Changes in the Arbuscular Mycorrhizal Fungal Community in the Roots of Eucalyptus grandis Plantations at Different Ages in Southern Jiangxi, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Sampling
2.2. Soil Properties
2.3. AM Fungi Colonization
2.4. Sequencing and Data Analysis
2.4.1. Sequencing
2.4.2. Sequencing Data Processing
2.4.3. OTU Cluster and Species Annotation
2.5. Statistical Analysis
3. Results
3.1. Growth Statuses of Eucalypts with Different Stand Ages
3.2. Soil Properties of Eucalypt Plantations with Different Stand Ages
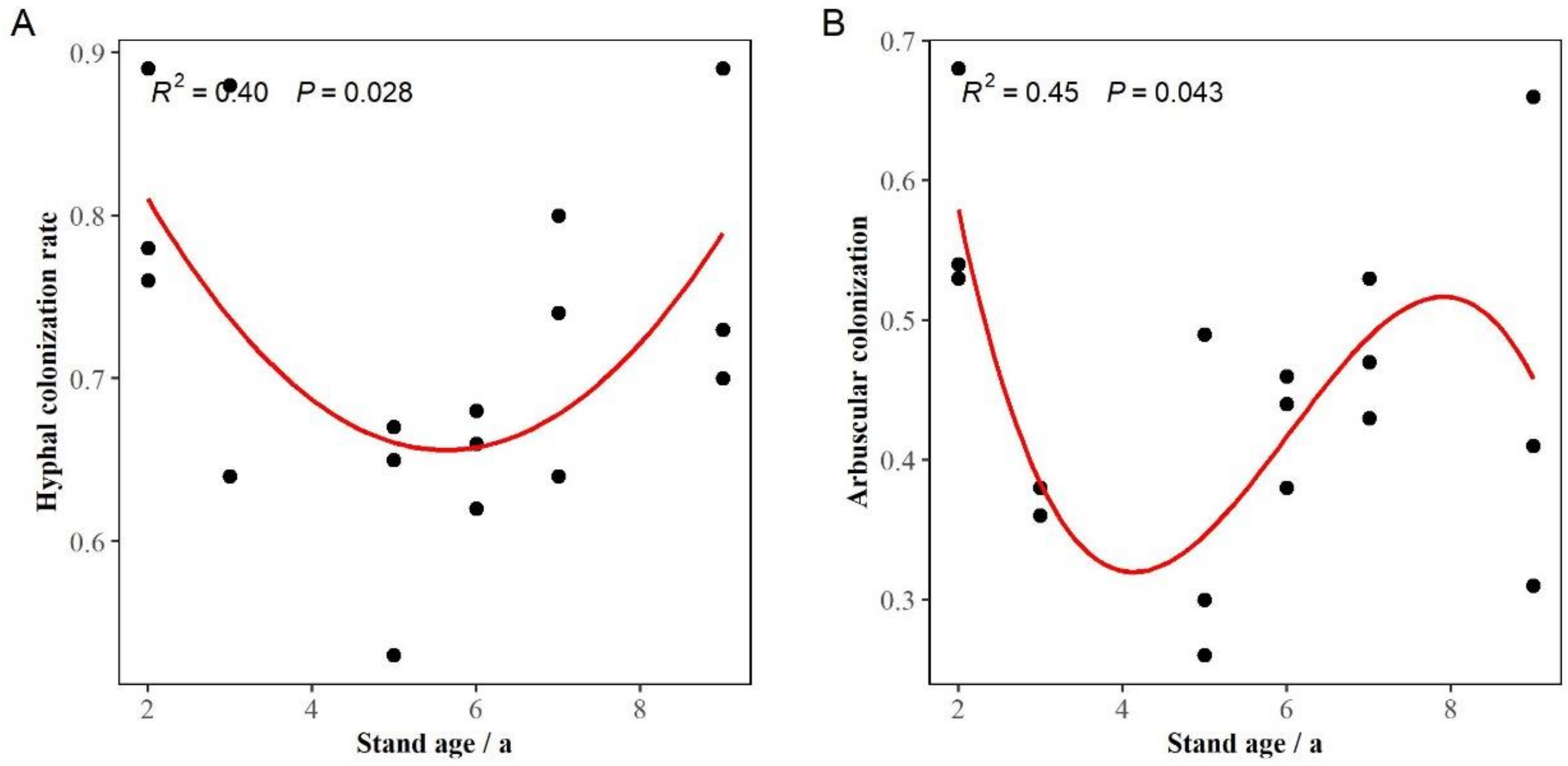
3.3. AM Fungal Colonization Rates in the Roots of Eucalypts with Different Stand Ages
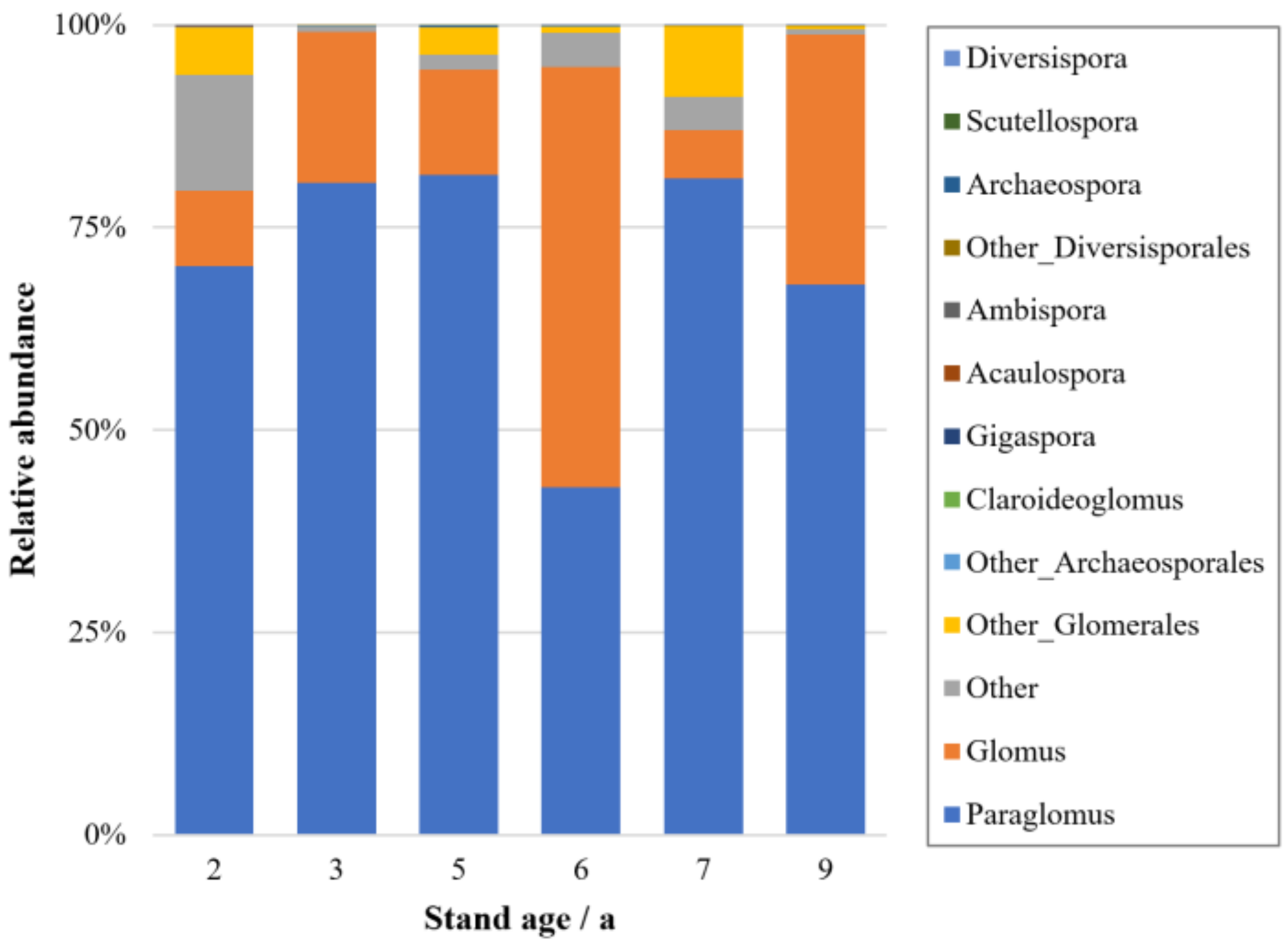
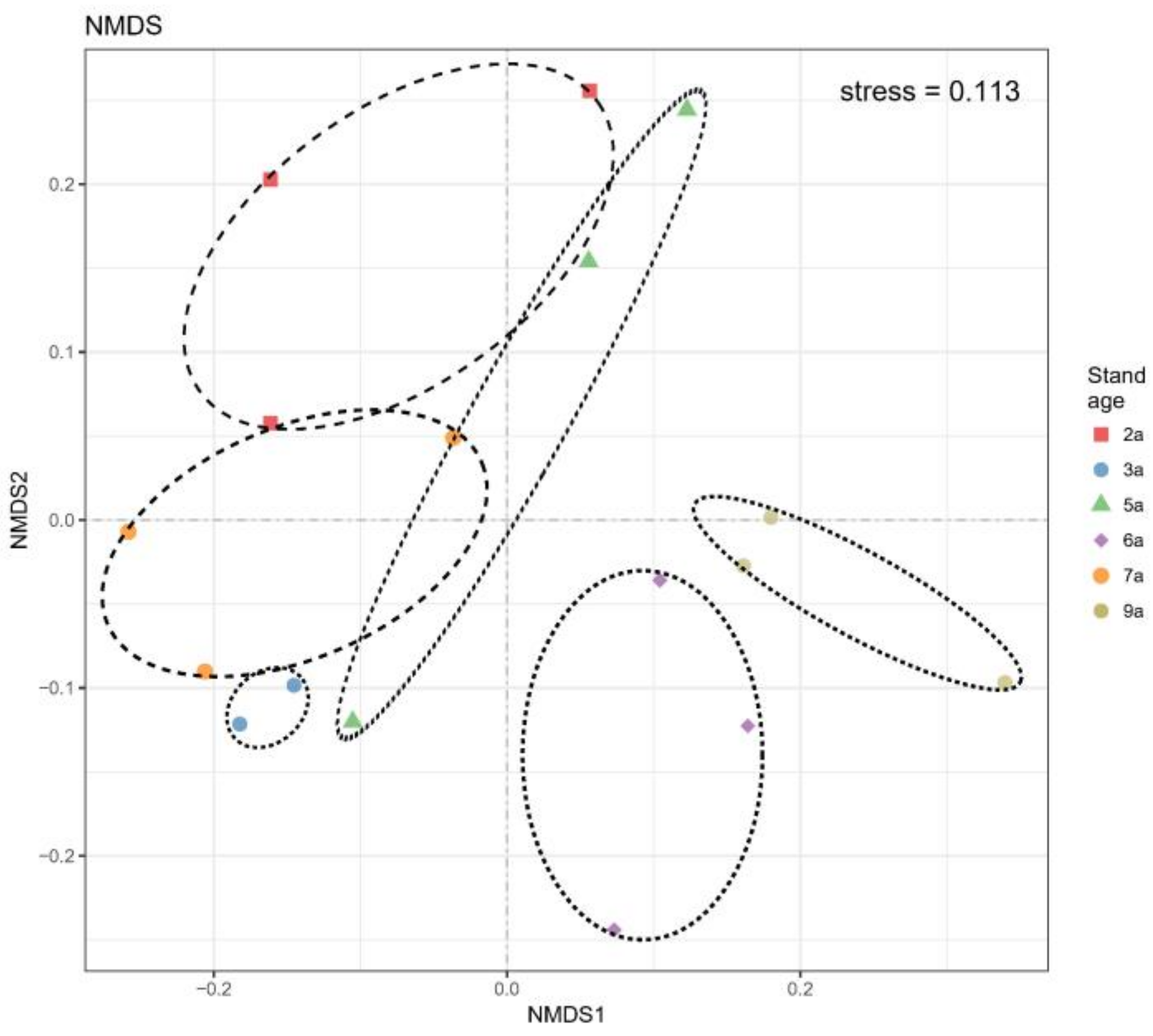
3.4. AM Fungal Identification and Community Composition with Different Stand Ages
3.5. Relationships among Plant Growth, Soil Properties, and AM Fungal Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis. Q. Rev. Biol. 2008, 3, 273–281. [Google Scholar]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; Tuinen, D.V.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Lima, F.d.S.; Sousa, C.d.S. Growth and nutrition of eucalyptus clones seedlings inoculated with mycorrhizal fungi. Pesqui. Agropecu. Trop. 2014, 44, 110–118. [Google Scholar] [CrossRef]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Frey, S.D.; Stinson, K.A. Tree seedling responses to multiple environmental stresses: Interactive effects of soil warming, nitrogen fertilization, and plant invasion. For. Ecol. Manag. 2017, 403, 44–51. [Google Scholar] [CrossRef]
- Vasconcellos, R.L.; Bonfim, J.A.; Baretta, D.; Cardoso, E.J. Arbuscular mycorrhizal fungi and glomalin-related soil protein as potential indicators of soil quality in a recuperation gradient of the Atlantic forest in Brazil. Land Degrad. Dev. 2016, 27, 325–334. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Sridhar, K.R.; Revanna, A. Arbuscular mycorrhizal fungi influence crop productivity, plant diversity, and ecosystem services. In Fungal Diversity, Ecology and Control Management; Springer: Singapore, 2022; pp. 345–362. [Google Scholar]
