Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology
Abstract
:1. Introduction
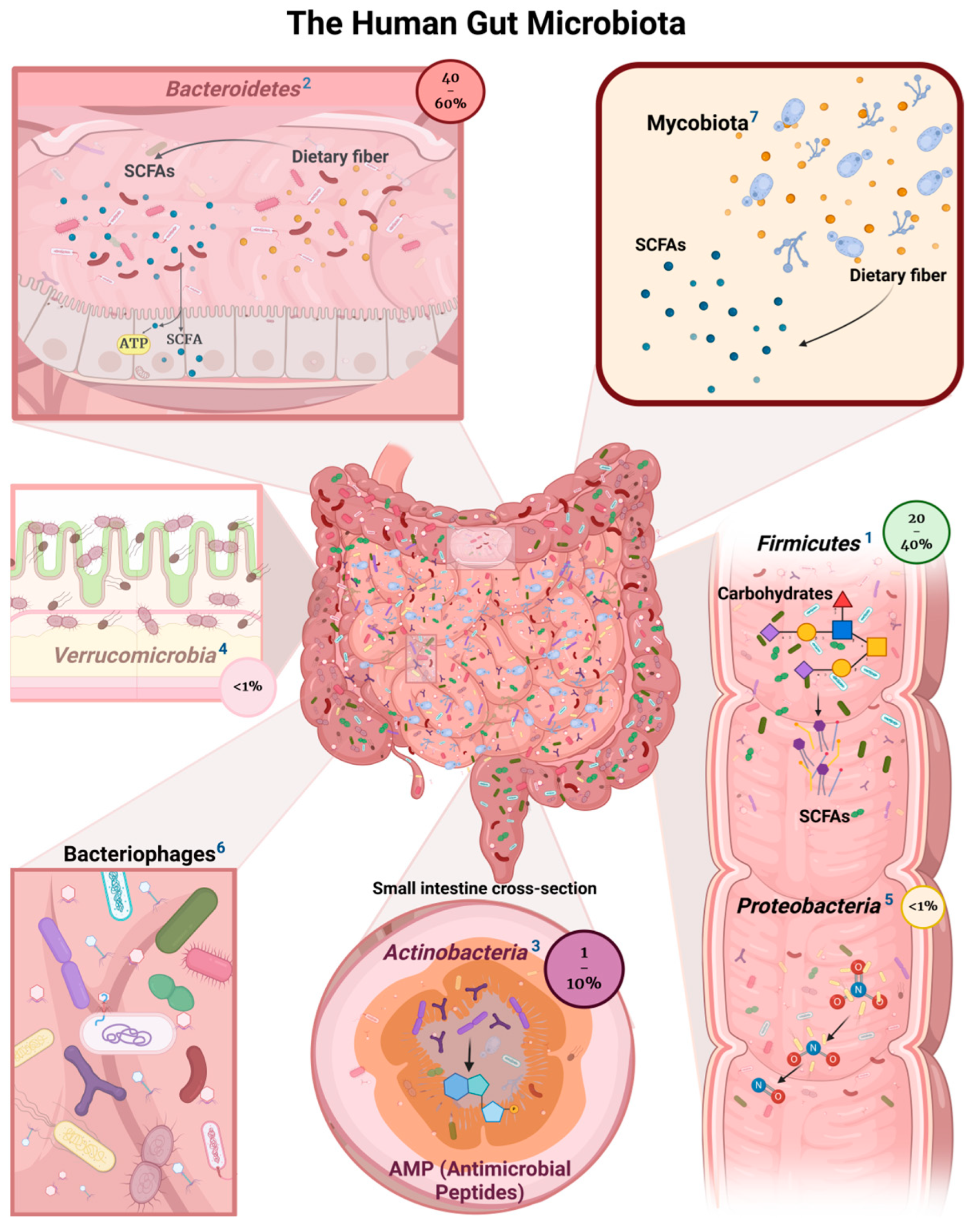
2. Bacterial Commensals
3. Fungal Polysaccharides
4. Bacterial Role in Fungal Polysaccharide Breakdown
5. Beta-Glucans and Their Impact on Gut Microbiota
6. Human Gut Bacteria’s Affinity for β-Glucan
7. Impact of Fungal Fiber Polysaccharides on Diabetes Care
8. Functional Fungal Polysaccharides and the Gut–Brain Connection in Preventing Neurodegenerative Diseases
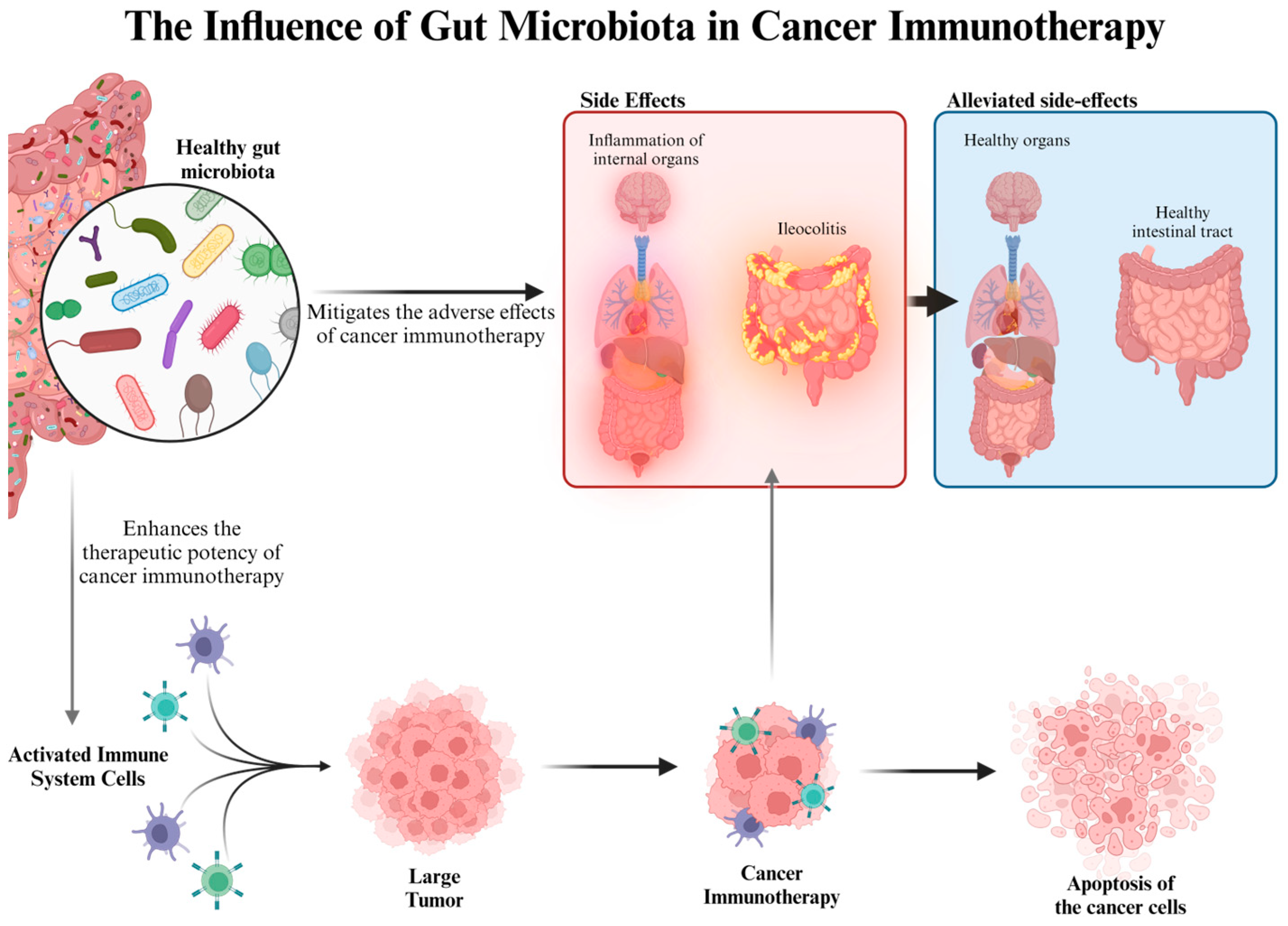
9. Fungal Fiber Polysaccharides and Cancer Treatment
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Starwood, J. Mushroom Wanderland: A Forager’s Guide to Finding, Identifying, and Using More Than 25 Wild Fungi; The Countryman Press: Woodstock, VT, USA, 2021. [Google Scholar]
- Yang, L.; Kang, X.; Dong, W.; Wang, L.; Liu, S.; Zhong, X.; Liu, D. Prebiotic properties of Ganoderma lucidum polysaccharides with special enrichment of Bacteroides ovatus and B. uniformis in vitro. J. Funct. Foods 2022, 92, 105069. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Chen, W.-J.; Wei, Y.-S.; Lo, H.-I.; Chu, H.-F.; Tseng, M.-C.; Lee, B.-H.; Shen, T.-L. Liquid-State Fermented Ganoderma lucidum GANO99 Regulates Gut Microbiota and Concomitantly Modulates the Behavioral Deficits and Neurohistopathological Hallmarks of Alzheimer’s Disease in a Preclinical Transgenic Mouse Model of Alzheimer’s Disease. J. Food Biochem. 2024, 2024, 6676977. [Google Scholar] [CrossRef]
- Su, L.; Xin, C.; Yang, J.; Dong, L.; Mei, H.; Dai, X.; Wang, Q. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 214, 312–323. [Google Scholar] [CrossRef]
- Yang, M.; Hu, D.; Cui, Z.; Li, H.; Man, C.; Jiang, Y. Lipid-lowering effects of Inonotus obliquus polysaccharide in vivo and in vitro. Foods 2021, 10, 3085. [Google Scholar] [CrossRef]
- Li, J.; Qu, C.; Li, F.; Chen, Y.; Zheng, J.; Xiao, Y.; Jin, Q.; Jin, G.; Huang, X.; Jin, D. Inonotus obliquus polysaccharide ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated cancer in mice via activation of the NLRP3 inflammasome. Front. Pharmacol. 2021, 11, 621835. [Google Scholar] [CrossRef]
- Cui, W.; Song, X.; Li, X.; Jia, L.; Zhang, C. Structural characterization of Hericium erinaceus polysaccharides and the mechanism of anti-T2DM by modulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 242, 125165. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Xu, T.; Zou, X.; Mao, R.; Pi, X.; Wu, W.; Huang, L.; Yang, K.; Zeng, X.; et al. Digestive Characteristics of Hericium erinaceus polysaccharides and their positive effects on fecal microbiota of male and female volunteers during in vitro fermentation. Front. Nutr. 2022, 9, 858585. [Google Scholar] [CrossRef]
- Priori, E.C.; Ratto, D.; De Luca, F.; Sandionigi, A.; Savino, E.; Giammello, F.; Romeo, M.; Brandalise, F.; Roda, E.; Rossi, P. Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice. Biology 2023, 13, 18. [Google Scholar] [CrossRef]
- Morales, D.; Shetty, S.A.; López-Plaza, B.; Gómez-Candela, C.; Smidt, H.; Marín, F.R.; Soler-Rivas, C. Modulation of human intestinal microbiota in a clinical trial by consumption of a β-D-glucan-enriched extract obtained from Lentinula edodes. Eur. J. Nutr. 2021, 60, 3249–3265. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jiang, P.; Zhao, J.; Shi, H.; Zhang, P.; Yang, X.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory properties of polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Duan, W.; Li, D.; Tang, X.; Duan, Z. Effects of polysaccharides from Auricularia auricula on the immuno-stimulatory activity and gut microbiota in immunosuppressed mice induced by cyclophosphamide. Front. Immunol. 2020, 11, 595700. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.; Chen, F.; Ma, L.; Hu, X.; Ji, J. Functional perspective of black fungi (Auricularia auricula): Major bioactive components, health benefits and potential mechanisms. Trends Food Sci. Technol. 2021, 114, 245–261. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Wen, C.; Liu, J.; Xu, X.; Liu, G.; Kan, J.; Qian, C.; Jin, C. New light on Grifola frondosa polysaccharides as biological response modifiers. Trends Food Sci. Technol. 2022, 119, 565–578. [Google Scholar] [CrossRef]
- Zhao, J.; He, R.; Zhong, H.; Liu, S.; Liu, X.; Hussain, M.; Sun, P. A cold-water extracted polysaccharide-protein complex from Grifola frondosa exhibited anti-tumor activity via TLR4-NF-κB signaling activation and gut microbiota modification in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2023, 239, 124291. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, D.; Gao, L.; Ouyang, Y.; Wen, Y.; Ai, C.; Chen, Y.; Zhao, C. Health benefits of Grifola frondosa polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Wellness 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Xiao, C.; Jiao, C.; Xie, Y.; Ye, L.; Li, Q.; Wu, Q. Grifola frondosa GF5000 improves insulin resistance by modulation the composition of gut microbiota in diabetic rats. J. Funct. Foods 2021, 77, 104313. [Google Scholar] [CrossRef]
- Törős, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.H.; Peles, F. Modulation of the gut microbiota with prebiotics and antimicrobial agents from Pleurotus ostreatus mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef]
