Genome-Wide Identification of SNARE Family Genes and Functional Characterization of an R-SNARE Gene BbSEC22 in a Fungal Insect Pathogen Beauveria bassiana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
2.2. Identification and Analysis of Predicted SNAREs Encoding Genes
2.3. Targeted Gene Deletion and Complement of BbSEC22
2.4. qRT-PCR Analysis of Gene Expressions
2.5. Assays for Vegetative Growth, Sporulation and Conidial Germination
2.6. Assays for Stress Tolerance of the Growing Colonies and Germling Conidia
2.7. Assays for Fungal Virulence
2.8. Statistical Analysis
3. Results
3.1. Identification of SNARE Family Genes in B. bassiana Genome
3.2. Structural and Expression Features of BbSec22
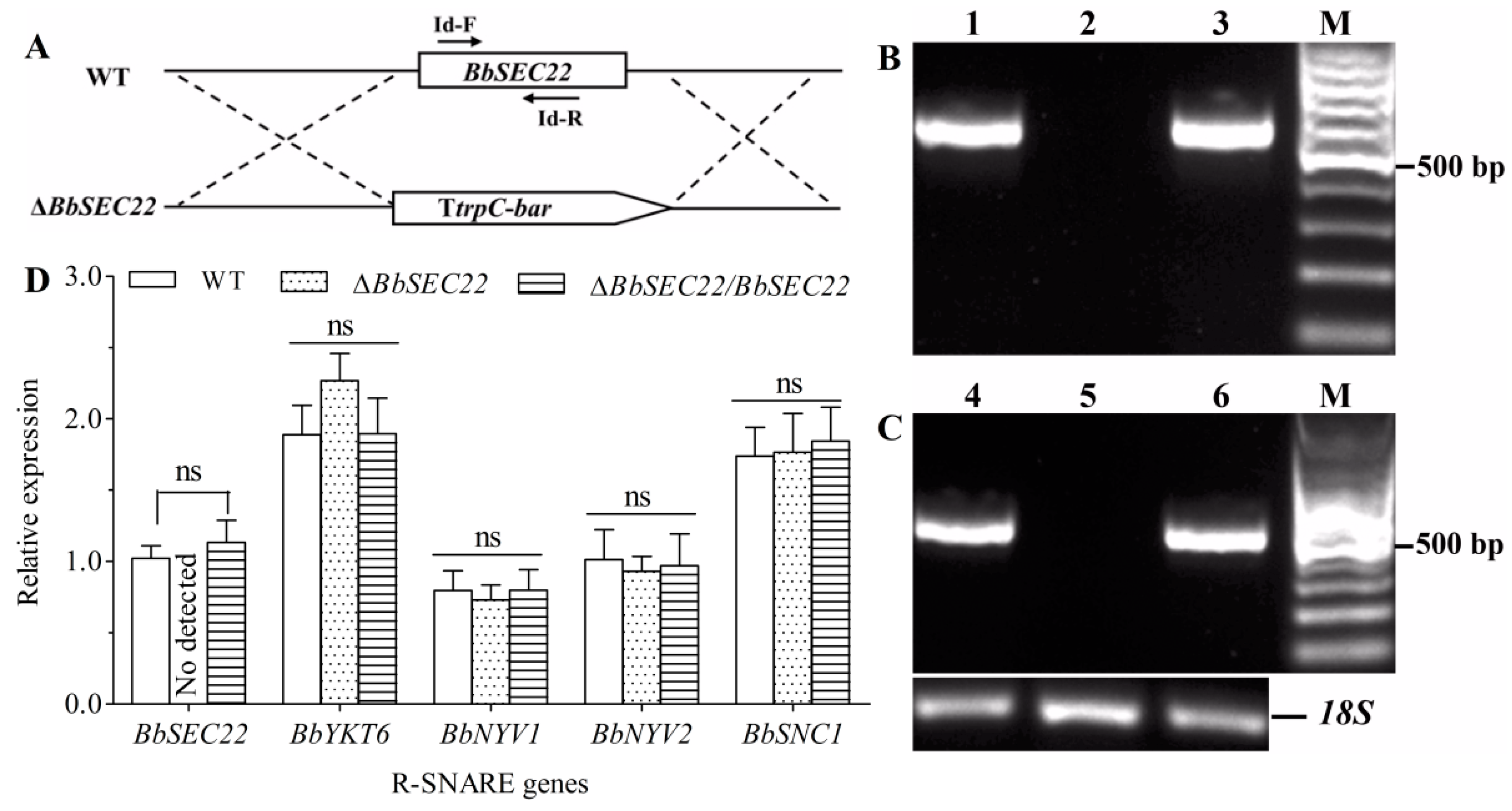
3.3. Targeted Deletion and Complementation of BbSEC22
3.4. Effects of the BbSEC22 Deletion on Mycelial Growth
3.5. Effects of the BbSEC22 Deletion on Sporulation and Conidial Germination
3.6. Effects of the BbSEC22 Deletion on Stress Tolerance
3.7. Effects of the BbSEC22 Deletion on Pathogenicity
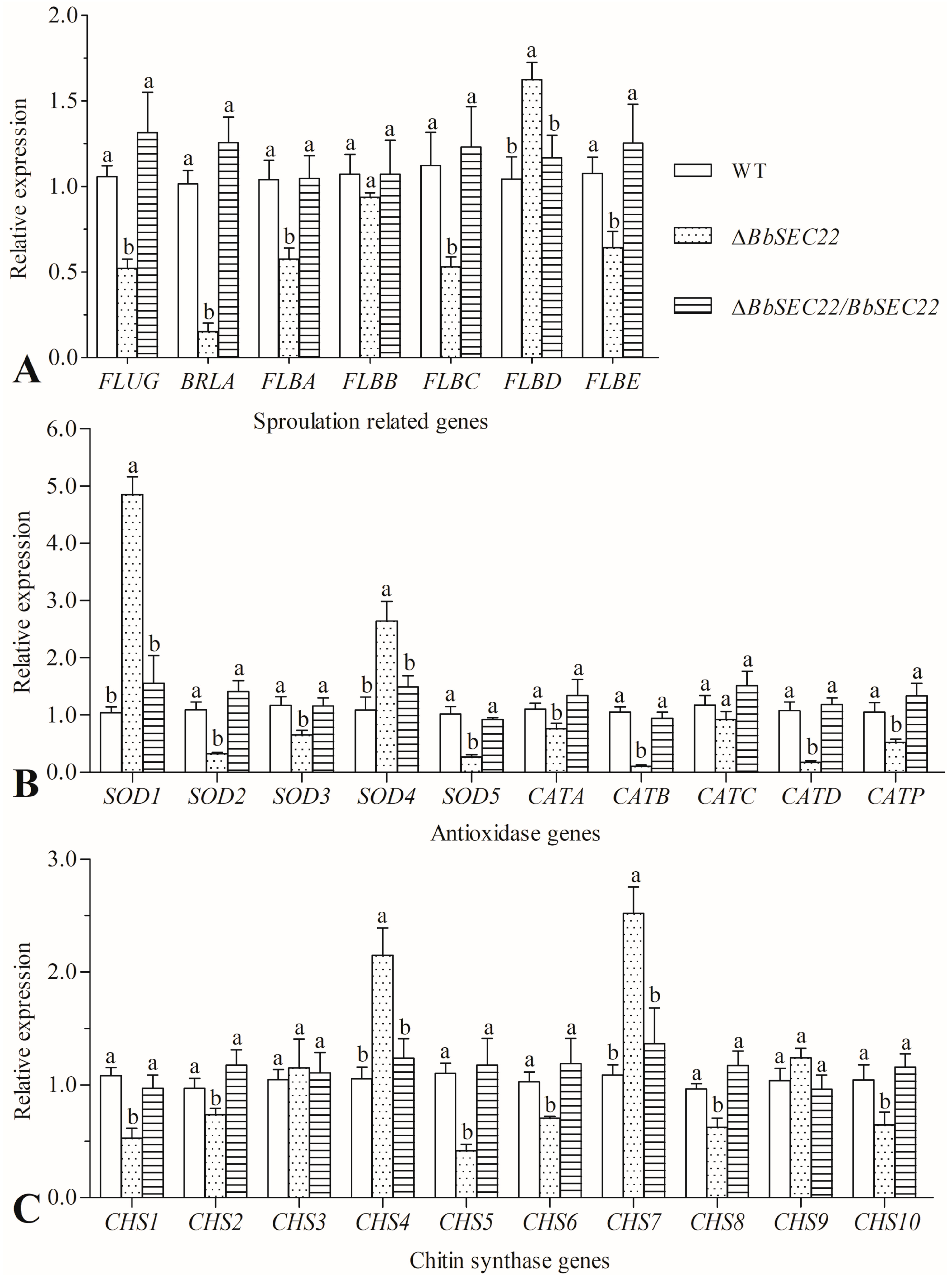
3.8. Transcript Changes of Phenotype-Associated Genes in the BbSEC22 Deletion Mutant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.A.; Scheller, R.H. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2001, 2, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.D.; Brent Heath, I. Predicting the distribution, conservation, and functions of SNAREs and related proteins in fungi. Fungal Genet. Biol. 2002, 36, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Islam, W.; Zhang, J.; Zheng, W.; Lu, G.D. Diverse role of SNARE protein Sec22 in vesicle trafficking, membrane fusion, and autophagy. Cells 2019, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Lithgow, T. A complete set of SNAREs in yeast. Traffic 2004, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kuratsu, M.; Taura, A.; Shoji, J.Y.; Kikuchi, S.; Arioka, M.; Kitamoto, K. Systematic analysis of SNARE localization in the filamentous fungus Aspergillus oryzae. Fungal Genet. Biol. 2007, 44, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Dong, S.; Ye, W.; Hua, C.; Meijer, H.J.; Dou, X.; Govers, F.; Wang, Y. Genome-wide identification of Phytophthora sojae SNARE genes and functional characterization of the conserved SNARE PsYKT6. Fungal Genet. Biol. 2011, 48, 241–251. [Google Scholar] [CrossRef]