- Adams, F.; Reddell, P.; Webb, M.J.; Shipton, W.A. Arbuscular mycorrhizas and ectomycorrhizas on Eucalyptus grandis (Myrtaceae) trees and seedlings in native forests of tropical north-eastern Australia. Aust. J. Bot. 2006, 54, 271–281. [Google Scholar] [CrossRef]
- Murugan, R.; Beggi, F.; Kumar, S. Belowground carbon allocation by trees, understory vegetation and soil type alter microbial community composition and nutrient cycling in tropical Eucalyptus plantations. Soil Biol. Biochem. 2014, 76, 257–267. [Google Scholar] [CrossRef]
- Liu, S.; Lu, X.; Yang, G.; He, C.; Shi, Y.; Li, C.; Liu, S.; Wang, Y.; Wang, Z.; Chen, L. Variation of arbuscular mycorrhizal fungi communities in the rhizosphere soil of Eucalyptus plantations based on different stand ages and its effect on phosphorus fractionation. Appl. Soil Ecol. 2023, 189, 104908. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Öpik, M.; Metsis, M.; Daniell, T.J.; Zobel, M.; Moora, M.J.N.P. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 2009, 184, 424–437. [Google Scholar] [CrossRef]
- Lin, G.; McCormack, M.L.; Guo, D. Arbuscular mycorrhizal fungal effects on plant competition and community structure. J. Ecol. 2015, 103, 1224–1232. [Google Scholar] [CrossRef]
- Cavagnaro, T.; Gao, L.; Smith, F.A.; Smith, S.E. Morphology of arbuscular mycorrhizas is influenced by fungal identity. New Phytol. 2001, 151, 469–475. [Google Scholar] [CrossRef]
- Vierheilig, H.; Schweiger, P.; Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 2005, 125, 393–404. [Google Scholar] [CrossRef]
- Egan, C.P.; Rummel, A.; Kokkoris, V.; Klironomos, J.; Lekberg, Y.; Hart, M. Using mock communities of arbuscular mycorrhizal fungi to evaluate fidelity associated with Illumina sequencing. Fungal Ecol. 2018, 33, 52–64. [Google Scholar] [CrossRef]
- Moebius-Clune, D.J.; Anderson, Z.U.; Pawlowska, T.E. Arbuscular mycorrhizal fungi associated with a single agronomic plant host across the landscape: The structure of an assemblage. Soil Biol. Biochem. 2013, 64, 181–190. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.H.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Hishi, T.; Tateno, R.; Fukushima, K.; Fujimaki, R.; Itoh, M.; Tokuchi, N. Changes in the anatomy, morphology and mycorrhizal infection of fine root systems of Cryptomeria japonica in relation to stand ageing. Tree Physiol. 2016, 37, 61–70. [Google Scholar]