- Song, X.; Feng, Z.; Tan, J.; Wang, Z.; Zhu, W. Dietary administration of Pleurotus ostreatus polysaccharides (POPS) modulates the non-specific immune response and gut microbiota diversity of Apostichopus japonicus. Aquac. Rep. 2021, 19, 100578. [Google Scholar] [CrossRef]
- Shao, Y.; Zhong, H.; Pérez-Ponce, A.; Chen, D.; Zhong, L. Pleurotus Ostreatus β-glucan Alleviates Cyclophosphamide-induced Immunosuppression by Regulating Gut Microbiota in Mice. Res. Sq. 2020, preprint. [Google Scholar]
- Liao, W.; Khan, I.; Huang, G.; Chen, S.; Liu, L.; Leong, W.K.; Li, X.A.; Wu, J.; Hsiao, W.L.W. Bifidobacterium animalis: The missing link for the cancer-preventive effect of Gynostemma pentaphyllum. Gut Microbes 2021, 13, 1847629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Huang, W.; Suo, J.; Chen, X.; Ding, K.; Sun, Q.; Zhang, H. Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. Int. J. Biol. Macromol. 2020, 145, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ouyang, Q.; Li, X.; Alolgal, R.N.; Fan, Y.; Sun, Y.; Gong, H.; Xiao, P.; Ma, G. The role of Gynostemma pentaphyllum in regulating hyperlipidemia. Am. J. Chin. Med. 2023, 51, 953–978. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, Z.; Wang, W.; Song, M.; Dong, R.; Zhou, Y.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Amanita caesarea and its pharmacological basis for application in Alzheimer’s disease: Endoplasmic reticulum stress. Food Funct. 2021, 12, 11009–11023. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Avila, D.; Avila, A.; Marcia, J.; Picha, D.; Aryana, K.; Montero-Fernández, I. Effects of Weevil (Rhynchophorus palmarum), Teosinte (Dioon mejiae) and Caesar’s Mushroom (Amanita caesarea) on the Properties of Lactobacillus acidophilus LA-K. Fermentation 2023, 9, 852. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, X.; Huang, S.; Feng, X.; Zhang, X.; Xiang, D. A monomeric polysaccharide from Polygonatum sibiricum improves cognitive functions in a model of Alzheimer’s disease by reshaping the gut microbiota. Int. J. Biol. Macromol. 2022, 213, 404–415. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, Y.; Liu, C.; Chen, B.; Ji, J.; Sun, J.; Zhang, Y.; Sun, X. Positive effects of steamed Polygonatum sibiricum polysaccharides including a glucofructan on fatty acids and intestinal microflora. Food Chem. 2023, 402, 134068. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, Q.; Lai, Y.; Lei, H.; Zhang, D.; Niu, H.; Wang, R.; Song, C. Polysaccharides from the leaves of Polygonatum sibiricum Red. regulate the gut microbiota and affect the production of short-chain fatty acids in mice. AMB Express 2022, 12, 35. [Google Scholar] [CrossRef]
- Rehman, A.U.; Siddiqui, N.Z.; Farooqui, N.A.; Alam, G.; Gul, A.; Ahmad, B.; Asim, M.; Khan, A.I.; Xin, Y.; Zexu, W.; et al. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front. Nutr. 2022, 9, 984695. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, J.; Liu, Y.; Cheng, H.; Nan, J.; Park, H.J.; Yang, L.; Li, J. Digestion profile, antioxidant, and antidiabetic capacity of Morchella esculenta exopolysaccharide: In vitro, in vivo and microbiota analysis. J. Sci. Food Agric. 2023, 103, 4401–4412. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Yan, C.; Zhang, C.; Pan, W.; Zhang, W.; Lu, Y.; Chen, L.; Chen, Y. Sarcodon aspratus polysaccharides ameliorated obesity-induced metabolic disorders and modulated gut microbiota dysbiosis in mice fed a high-fat diet. Food Funct. 2020, 11, 2588–2602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiang, M.; Jiang, Y.; Wu, F.; Chen, H.; Sun, M.; Zhang, L.; Du, X.; Chen, L. The protective effect of polysaccharide SAFP from Sarcodon aspratus on water immersion and restraint stress-induced gastric ulcer and modulatory effects on gut microbiota dysbiosis. Foods 2022, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Chen, J.; Wang, Y.; Yan, C.-C.; Zhang, C.; Mehmood, S.; Pan, W.-J.; Zhang, W.-N.; Lu, Y.-M.; Wu, Q.-X.; et al. Reduction of 5-fluorouracil-induced toxicity by Sarcodon aspratus polysaccharides in Lewis tumor-bearing mice. Int. J. Biol. Macromol. 2020, 163, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Zhou, X.; Zhao, X.; Lv, X.; Zhu, X.; Gao, N.; Jiang, Y.; Wu, M.; Sun-Waterhouse, D.; Li, D. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total Environ. 2023, 877, 162910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ji, Y.; Chen, X.; Su, A.; Ma, G.; Chen, G.; Hu, Q.; Zhao, L. Effects of a β-type glycosidic polysaccharide from Flammulina velutipes on anti-inflammation and gut microbiota modulation in colitis mice. Food Funct. 2020, 11, 4259–4274. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Y.; Yue, S.-R.; Lu, A.-P.; Zhang, L.; Ji, G.; Liu, B.-C.; Wang, R.-R. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis. Phytomedicine 2022, 103, 154208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ye, C.; Hu, B.; Xia, H.; Bian, Q.; Liu, Y.; Kong, M.; Zhou, S.; Liu, H. Regulation of gut microbiota and intestinal metabolites by Poria cocos oligosaccharides improves glycolipid metabolism disturbance in high-fat diet-fed mice. J. Nutr. Biochem. 2022, 107, 109019. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Liu, J.; Hong, Z.; Wong, K.-H.; Chiou, J.-C.; Xu, B.; Cespedes-Acuna, C.L.; Bai, W.; Tian, L. Structural characteristics and in vitro fermentation patterns of polysaccharides from Boletus mushrooms. Food Funct. 2023, 14, 7912–7923. [Google Scholar] [CrossRef]
