NO | ≥1 (I) | ≥1 (I) | ≥1 (I) | ≥1 (I) | |
| DETA/NO | ≥1 (I) | ≥1 (I) | 0.500 (A) | ≥1 (I) | |
| Miconazole | MD3 | 0.530 (A) | 0.562 (A) | ≥1 (I) | ≥1 (I) |
| SPER/NO | 0.265 (S) | 0.375 (S) | 0.500 (S) | ≥1 (I) | |
| DPTA/NO | ≥1 (I) | 0.625 (A) | 0.375 (S) | ≥1 (I) | |
| DETA/NO | 0.375 (S) | ≥1 (I) | 0.562 (A) | ≥1 (I) |
| Fungi | Strain | Selectivity Index | |||
|---|---|---|---|---|---|
| MD3 | SPER/NO | DPTA/NO | DETA/NO | ||
| C. albicans | ATCC MYA-2876 | 267 | 164 | 35 | 383 |
| ATCC 18804 | 400 | 137 | 28 | 77 | |
| ATCC 14053 | 107 | 82 | 28 | 77 | |
| C. auris | ATCC MYA-5000 | 533 | 164 | 28 | 77 |
| ATCC MYA-5001 | 800 | 82 | 35 | 1595 | |
| ATCC MYA-5003 | 400 | 164 | 57 | 154 | |
| C. neoformans | ATCC 208821 | 533 | 41 | 18 | 191 |
| ATCC MYA-4566 | 842 | 1079 | 28 | 613 | |
| ATCC MYA-4567 | 800 | 263 | 28 | 154 | |
| A. fumigatus | ATCC 1022 | 228 | 293 | 14 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grayton, Q.E.; Conlon, I.L.; Broberg, C.A.; Schoenfisch, M.H. Impact of Nitric Oxide-Release Kinetics on Antifungal Activity. J. Fungi 2024, 10, 308. https://doi.org/10.3390/jof10050308
Grayton QE, Conlon IL, Broberg CA, Schoenfisch MH. Impact of Nitric Oxide-Release Kinetics on Antifungal Activity. Journal of Fungi. 2024; 10(5):308. https://doi.org/10.3390/jof10050308
Chicago/Turabian StyleGrayton, Quincy E., Ivie L. Conlon, Christopher A. Broberg, and Mark H. Schoenfisch. 2024. "Impact of Nitric Oxide-Release Kinetics on Antifungal Activity" Journal of Fungi 10, no. 5: 308. https://doi.org/10.3390/jof10050308
APA StyleGrayton, Q. E., Conlon, I. L., Broberg, C. A., & Schoenfisch, M. H. (2024). Impact of Nitric Oxide-Release Kinetics on Antifungal Activity. Journal of Fungi, 10(5), 308. https://doi.org/10.3390/jof10050308