- Nakano-Hylander, A.; Olsson, P.A. Carbon allocation in mycelia of arbuscular mycorrhizal fungi during colonisation of plant seedlings. Soil Biol. Biochem. 2007, 39, 1450–1458. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef]
- Jerbi, M.; Labidi, S.; Bahri, B.A.; Laruelle, F.; Tisserant, B.; Jeddi, F.B.; Sahraoui, A.L.-H. Soil properties and climate affect arbuscular mycorrhizal fungi and soil microbial communities in Mediterranean rainfed cereal cropping systems. Pedobiologia 2021, 87, 150748. [Google Scholar] [CrossRef]
- Carrenho, R.; Trufem, S.F.B.; Bononi, V.L.R.; Silva, E.S. The effect of different soil properties on arbuscular mycorrhizal colonization of peanuts, sorghum and maize. Acta Bot. Bras. 2007, 21, 723–730. [Google Scholar] [CrossRef]
- Xu, R.K.; Zhao, A.Z.; Li, Q.M.; Kong, X.L.; Ji, G.L. Acidity regime of the Red Soils in a subtropical region of southern China under field conditions. Geoderma 2003, 115, 75–84. [Google Scholar] [CrossRef]
- Wang, L.; Yan, H.; Wang, X.; Wang, Z.; Yu, S.; Wang, T.; Shi, Z. The potential for soil erosion control associated with socio-economic development in the hilly red soil region, southern China. Catena 2020, 194, 104678. [Google Scholar] [CrossRef]
- Turnbull, J.W. Eucalypt plantations. New For. 1999, 17, 37–52. [Google Scholar] [CrossRef]
- Cuong, T.; Chinh, T.T.Q.; Zhang, Y.; Xie, Y. Economic performance of forest plantations in Vietnam: Eucalyptus, Acacia mangium, and Manglietia conifera. Forests 2020, 11, 284. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Rockville, MA, USA, 1995; Volume 1. [Google Scholar]
- Jorhem, L. Determination of Metals in Foods by Atomic Absorption Spectrometry after Dry Ashing: NMKL1 Collaborative Study. J. AOAC Int. 2000, 83, 1204–1211. [Google Scholar] [CrossRef]
- Grattan, S.; Shannon, M.; Grieve, C.; Poss, J.; Suarez, D.; Leland, F. Interactive effects of salinity and boron on the performance and water use of eucalyptus. In II International Symposium on Irrigation of Horticultural Crops 449; ISHS: Leuven, Belgium, 1996; pp. 607–614. [Google Scholar]