- Avram, I.; Pelinescu, D.; Gatea, F.; Ionescu, R.; Barcan, A.; Rosca, R.; Zanfirescu, A.; Vamanu, E. Boletus edulis Extract—A New Modulator of Dysbiotic Microbiota. Life 2023, 13, 1481. [Google Scholar] [CrossRef]
- Kristófi, R.; Bodegard, J.; Norhammar, A.; Thuresson, M.; Nathanson, D.; Nyström, T.; Birkeland, K.I.; Eriksson, J.W. Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: A two-country nationwide observational study. Diabetes Care 2021, 44, 1211–1218. [Google Scholar] [CrossRef]
- Bhattacharya, S. Extravaganza of Nanobiotechnology in the Diagnosis and Treatment of Dementia Patients. Curr. Pharm. Biotechnol. 2023, 24, 1108–1121. [Google Scholar] [CrossRef]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef] [PubMed]
- Ayeka, P.A. Potential of Mushroom Compounds as Immunomodulators in Cancer Immunotherapy: A Review. Evid.-Based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, R.; Yang, Z.; Wen, Q.; Cao, X.; Zhao, N.; Yan, J. Protective effects of polysaccharides in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 917629. [Google Scholar] [CrossRef]
- Dan, X.; Mushi, Z.; Baili, W.; Han, L.; Enqi, W.; Huanhu, Z.; Shuchun, L. Differential analysis of hypertension-associated intestinal microbiota. Int. J. Med. Sci. 2019, 16, 872–881. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current prospects of nutraceuticals: A review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Vieites, E.; Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.d.C.; Lamas, A.; Cardelle-Cobas, A.; Nebot, C.; Franco, C.M.; Cepeda, A. Influence of the intestinal microbiota on diabetes management. Curr. Pharm. Biotechnol. 2020, 21, 1603–1615. [Google Scholar] [CrossRef]
- de Oliveira, B.F.R.; Freitas-Silva, J.; Canellas, A.L.B.; Costa, W.F.; Laport, M.S. Time for a Change! A Spotlight on Many Neglected Facets of Sponge Microbial Biotechnology. Curr. Pharm. Biotechnol. 2023, 24, 471–485. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Shariati, M.A.; Akram, M.; Zainab, R.; Daniyal, M.; Bankole, M.M.; Rebezov, M.; Okuskhanova, E. Herbal medicine for the management of laxative activity. Curr. Pharm. Biotechnol. 2022, 23, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.D.; Ihekweazu, F.D.; Haidacher, S.J.; Ruan, W.; Engevik, K.A.; Fultz, R.; Hoch, K.M.; Luna, R.A.; Oezguen, N.; Spinler, J.K.; et al. Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. iScience 2022, 25, 104158. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.V.; Gohel, S.D. Molecular diversity and pharmaceutical applications of free-living and rhizospheric marine actinobacteria. In Marine Niche: Applications in Pharmaceutical Sciences; Translational Research; Springer: Singapore, 2020; pp. 111–131. [Google Scholar]
- Arn, F.; Frasson, D.; Kroslakova, I.; Rezzonico, F.; Pothier, J.F.; Riedl, R.; Sievers, M. Isolation and identification of actinomycetes strains from switzerland and their biotechnological potential. Chimia 2020, 74, 382–390. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Wang, Z.; Li, Z.; Geng, Y. Effects of Pine Pollen Polysaccharides and Sulfated Polysaccharides on Ulcerative Colitis and Gut Flora in Mice. Polymers 2023, 15, 1414. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.; Li, X.-A.; Liao, W.; Leong, W.K.; Xia, W.; Bian, X.; Wu, J.; Hsiao, W.L.W. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in ApcMin/+ mice. Pharmacol. Res. 2019, 148, 104448. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Neuroprotective effects of polysaccharide from Sparassis crispa on Alzheimer’s disease-like mice: Involvement of microbiota-gut-brain axis. Int. J. Biol. Macromol. 2023, 225, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, A.; Dawoud, A.; Makky, S.; Nofal, R.; Aziz, R.; El-Shibiny, A. Bacteriophages: From isolation to application. Curr. Pharm. Biotechnol. 2022, 23, 337–360. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Mikhel, I.B.; Kondratieva, V.M.; Demina, N.B.; Grebennikova, T.V.; Krasnyuk, I.I., Jr.; Krasnyuk, I.I. Main Aspects of Pharmaceutical Development of In Situ Immunobiological Drugs for Intranasal Administration. Curr. Pharm. Biotechnol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Xiang, J.-Y.; Chi, Y.-Y.; Han, J.-X.; Kong, P.; Liang, Z.; Wang, D.; Xiang, H.; Xie, Q. Litchi chinensis seed prevents obesity and modulates the gut microbiota and mycobiota compositions in high-fat diet-induced obese zebrafish. Food Funct. 2022, 13, 2832–2845. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhang, X.; Zhang, F.; Linhardt, R.J. Structural and immunological studies on the polysaccharide from spores of a medicinal entomogenous fungus Paecilomyces cicadae. Carbohydr. Polym. 2021, 254, 117462. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zou, Y.; Chi, H.; Liao, D. Lipid-Modifying Effects of Chitosan Supplementation in Humans: A Pooled Analysis with Trial Sequential Analysis. Mol. Nutr. Food Res. 2018, 62, e1700842. [Google Scholar] [CrossRef] [PubMed]