- Yang, X.; Ben, S.; Sun, Y.; Fan, X.; Tian, C.; Wang, Y. Genome-wide identification, phylogeny and expression profile of vesicle fusion components in Verticillium dahliae. PLoS ONE 2013, 87, e68681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, B.; Fang, Q.; Li, Y.; Zheng, X.; Zhang, Z. SNARE protein FgVam7 controls growth, asexual and sexual development, and plant infection in Fusarium graminearum. Mol. Plant Pathol. 2016, 17, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Hong, W. SNAREs and traffic. Biochim. Biophys. Acta 2005, 1744, 120–144. [Google Scholar] [CrossRef]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The multifaceted role of SNARE proteins in membrane fusion. Front. Physiol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs-engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Sollner, T.; Bennett, M.K.; Whiteheart, S.W.; Scheller, R.H.; Rothman, J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 1993, 75, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.B.; Matern, H.T.; Peden, A.A.; Scheller, R.H. A genomic perspective on membrane compartment organization. Nature 2001, 409, 839–841. [Google Scholar] [CrossRef]
- Fasshauer, D.; Eliason, W.K.; Brunger, A.T.; Jahn, R. Identification of a minimal core of the synaptic SNARE complex sufficient for reversible assembly and disassembly. Biochemistry 1998, 37, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.Y.; Munson, M. SNARE complex assembly and disassembly. Curr. Biol. 2018, 28, R397–R401. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Ueda, T. Plant vacuolar trafficking driven by RAB and SNARE proteins. Curr. Opin. Plant Biol. 2014, 22, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Feyder, S.; De Craene, J.-O.; Bär, S.; Bertazzi, D.; Friant, S. Membrane trafficking in the yeast Saccharomyces cerevisiae model. Int. J. Mol. Sci. 2015, 16, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Zhao, R.; Kou, R.; Zheng, X.; Zhang, H. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS Pathog. 