- Hodecker, B.E.R.; De Barros, N.F.; Da Silva, I.R.; Diola, V.; Sarkis, J.E.S.; Loureiro, M.E. Boron delays dehydration and stimulates root growth in Eucalyptus urophylla (Blake, ST) under osmotic stress. Plant Soil 2014, 384, 185–199. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for cleaning and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Yan, L.; Mu, X.; Han, B.; Zhang, S.; Qiu, C.; Ohore, O.E. Ammonium loading disturbed the microbial food webs in biofilms attached to submersed macrophyte Vallisneria natans. Sci. Total Environ. 2019, 659, 691–698. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kanzaki, N.; Tamai, S.; Yamanaka, N.; Futai, K.J.N.P. Does ectomycorrhizal fungal community structure vary along a Japanese black pine (Pinus thunbergii) to black locust (Robinia pseudoacacia) gradient? New Phytol. 2007, 173, 322–334. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Luo, C.-Y.; Zhang, Z.-H.; Wang, S.-P.; Guo, L.-D. Plant Identity Exerts Stronger Effect than Fertilization on Soil Arbuscular Mycorrhizal Fungi in a Sown Pasture. Microb. Ecol. 2016, 72, 647–658. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, W.; Ouyang, S.; Xiao, W.; Li, S.; Chen, L.; Lei, P.; Deng, X.; Zeng, Y.; Zeng, L. Tree growth rate and soil nutrient status determine the shift in nutrient-use strategy of Chinese fir plantations along a chronosequence. For. Ecol. Manag. 2020, 460, 117896. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust. J. Plant Physiol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Laclau, J.P.; Almeida, J.C.; Gonçalves, J.L.; Saint-André, L.; Ventura, M.; Ranger, J.; Moreira, R.M.; Nouvellon, Y. Influence of nitrogen and potassium fertilization on leaf lifespan and allocation of above-ground growth in Eucalyptus plantations. Tree Physiol. 2009, 29, 111. [Google Scholar] [CrossRef]