- Vlasenko, V.A.; Ilyicheva, T.; Teplyakova, T.; Svyatchenko, S.; Asbaganov, S.; Zmitrovich, I.; Vlasenko, A.V. Antiviral activity of total polysaccharide fraction of water and ethanol extracts of Pleurotus pulmonarius against the influenza A virus. Curr. Res. Environ. Appl. Mycol. (J. Fungal Biol.) 2020, 10, 224–235. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Li, J.; Liu, Y.; Park, H.J.; Yang, L. Recent advances on bioactive ingredients of Morchella esculenta. Appl. Biochem. Biotechnol. 2021, 193, 4197–4213. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, A.; Betül, Ö.Z.; Mustafa, S.; Emre Cem, E.; Zeliha, S.; Celal, B.A.L. Phenolic compound and antioxidant potential of Hebeloma sinapizans Mushroom. AgroLife Sci. J. 2023, 12, 12–17. [Google Scholar]
- Alvandi, H.; Hatamian-Zarmi, A.; Hosseinzadeh, B.E.; Mokhtari-Hosseini, Z.B.; Langer, E.; Aghajani, H. Improving the biological properties of Fomes fomentarius MG835861 exopolysaccharide by bioincorporating selenium into its structure. Carbohydr. Polym. Technol. Appl. 2021, 2, 100159. [Google Scholar] [CrossRef]
- Wan, X.; Jin, X.; Xie, M.; Liu, J.; Gontcharov, A.A.; Wang, H.; Lv, R.; Liu, D.; Wang, Q.; Li, Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 163, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Chandra, S.; Acharya, K. Hot alkali-extracted antioxidative crude polysaccharide from a novel mushroom enhances immune response via TLR-mediated NF-κB activation: A strategy for full utilization of a neglected tribal food. J. Food Biochem. 2021, 45, e13594. [Google Scholar] [CrossRef]
- Thomas, S.; Patil, A.B.; Salgaonkar, P.N.; Shrivastava, S.; Nigam, P.S.-N. Screening of bacterial isolates from seafood-wastes for chitin degrading enzyme activity. Chem. Eng. Process Tech. 2020, 5, 1059. [Google Scholar]
- Ullah, K.; Khan, S.A.; Mannan, A.; Khan, R.; Murtaza, G.; Yameen, M.A. Enhancing the antibacterial activity of erythromycin with titanium dioxide nanoparticles against MRSA. Curr. Pharm. Biotechnol. 2020, 21, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Sun, J.; Jian, T.; Zhang, G.; Ling, J. Immunomodulatory and antioxidant effects of polysaccharides from the parasitic fungus Cordyceps kyushuensis. BioMed Res. Int. 2020, 2020, 8257847. [Google Scholar] [CrossRef] [PubMed]
- Bolaniran, T.; Jamiu, A.T.; Garuba, T.; Wudil, A.M.; Adeola, H.A.; Sabiu, S. An appraisal of the metabolites, pharmacological and biotechnological significance of edible mushrooms. Trans. R. Soc. S. Afr. 2021, 76, 257–272. [Google Scholar] [CrossRef]
- Nieto-Domínguez, M.; Martínez-Fernández, J.A.; de Toro, B.F.; Méndez-Líter, J.A.; Cañada, F.J.; Prieto, A.; de Eugenio, L.I.; Martínez, M.J. Exploiting xylan as sugar donor for the synthesis of an antiproliferative xyloside using an enzyme cascade. Microb. Cell Factories 2019, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Thimmaraju, A.; Govindan, S. Novel studies of characterization, antioxidant, anticoagulant and anticancer activity of purified polysaccharide from Hypsizygus ulmarius mushroom. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100308. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.; Gao, H.; Fan, L.; Chen, H.; Nie, S. Polysaccharide from Plantago asiatica L. attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocoll. 2019, 86, 34–42. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Rasanen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietilainen, K.H.; et al. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metab. 2018, 27, 559–571.e5. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Huang, G.; Li, X.; Leong, W.; Xia, W.; Hsiao, W.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Su, A.; Ma, G.; Xie, M.; Ji, Y.; Li, X.; Zhao, L.; Hu, Q. Characteristic of polysaccharides from Flammulina velutipes in vitro digestion under salivary, simulated gastric and small intestinal conditions and fermentation by human gut microbiota. Int. J. Food Sci. Technol. 2019, 54, 2277–2287. [Google Scholar] [CrossRef]
- Zhao, R.; Cheng, N.; Nakata, P.A.; Zhao, L.; Hu, Q. Consumption of polysaccharides from Auricularia auricular modulates the intestinal microbiota in mice. Food Res. Int. 2019, 123, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X. Lentinula edodes-derived polysaccharide alters the spatial structure of gut microbiota in mice. PLoS ONE 2015, 10, e0115037. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Khanday, F.A.; Masoodi, F. Biological and pharmaceutical activities of mushroom β-glucan discussed as a potential functional food ingredient. Bioact. Carbohydr. Diet. Fibre 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Zheng, H.; Joglekar, P.; Higginbottom, S.K.; Firbank, S.J.; Bolam, D.N.; Sonnenburg, J.L. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 2010, 141, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lai, L.; Teng, L.; Li, Y.; Cheng, J.; Chen, J.; Deng, C. Mechanism of the anti-inflammatory activity by a polysaccharide from Dictyophora indusiata in lipopolysaccharide-stimulated macrophages. Int. J. Biol. Macromol. 2019, 126, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Wang, L.; Yu, C.; Zhang, G.; Zhu, H.; Wang, C.; Zhao, S.; Hu, C.-A.A.; Liu, Y. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Hu, H.; Xiao, X.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model. Food Funct. 2019, 10, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuan, S.; Ye, J.; Wang, X.; Zhang, X.; Shen, J.; Yuan, M.; Liao, W. Polysaccharide from flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int. J. Biol. Macromol. 2020, 149, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Yoon, Y.; Lee, J. The Immunomodulatory Effect of β-Glucan Depends on the Composition of the Gut Microbiota. Foods 2023, 12, 3148. [Google Scholar] [CrossRef]