2019, 15, e1007754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, D.; Bai, N.; Liu, Q.; Zhao, N.; Yang, J. SNARE protein AoSec22 orchestrates mycelial growth, vacuole assembly, trap formation, stress response, and secondary metabolism in Arthrobotrys oligospora. J. Fungi 2023, 9, 75. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Noman, A.; Hussain, A.; Anwar, M.; Khan, M.U.; Akram, W.; Ashraf, M.F.; Raza, M.F. Q-SNARE protein FgSyn8 plays important role in growth, DON production and pathogenicity of Fusarium graminearum. Microb. Pathog. 2020, 140, 103948. [Google Scholar] [CrossRef]
- Gupta, G.D.; Free, S.J.; Levina, N.N.; Keränen, S.; Heath, I.B. Two divergent plasma membrane syntaxin-like SNAREs, nsyn1 and nsyn2, contribute to hyphal tip growth and other developmental processes in Neurospora crassa. Fungal Genet. Biol. 2003, 40, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; So, J.; Lee, J.; Min, K.; Son, H.; Park, C.; Yun, S.H.; Lee, Y.W. Functional analyses of two syntaxin-like SNARE genes, GzSYN1 and GzSYN2, in the ascomycete Gibberella zeae. Fungal Genet. Biol. 2010, 47, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, H.; Shim, W.B. Fusarium verticillioides SNARE protein FvSyn1 harbours two key functional motifs that play selective roles in fungal development and virulence. Microbiology 2019, 165, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Song, W.W.; Dou, X.Y.; Qi, Z.Q.; Wang, Q.; Zhang, X.; Zhang, H.F.; Guo, M.; Dong, S.M.; Zhang, Z.G.; Wang, P.; et al. R-SNARE homolog MoSec22 is required for conidiogenesis, cell wall integrity, and pathogenesis of Magnaporthe oryzae. PLoS ONE 2010, 5, e13193. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Wang, Q.; Qi, Z.; Song, W.; Wang, W.; Guo, M.; Zhang, H.; Zhang, Z.; Wang, P.; Zheng, X. MoVam7, a conserved SNARE involved in vacuole assembly, is required for growth, endocytosis, ROS accumulation, and pathogenesis of Magnaporthe oryzae. PLoS ONE 2011, 6, e16439. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, M.; Dong, Y.; Zhu, Q.; Li, L.; Li, B.; Yang, J.; Li, Y.; Ru, Y.; Zhang, H.F.; et al. The syntaxin protein (MoSyn8) mediates intracellular trafficking to regulate conidiogenesis and pathogenicity of rice blast fungus. New Phytol. 