- de Moraes Goncalves, J.L.; Stape, J.L.; Laclau, J.P.; Smethurst, P.; Gava, J.L. Silvicultural effects on the productivity and wood quality of eucalypt plantations. For. Ecol. Manag. 2004, 193, 45–61. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Tiwari, G.; Mishra, P.K.; Pattanayak, A.; Bisht, J.K. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2020, 202, 1581–1596. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Metcalfe, D.B.; Inselsbacher, E.; Stangl, Z.; Oren, R.; Näsholm, T.; Högberg, P. Greater carbon allocation to mycorrhizal fungi reduces tree nitrogen uptake in a boreal forest. Ecology 2016, 97, 1012–1022. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.; Xue, S. Effect of black locust (Robinia pseudoacacia) on soil chemical and microbiological properties in the eroded hilly area of China’s Loess Plateau. Environ. Earth Sci. 2012, 65, 597–607. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Dai, Y.; Liu, Q.; Tang, J.; Bian, X.; Chen, X. Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: A meta-analysis. Plant Soil 2015, 389, 361–374. [Google Scholar] [CrossRef]
- Gonçalves, J.D.M.; Barros, N.F.; Nambiar, E.K.S.; Novais, R.F. Management of soil, nutrients and water in tropical plantation forests. In Soil and Stand Management for Short-Rotation Plantations; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1997. [Google Scholar]
- Laclau, J.P.; Ranger, J.; Deleporte, P.; Nouvellon, Y.; Saint-André, L.; Marlet, S.; Bouillet, J.-P. Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo: 3. Input–output budgets and consequences for the sustainability of the plantations. For. Ecol. Manag. 2003, 210, 375–391. [Google Scholar] [CrossRef]
- Carrenho, R.; Barbosa, F.F.; Araújo, C.V.; Alves, L.J.; Santos, O.M. Mycorrhizal associations in Eucalyptus spp.: Status and needs. Tree For. Sci. Biotechnol. 2008, 2, 57–67. [Google Scholar]
- Khaekhum, S.; Lumyong, S.; Kuyper, T.W.; Boonlue, S. Species richness and composition of arbuscular mycorrhizal fungi occurring on eucalypt trees (Eucalyptus camaldulensis Dehnh.) in rainy and dry season. Curr. Res. Environ. Appl. Mycol. 2017, 7, 282–292. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, P.; Wang, P.; Wang, X.; Ji, B.; Mu, J. Environmental factors affect the arbuscular mycorrhizal fungal community through the status of host plants in three patterns of Chinese fir in southern China. Glob. Ecol. Conserv. 2022, 36, e02121. [Google Scholar] [CrossRef]