- Neun, B.W.; Cedrone, E.; Potter, T.M.; Crist, R.M.; Dobrovolskaia, M.A. Detection of beta-glucan contamination in nanotechnology-based formulations. Molecules 2020, 25, 3367. [Google Scholar] [CrossRef]
- Eker, F.D.; Mutlu, A. Oat, Buckwheat and Whole Brown Rice Flours as a Potential Prebiotic for Lactobacillus acidophilus (LA5), Lactobacillus casei and Bifidobacterium animalis subsp. lactis (BB-12). Harran Üniversitesi Mühendislik Derg. 2022, 7, 91–98. [Google Scholar]
- Lao, E.J.; Dimoso, N.; Raymond, J.; Mbega, E.R. The prebiotic potential of brewers’ spent grain on livestock’s health: A review. Trop. Anim. Health Prod. 2020, 52, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Kostopoulos, I.; Geerlings, S.Y.; Smidt, H.; de Vos, W.M.; Belzer, C. Dynamic metabolic interactions and trophic roles of human gut microbes identified using a minimal microbiome exhibiting ecological properties. ISME J. 2022, 16, 2144–2159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Heath, A.-L.; Galland, B.; Rehrer, N.; Drummond, L.; Wu, X.-Y.; Bell, T.J.; Lawley, B.; Sims, I.M.; Tannock, G.W. Substrate use prioritization by a coculture of five species of gut bacteria fed mixtures of arabinoxylan, xyloglucan, beta-glucan, and pectin. Appl. Environ. Microbiol. 2020, 86, e01905-19. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Yokoi, T.; Sugiyama, M.; Osawa, R.; Mukai, T.; Okada, N. Roles of the cell surface architecture of bacteroides and bifidobacterium in the gut colonization. Front. Microbiol. 2021, 12, 754819. [Google Scholar] [CrossRef] [PubMed]
- Dyksma, S.; Gallert, C. Effect of magnetite addition on transcriptional profiles of syntrophic Bacteria and Archaea during anaerobic digestion of propionate in wastewater sludge. Environ. Microbiol. Rep. 2022, 14, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kedar, S.; Keizer, D.; Schwartz, S.; Rabinowitz, K.M.; Kaboub, K.; Barda, E.S.; Sadot, E.; Wolff-Bar, M.; Shaltiel, T.; Dotan, I. Commensal fungi and their cell-wall β-glucans direct differential responses in human intestinal epithelial cells. Eur. J. Immunol. 2021, 51, 864–878. [Google Scholar] [CrossRef] [PubMed]
- White, J.B.; Silale, A.; Feasey, M.; Heunis, T.; Zhu, Y.; Zheng, H.; Gajbhiye, A.; Firbank, S.; Baslé, A.; Trost, M.; et al. Outer membrane utilisomes mediate oligosaccharide uptake in gut Bacteroidetes. bioRxiv 2023, 618, 583–589. [Google Scholar]
- Wu, B.; Jin, C.; Wang, Y.; Zhang, Y.; Zheng, T.; Zhou, B.; Guan, L.; Yu, L. Screening and Verification of Host Proteins Interacting with Neospora caninum AMA1 Using a Yeast Two-hybrid System. Res. Sq. 2020. preprint. [Google Scholar]
- Telle-Hansen, V.H.; Gaundal, L.; Hogvard, B.; Ulven, S.M.; Holven, K.B.; Byfuglien, M.G.; Mage, I.; Knutsen, S.H.; Ballance, S.; Rieder, A.; et al. A Three-day intervention with granola containing cereal beta-glucan improves glycemic response and changes the gut microbiota in healthy individuals: A crossover study. Front. Nutr. 2022, 9, 796362. [Google Scholar] [CrossRef]
- Golisch, B.; Lei, Z.; Tamura, K.; Brumer, H. Configured for the human gut microbiota: Molecular mechanisms of dietary β-glucan utilization. ACS Chem. Biol. 2021, 16, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Bhardwaj, A. β-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulou, M.; Yannakoulia, M.; Pletsa, V.; Zervakis, G.I.; Kyriacou, A. Effects of fungal beta-glucans on health—A systematic review of randomized controlled trials. Food Funct. 2021, 12, 3366–3380. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, N.; Hrynkiewicz, K.; Thiem, D.; Randazzo, C.; Walsh, A.M.; Guinan, K.J.; O’sullivan, J.T.; Stadnicka, K. Evaluation of probiotic growth stimulation using prebiotic ingredients to optimize compounds for in ovo delivery. Front. Microbiol. 2023, 14, 1242027. [Google Scholar] [CrossRef] [PubMed]
- Ejby, M.; Guskov, A.; Pichler, M.J.; Zanten, G.; Schoof, E.; Saburi, W.; Slotboom, D.J.; Abou Hachem, M. Two binding proteins of the ABC transporter that confers growth of Bifidobacterium animalis subsp. lactis ATCC27673 on β-mannan possess distinct manno-oligosaccharide-binding profiles. Mol. Microbiol. 2019, 112, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Theilmann, M.C.; Fredslund, F.; Svensson, B.; Leggio, L.L.; Abou Hachem, M. Substrate preference of an ABC importer corresponds to selective growth on β-(1,6)-galactosides in Bifidobacterium animalis subsp. lactis. J. Biol. Chem. 2019, 294, 11701–11711. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Ojima, M.N.; Sakanaka, M.; Ashida, H.; Gotoh, A.; Katayama, T. Enzymatic adaptation of Bifidobacterium bifidum to host glycans, viewed from glycoside hydrolyases and carbohydrate-binding modules. Microorganisms 2020, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, M.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Edible fungal polysaccharides, the gut microbiota, and host health. Carbohydr. Polym. 2021, 273, 118558. [Google Scholar] [CrossRef]