2016, 209, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.S.; Yang, J.; Wang, D.W.; He, D.; Chu, Y.; Chen, X.L.; Zhou, W.; Hsiang, T.; Peng, Y.L. MoTlg2, a t-SNARE component is important for formation of the Spitzenkrper and polar deposition of chitin in Magnaporthe oryzae. Physiol. Mol. Plant 2014, 87, 9–18. [Google Scholar] [CrossRef]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1996. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Fang, W.; Sun, P.; Zheng, Y.; Abubakar, Y.S.; Zhang, J.; Lou, Y.; Zheng, W.; Lu, G.D. R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum. Curr. Genet. 2020, 66, 421–435. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Zhang, D.D.; Short, D.P.G.; Zhou, L.; Song, S.S.; Liu, Y.; Wang, D.; Kong, Z.Q.; Cui, W.Y.; et al. SNARE-encoding genes VdSec22 and VdSso1 mediate protein secretion required for full virulence in Verticillium dahliae. Mol. Plant Microbe Interact. 2018, 31, 651–664. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Fan, Y.; Xiang, M.; Kang, S.; Wei, D.; Liu, X. SNARE Protein DdVam7 of the Nematode-trapping fungus Drechslerella dactyloides regulates vegetative growth, conidiation, and the predatory process via vacuole assembly. Microbiol. Spectr. 2022, 10, e0187222. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef] [PubMed]
- De Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Lu, H.L.; St Leger, R.J. Insect immunity to entomopathogenic fungi. Adv. Genet. 2016, 94, 251–285. [Google Scholar] [PubMed]
- Xiao, G.H.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.W.; Xie, X.Q.; Shang, Y.F.; St. Leger, R.J.; Zhao, G.P.; Wang, C.S.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; Mc William, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Zhang, L.B.; Ying, S.H.; Feng, M.G. Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ. Microbiol. 2013, 15, 21409–21418. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Harris, S.D.; Morrell, J.L.; Hamer, J.E. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 1994, 136, 517–532. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.G. Intraspecific tolerance of Metarhizium anisopliae conidia to the upper thermal limits of summer with a description of a quantitative assay system. Mycol. Res. 2009, 113, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Zhang, C.X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect. Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.J.; Mou, Y.N.; Tong, S.M.; Ying, S.H.; Feng, M.G. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence 2020, 11, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Anwar, W.; Javed, M.A.; Shahid, A.A.; Nawaz, K.; Akhter, A.; Ur Rehman, M.Z.; Hameed, U.; Iftikhar, S.; Haider, M.S. Chitinase genes from Metarhizium anisopliae for the control of whitefly in cotton. R. Soc. Open Sci. 2019, 6, 190412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef] [PubMed]
- Sowjanya Sree, K.; Padmaja, V.; Murthy, Y.L. Insecticidal activity of destruxin, a mycotoxin from Metarhizium anisopliae (Hypocreales), against Spodoptera litura (Lepidoptera: Noctuidae) larval stages. Pest Manag. Sci. 2008, 64, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Lu, J.D.; Feng, M.G. Primary roles of two dehydrogenases in the mannitol metabolism and multi-stress tolerance of entomopathogenic fungus Beauveria bassiana. Environ. Microbiol. 2012, 14, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ying, S.H.; Feng, M.G.; Jiang, X.H. Physiological implication of intracellular trehalose and mannitol changes in response of entomopathogenic fungus Beauveria bassiana to thermal stress. Antonie Van Leeuwenhoek 2009, 95, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Morishita, M.; Schwartz, C.L.; Coluccio, A.; Engebrecht, J.; Neiman, A.M. Phospholipase D and the SNARE Sso1p are necessary for vesicle fusion during sporulation in yeast. J. Cell Sci. 2006, 119, 1406–1415. [Google Scholar] [CrossRef]
- Zhang, A.X.; Mouhoumed, A.Z.; Tong, S.M.; Ying, S.H.; Feng, M.G. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems 2019, 4, e00140-19. [Google Scholar] [CrossRef]
- Jia, L.; Yu, J.H.; Chen, F.; Chen, W. Characterization of the asexual developmental genes brlA and wetA in Monascus ruber M7. Fungal Genet. Biol. 2021, 151, 103564. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.G.H. Fruiting in the higher fungi. Adv. Microb. Physiol. 1993, 34, 147–202. [Google Scholar] [PubMed]