- Weber, O.B.; da Silva, M.C.B.; da Silva, C.F.; Correia, D.; dos Santos Garruti, D.; Pagano, M.C. Diversity of mycorrhizal fungi and soil indicative species in coastal plantations of northeast Brazil. J. For. Res. 2021, 32, 1203–1211. [Google Scholar] [CrossRef]
- Schwarzott, D.; Walker, C.; Schüßler, A. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales), is nonmonophyletic. Mol. Phylogenet. Evol. 2001, 21, 190–197. [Google Scholar] [CrossRef]
- Rodrigues, K.; Rodrigues, B. Glomus (Chapter 27); Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Błaszkowski, J.; Kovács, G.M.; Gáspár, B.K.; Balázs, T.K.; Buscot, F.; Ryszka, P. The arbuscular mycorrhizal Paraglomus majewskii sp. nov. represents a distinct basal lineage in Glomeromycota. Mycologia 2012, 104, 148–156. [Google Scholar] [CrossRef]
- Gosling, P.; Proctor, M.; Jones, J.; Bending, G.D. Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 2014, 24, 1–11. [Google Scholar] [CrossRef]
- Freitas, R.D.; Buscardo, E.; Nagy, L.; Maciel, A.B.D.; Carrenho, R.; Luizao, R.C.C. Arbuscular mycorrhizal fungal communities along a pedo-hydrological gradient in a Central Amazonian terra firme forest. Mycorrhiza 2014, 24, 21–32. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Bräu, L.; Davison, J.; Jairus, T.; Robain, H.; Öpik, M. Diversity of root-associated arbuscular mycorrhizal fungal communities in a rubber tree plantation chronosequence in Northeast Thailand. Mycorrhiza 2016, 26, 863–877. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar] [CrossRef]
- Fu, W.; Chen, B.; Rillig, M.C.; Jansa, J.; Ma, W.; Xu, C.; Luo, W.; Wu, H.; Hao, Z.; Wu, H. Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. New Phytol. 2022, 234, 2003–2017. [Google Scholar] [CrossRef]
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 2020, 101, e02978. [Google Scholar] [CrossRef]
- Qin, H.; Chen, J.H.; Wu, Q.F.; Niu, L.M.; Li, Y.C.; Liang, C.F.; Shen, Y.; Xu, Q.F. Intensive management decreases soil aggregation and changes the abundance and community compositions of arbuscular mycorrhizal fungi in Moso bamboo (Phyllostachys pubescens) forests. For. Ecol. Manage. 2017, 400, 246–255. [Google Scholar] [CrossRef]