- Gudi, R.; Perez, N.; Johnson, B.M.; Sofi, M.H.; Brown, R.; Quan, S.; Karumuthil-Melethil, S.; Vasu, C. Complex dietary polysaccharide modulates gut immune function and microbiota, and promotes protection from autoimmune diabetes. Immunology 2019, 157, 70–85. [Google Scholar] [CrossRef]
- Guo, W.-L.; Chen, M.; Pan, W.-L.; Zhang, Q.; Xu, J.-X.; Lin, Y.-C.; Li, L.; Liu, B.; Bai, W.-D.; Zhang, Y.-Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, Y.; Lu, X.; Chang, Y. Auricularia auricula-judae (Bull.) polysaccharides improve type 2 diabetes in HFD/STZ-induced mice by regulating the AKT/AMPK signaling pathways and the gut microbiota. J. Food Sci. 2021, 86, 5479–5494. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lei, Y.; Guo, M.; Wang, L. Research Progress on the Hypoglycemic Effects and Mechanisms of Action of Momordica charantia polysaccharide. J. Food Biochem. 2023, 2023, 8867155. [Google Scholar] [CrossRef]
- Frommhagen, M.; Sforza, S.; Westphal, A.H.; Visser, J.; Hinz, S.W.A.; Koetsier, M.J.; van Berkel, W.J.H.; Gruppen, H.; Kabel, M.A. Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase. Biotechnol. Biofuels 2015, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, D.; Guan, J.; Chen, J.; Xu, X. Mushroom polysaccharides with potential in anti-diabetes: Biological mechanisms, extraction, and future perspectives: A review. Front. Nutr. 2022, 9, 1087826. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-Z.; Chen, K.-W.; Huang, Z.-F.; Liu, Y.; Jia, R.-B.; Liu, B. Sanghuangporus vaninii fruit body polysaccharide alleviates hyperglycemia and hyperlipidemia via modulating intestinal microflora in type 2 diabetic mice. Front. Nutr. 2022, 9, 1013466. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Du, H.; Hu, Q.; Yang, W.; Pei, F.; Xiao, H. Health benefits of edible mushroom polysaccharides and associated gut microbiota regulation. Crit. Rev. Food Sci. Nutr. 2021, 62, 6646–6663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Zhang, X. Modulation of intestinal flora by dietary polysaccharides: A novel approach for the treatment and prevention of metabolic disorders. Foods 2022, 11, 2961. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.B.; Vasu, C. Impact of prebiotic β-glucan treatment at juvenile age on the gut microbiota composition and the eventual type 1 diabetes onset in non-obese diabetic mice. Front. Nutr. 2021, 8, 769341. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.S.; Brennan, M.A.; Binosha Fernando, W.; Martins, R.N. Current Understanding of Alzheimer’s Disease and Other Neurodegenerative Diseases, and the Potential Role of Diet and Lifestyle in Reducing the Risks of Alzheimer’s Disease and Cognitive Decline. In Neurodegeneration and Alzheimer’s Disease: The Role of Diabetes, Genetics, Hormones, and Lifestyle; Wiley: Hoboken, NJ, USA, 2019; pp. 1–8. [Google Scholar]
- Solano-Aguilar, G.I.; Lakshman, S.; Jang, S.; Gupta, R.; Molokin, A.; Schroeder, S.G.; Gillevet, P.M.; Urban, J.F. The Effects of Consuming White Button Mushroom Agaricus bisporus on the Brain and Liver Metabolome Using a Targeted Metabolomic Analysis. Metabolites 2021, 11, 779. [Google Scholar] [CrossRef]
- Foster, J.A.; Baker, G.B.; Dursun, S.M. The Relationship Between the Gut Microbiome-Immune System-Brain Axis and Major Depressive Disorder. Front. Neurol. 2021, 12, 721126. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Chaudhuri, K.R.; Reynolds, R.; Tan, E.-K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain J. Neurol. 2021, 144, 2571–2593. [Google Scholar] [CrossRef]
- Chait, Y.A.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Nutritional and therapeutic approaches for protecting human gut microbiota from psychotropic treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 108, 110182. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Yuan, H.; Huang, S.; Park, S. Mitigation of memory impairment with fermented fucoidan and λ-carrageenan supplementation through modulating the gut microbiota and their metagenome function in hippocampal amyloid-β infused rats. Cells 2022, 11, 2301. [Google Scholar] [CrossRef]
- Sharvin, B.L.; Aburto, M.R.; Cryan, J.F. Decoding the neurocircuitry of gut feelings: Region-specific microbiome-mediated brain alterations. Neurobiol. Dis. 2023, 179, 106033. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ueyama, J.; Kashihara, K.; Ito, M.; Hamaguchi, T.; Maeda, T.; Tsuboi, Y.; Katsuno, M.; Hirayama, M.; Ohno, K. Gut microbiota in dementia with Lewy bodies. NPJ Park. Dis. 2022, 8, 169. [Google Scholar] [CrossRef]
- Yıldırım, S.; Nalbantoğlu, U.; Bayraktar, A.; Ercan, F.B.; Gündoğdu, A.; Velioğlu, H.A.; Göl, M.F.; Soylu, A.E.; Koç, F.; Gülpınar, E.A.; et al. Stratification of the gut microbiota composition landscape across the alzheimer’s disease continuum in a turkish cohort. mSystems 2022, 7, e0000422. [Google Scholar] [CrossRef]
- Sahu, N.; Upadhyay, P.; Mishra, S.K. Role of Short-Chain Fatty Acids from Gut Microbiota in Neuroendocrine Pathogenesis Management. In Gut Microbiome in Neurological Health and Disorders; Springer: Berlin/Heidelberg, Germany, 2022; pp. 139–151. [Google Scholar]