- Zhang, L.B.; Feng, M.G. Antioxidant enzymes and their contributions to biological control potential of fungal insect pathogens. Appl. Microbiol. Biotechnol. 2018, 102, 4995–5004. [Google Scholar] [CrossRef] [PubMed]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, K.Q. Chitin synthesis and degradation in fungi: Biology and enzymes. Adv. Exp. Med. Biol. 2019, 1142, 153–167. [Google Scholar] [PubMed]
- Sietsma, J.H.; Beth Din, A.; Ziv, V.; Sjollema, K.A.; Yarden, O. The localization of chitin synthase in membranous vesicles (chitosomes) in Neurospora crassa. Microbiology. 1996, 142, 1591–1596. [Google Scholar] [CrossRef]
- Lesage, G.; Shapiro, J.; Specht, C.A.; Sdicu, A.M.; Ménard, P.; Hussein, S.; Tong, A.H.; Boone, C.; Bussey, H. An interactional network of genes involved in chitin synthesis in Saccharomyces cerevisiae. BMC Genet. 2005, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, U.; Hause, G.; Schuchardt, I.; Steinberg, G. Endocytosis is essential for pathogenic development in the corn smut fungus Ustilago maydis. Plant Cell. 2006, 18, 2066–2081. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Extracellular enzyme activity of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and their pathogenicity potential as a bio-control agent against whitefly pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC Res. Notes 2022, 15, 117. [Google Scholar] [CrossRef]
- Cito, A.; Barzanti, G.P.; Strangi, A.; Francardi, V.; Zanfini, A.; Dreassi, E. Cuticle-degrading proteases and toxins as virulence markers of Beauveria bassiana (Balsamo) Vuillemin. J. Basic Microbiol. 2016, 56, 941–948. [Google Scholar] [CrossRef]
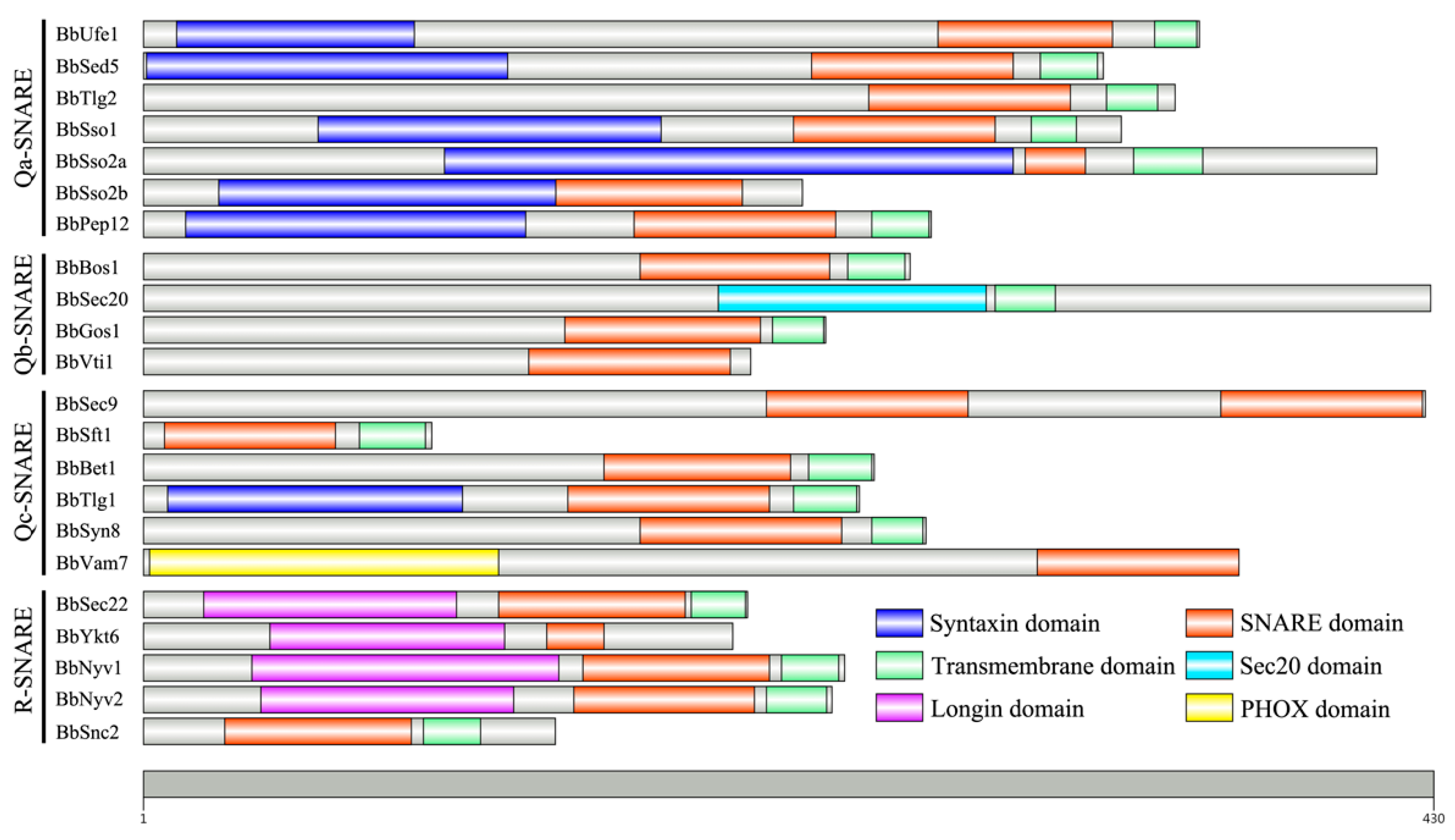



| Type | Name | Protein_ID | aa | Gene_ID | Gene (bp) | CDS (bp) a | Intron | Zero Layer Residue b | Homologs in S. cerevisiae |
|---|---|---|---|---|---|---|---|---|---|
| Qa-SNARE | BbUfe1 | EJP69482 | 352 | BBA_01447 | 1179 | 1059 | 2 | Q | Ufe1 |
| BbSed5 | EJP63375 | 320 | BBA_07769 | 1074 | 963 | 2 | Q | Sed5 | |
| BbTlg2 | EJP63918 | 344 | BBA_07242 | 1161 | 1035 | 2 | Q | Tlg2 | |
| BbSso1 | EJP64917 | 326 | BBA_06092 | 1377 | 981 | 2 | Q | Sso1 | |