- Ghaffari, S.; Abbasi, A.; Somi, M.H.; Moaddab, S.Y.; Nikniaz, L.; Kafil, H.S.; Leylabadlo, H.E. Akkermansia muciniphila: From its critical role in human health to strategies for promoting its abundance in human gut microbiome. Crit. Rev. Food Sci. Nutr. 2023, 63, 7357–7377. [Google Scholar] [CrossRef]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef]
- Swallah, M.S.; Yu, H.; Piao, C.; Fu, H.; Yakubu, Z.; Sossah, F.L. Synergistic Two-Way Interactions of dietary polyphenols and dietary components on the gut microbial composition: Is there a positive, negative, or neutralizing effect in the prevention and management of metabolic diseases? Curr. Protein Pept. Sci. 2021, 22, 313–327. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Plaza-Diaz, J. Dietary Polysaccharides as Modulators of the Gut Microbiota Ecosystem: An Update on Their Impact on Health. Nutrients 2022, 14, 4116. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Qian, C.; Hussain, M.; Liu, S.; Zhang, A.; He, R.; Sun, P. The Interaction between Mushroom Polysaccharides and Gut Microbiota and Their Effect on Human Health: A Review. Biology 2023, 12, 122. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Zhao, Q.; Manzi, H.P.; Su, L.; Liu, D.; Huang, X.; Long, D.; Tang, Z.; Zhang, Y. The benefits of edible mushroom polysaccharides for health and their influence on gut microbiota: A review. Front. Nutr. 2023, 10, 1213010. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Li, L.-X.; Feng, X.; Tao, M.-T.; Paulsen, B.S.; Huang, C.; Feng, B.; Liu, W.; Yin, Z.-Q.; Song, X.; Zhao, X.; et al. Benefits of neutral polysaccharide from rhizomes of Polygonatum sibiricum to intestinal function of aged mice. Front. Nutr. 2022, 9, 992102. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Han, C.; Nie, S. Advances in Polygonatum sibiricum polysaccharides: Extraction, purification, structure, biosynthesis, and bioactivity. Front. Nutr. 2022, 9, 1074671. [Google Scholar] [CrossRef]
- Han, Y.; Nan, S.; Fan, J.; Chen, Q.; Zhang, Y. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int. J. Biol. Macromol. 2019, 131, 769–778. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Kiddane, A.T.; Kim, G.-D. Anticancer and immunomodulatory effects of polysaccharides. Nutr. Cancer 2021, 73, 2219–2231. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, Y.; Yao, L.; Peng, J.; Tu, Y.; He, B. Research progress on the prevention of tumor by fungal polysaccharides. Trends Food Sci. Technol. 2024, 147, 104422. [Google Scholar] [CrossRef]
- Tortora, S.C.; Bodiwala, V.M.; Quinn, A.; Martello, L.A.; Vignesh, S. Microbiome and colorectal carcinogenesis: Linked mechanisms and racial differences. World J. Gastrointest. Oncol. 2022, 14, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zheng, S.; Li, M.; Xu, C.; Jia, D.; Qi, Y.; Hou, T.; Wang, L.; Wang, B.; et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes 2022, 14, 2038852. [Google Scholar] [CrossRef] [PubMed]
- Aidid, E.M.; Shalihin, M.S.E.; Nor, A.M.; Hamzah, H.A.; Ab Hamid, N.F.; Bahri, N.A.N.S.; Ghani, N.D.A. Risk of colorectal cancer due to Streptococcus gallolyticus: A systematic review. Med. J. Malays. 2023, 78, 404–410. [Google Scholar]
- Williamson, A.J.; Jacobson, R.; van Praagh, J.; Gaines, S.; Koo, H.Y.; Lee, B.; Chan, W.-C.; Weichselbaum, R.; Alverdy, J.C.; Zaborina, O.; et al. Enterococcus faecalis promotes a migratory and invasive phenotype in colon cancer cells. Neoplasia 2022, 27, 100787. [Google Scholar] [CrossRef] [PubMed]
- Oliero, M.; Hajjar, R.; Cuisiniere, T.; Fragoso, G.; Calvé, A.; Dagbert, F.; Loungnarath, R.; Sebajang, H.; Schwenter, F.; Wassef, R.; et al. Prevalence of pks+ bacteria and enterotoxigenic Bacteroides fragilis in patients with colorectal cancer. Gut Pathog. 2022, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; Van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Bezem, M.T. Functionalization of Nanoparticles with Tyrosine Hydroxylase: Biotechnological and Therapeutic Implications. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2022. [Google Scholar]
- Wei, Y.; Song, D.; Wang, R.; Li, T.; Wang, H.; Li, X. Dietary fungi in cancer immunotherapy: From the perspective of gut microbiota. Front. Oncol. 2023, 13, 1038710. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Liu, C.; Hua, H.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Comprehensive analysis of Sparassis crispa polysaccharide characteristics during the in vitro digestion and fermentation model. Food Res. Int. 2022, 154, 111005. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Doocey, C.M.; Finn, K.; Murphy, C.; Guinane, C.M. The impact of the human microbiome in tumorigenesis, cancer progression, and biotherapeutic development. BMC Microbiol. 2022, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Cancemi, G.; Caserta, S.; Gangemi, S.; Pioggia, G.; Allegra, A. Exploring the Therapeutic Potential of Ganoderma lucidum in Cancer. J. Clin. Med. 2024, 13, 1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Xie, D. Targeting the gut microbiota to enhance the antitumor efficacy and attenuate the toxicity of CAR-T cell therapy: A new hope? Front. Immunol. 2024, 15, 1362133. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, W.; Zhao, L. Regulatory Effects of Ganoderma lucidum, Grifola frondosa, and American ginseng Extract Formulation on Gut Microbiota and Fecal Metabolomics in Mice. Foods 2023, 12, 3804. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Tang, L.; Zhou, Y.; Zhao, S.; Zhu, H. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomisation study. BMC Med. 2023, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Xue, X.; Bukhari, I.; Qiu, C.; Li, Y.; Zheng, P.; Mi, Y. Gut microbiota and its therapeutic implications in tumor microenvironment interactions. Front. Microbiol. 2024, 15, 1287077. [Google Scholar] [CrossRef] [PubMed]