| BbSso2a | EJP63095 | 411 | BBA_07900 | 1441 | 1236 | 2 | Q | Sso2 | |
| BbSso2b | EJP61869 | 220 | BBA_09206 | 775 | 663 | 1 | Q | Sso2 | |
| BbPep12 | EJP66927 | 263 | BBA_04220 | 849 | 792 | 1 | Q | Pep12 | |
| Qb-SNARE | BbSec20 | EJP61319 | 429 | BBA_09714 | 1390 | 1290 | 1 | Q | Sec20 |
| BbBos1 | EJP65062 | 256 | BBA_05832 | 908 | 771 | 1 | Q | Bos1 | |
| BbGos1 | EJP69616 | 228 | BBA_01581 | 830 | 687 | 2 | Q | Gos1 | |
| BbVti1 | EJP64999 | 203 | BBA_06174 | 742 | 612 | 2 | Q | Vti1 | |
| Qc-SNARE | BbSft1 | EJP63515 | 97 | BBA_07441 | 406 | 294 | 1 | D | Sft1 |
| BbBet1 | EJP67882 | 244 | BBA_02778 | 1754 | 1230 | 2 | S | Bet1 | |
| BbTlg1 | EJP64309 | 239 | BBA_06691 | 779 | 720 | 1 | Q | Tlg1 | |
| BbSec9 | EJP65468 | 427 | BBA_05799 | 1336 | 1284 | 1 | Q | Sec9 | |
| BbSyn8 | EJP60748 | 261 | BBA_10304 | 860 | 786 | 1 | H | Syn8 | |
| BbVam7 | EJP66712 | 365 | BBA_04005 | 1098 | 1098 | 0 | Q | Vam7 | |
| R-SNARE | BbSec22 | EJP64509 | 202 | BBA_06503 | 696 | 609 | 1 | R | Sec22 |
| BbYkt6 | EJP70865 | 197 | BBA_00495 | 984 | 594 | 4 | R | Ykt6 | |
| BbNyv1 | EJP61800 | 234 | BBA_09220 | 799 | 705 | 1 | R | Nyv1 | |
| BbNyv2 | EJP61468 | 230 | BBA_09604 | 819 | 693 | 2 | R | Nyv2 | |
| BbSnc1 | EJP66249 | 138 | BBA_04742 | 884 | 417 | 1 | R | Snc1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhang, J.; Zhong, H.; Yu, K.; Chen, J. Genome-Wide Identification of SNARE Family Genes and Functional Characterization of an R-SNARE Gene BbSEC22 in a Fungal Insect Pathogen Beauveria bassiana. J. Fungi 2024, 10, 393. https://doi.org/10.3390/jof10060393
Li F, Zhang J, Zhong H, Yu K, Chen J. Genome-Wide Identification of SNARE Family Genes and Functional Characterization of an R-SNARE Gene BbSEC22 in a Fungal Insect Pathogen Beauveria bassiana. Journal of Fungi. 2024; 10(6):393. https://doi.org/10.3390/jof10060393
Chicago/Turabian StyleLi, Fang, Juefeng Zhang, Haiying Zhong, Kaili Yu, and Jianming Chen. 2024. "Genome-Wide Identification of SNARE Family Genes and Functional Characterization of an R-SNARE Gene BbSEC22 in a Fungal Insect Pathogen Beauveria bassiana" Journal of Fungi 10, no. 6: 393. https://doi.org/10.3390/jof10060393