- Zyoud, S.H.; Al-Jabi, S.W.; Amer, R.; Shakhshir, M.; Shahwan, M.; Jairoun, A.A.; Akkawi, M.; Abu Taha, A. Global research trends on the links between the gut microbiome and cancer: A visualization analysis. J. Transl. Med. 2022, 20, 83. [Google Scholar] [CrossRef]
- Dumitru, M.; Sorescu, I.; Hăbeanu, M.; Tabuc, C.; Jurcoane, Ş. Preliminary characterization in vitro of Bacillus licheniformis strain for used as dietary probiotic. Sci. Bull. Ser. F Biotechnol. 2019, 23. [Google Scholar]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef] [PubMed]
- Micu, G.; Boiu-Sicuia, O.A.; Cornea, C.P. Genetic approaches to select L-asparaginase producing Bacillus strains. Sci. Bull. Ser. F Biotechnol. 2022, 26. [Google Scholar]
- Viswanathan, S.; Parida, S.; Lingipilli, B.T.; Krishnan, R.; Podipireddy, D.R.; Muniraj, N. Role of Gut Microbiota in Breast Cancer and Drug Resistance. Pathogens 2023, 12, 468. [Google Scholar] [CrossRef] [PubMed]


| Study Type | Functions and Health Benefits | References | |
|---|---|---|---|
| Ganoderma lucidum Polysaccharides | In Vivo, In Vitro | Enhances glucose metabolism, reduces colorectal cancer growth, promotes gut health, and protects against Alzheimer’s. | [2,3,4,5] |
| Inonotus obliquus Polysaccharides | In Vivo, In Vitro | Ameliorates type 2 diabetes, lowers lipids, and inhibits tumor growth in colitis-associated cancer. | [6,7,8] |
| Hericium erinaceus Polysaccharides | In Vivo, In Vitro | Regulates glucose and lipid metabolism, enhances gut and liver health, improves cognitive function in mice. | [9,10,11] |
| Lentinula edodes Polysaccharides | Clinical Trial, In Vivo | Evaluated in subjects with high cholesterol; no lipid change but improved dietary fiber intake and gut microbiota. Protects against diet-induced cognitive decline in mice, enhancing gut–brain axis and synaptic functions. | [12,13,14] |
| Auricularia auricula Polysaccharides | In Vivo | Boosts immune function in treated mice, enhances cytokines and tight junction proteins, and restores gut microbiota, suggesting use in food and pharmaceuticals. | [15,16] |
| Grifola frondosa Polysaccharides | In Vivo | Shows anti-hepatocellular carcinoma effects; modulates gut microbiota and SCFA in diabetic mice, improving intestinal and microbial balance. | [17,18,19,20] |
| Pleurotus ostreatus Polysaccharides | In Vivo | Supports gut health in A. japonicus and immunosuppressed mice and enhances immune response, SCFAs, and gut microbiota diversity. | [21,22,23] |
| Gynostemma pentaphyllum Polysaccharides | In Vivo | Modulates gut microbiota, enhances anticancer effects in mice. Reduces oxidative stress and inflammation in diabetic mice. | [24,25,26] |
| Amanita caesarea Polysaccharides | In Vivo | Polysaccharides enhance cognitive function, alleviate inflammation, and modulate oxidative stress in Alzheimer’s models. | [27,28] |
| Polygonatum sibiricum Polysaccharides | In Vivo, In Vitro | Improves memory and cognition in Alzheimer’s mice, enhances SCFAs and LCFAs, and modulates gut microbiota balance. | [29,30,31] |
| Morchella esculenta Polysaccharides | In Vivo, In Vitro | Regulates hyperglycemia and hyperlipidemia, improves insulin sensitivity and gut microbiota, reduces inflammation, and enhances intestinal health. | [32,33] |
| Sarcodon aspratus Polysaccharides | In Vivo | Ameliorates obesity-related metabolic disorders, modulates gut microbiota, enhances probiotics, and reduces stress-triggered bacteria. | [34,35,36] |
| Flammulina velutipes Polysaccharides | In Vivo | Mitigates CdCl2-induced intestinal inflammation and ulcerative colitis in mice, modulates gut microbiota and SCFAs, and enhances metabolic capacity. | [37,38] |
| Poria cocos Polysaccharides | In Vivo | Improves glucose intolerance, insulin resistance, and lipid metabolism. Prevents NASH progression by modulating gut microbiota and suppressing inflammation. | [39,40] |
| Boletus edulis Polysaccharides | In Vitro, In Vivo | Modulates human microbiota, reduces inflammation, enhances antioxidant capacity, and mitigates antibiotic-induced and diabetes-associated dysbiosis. | [41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. https://doi.org/10.3390/jof10060394
Barcan AS, Barcan RA, Vamanu E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. Journal of Fungi. 2024; 10(6):394. https://doi.org/10.3390/jof10060394
Chicago/Turabian StyleBarcan, Alexandru Stefan, Rares Andrei Barcan, and Emanuel Vamanu. 2024. "Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology" Journal of Fungi 10, no. 6: 394. https://doi.org/10.3390/jof10060394