- Gao, S.; He, Q.; Huang, D.; Wang, Z.; Mao, J.; Xie, X.; Su, Y.; Qiu, Q.; Li, J.; Chen, Z. Responses of fungal community structure and functional composition to short-term fertilization and dry season irrigation in Eucalyptus urophylla × Eucalyptus grandis plantation soils. Forests 2022, 13, 854. [Google Scholar] [CrossRef]
- Garcia de Leon, D.; Moora, M.; Opik, M.; Neuenkamp, L.; Gerz, M.; Jairus, T.; Vasar, M.; Bueno, C.G.; Davison, J.; Zobel, M. Symbiont dynamics during ecosystem succession: Co-occurring plant and arbuscular mycorrhizal fungal communities. FEMS Microbiol. Ecol. 2016, 92, fiw097. [Google Scholar] [CrossRef]
- Pagano, M.C.; Scotti, M.R. Arbuscular and ectomycorrhizal colonization of two Eucalyptus species in semiarid Brazil. Mycoscience 2008, 49, 379–384. [Google Scholar] [CrossRef]
- Frater, P.N.; Borer, E.T.; Fay, P.A.; Jin, V.; Knaeble, B.; Seabloom, E.; Sullivan, L.; Wedin, D.A.; Harpole, W.S. Nutrients and environment influence arbuscular mycorrhizal colonization both independently and interactively in Schizachyrium scoparium. Plant Soil 2018, 425, 493–506. [Google Scholar] [CrossRef]
- Ezawa, T.; Smith, S.E.; Smith, F.A. P metabolism and transport in AM fungi. Plant Soil 2002, 244, 221–230. [Google Scholar] [CrossRef]
- Bücking, H.; Kafle, A. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.-T.; Zhu, J.-J.; Yang, K.; Yu, L.-Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Yu, Z.; Peñuelas, J.; Sardans, J.; Chen, Q.; Xu, J.; Zhou, G. Carbon, nitrogen and phosphorus stoichiometry in natural and plantation forests in China. Forests 2022, 13, 755. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]




| Stand Age (Years) | pH Value | Soil Moisture (%) | Bulk Density | Sand Content % (2–0.05 mm) | Powder Content % (0.05– 0.002 mm) | Clay Content % (<0.002 mm) |
|---|---|---|---|---|---|---|
| 2a | 4.74 ± 0.22 | 0.20 ± 0.02 | 1.38 ± 0.03 | 46.33 ± 3.79 | 35.00 ± 5.20 | 18.67 ± 1.53 |
| 3a | 4.22 ± 0.00 | 0.24 ± 0.04 | 1.36 ± 0.09 | 32.50 ± 4.95 | 42.00 ± 2.83 | 25.50 ± 2.12 |
| 5a | 4.52 ± 0.03 | 0.22 ± 0.02 | 1.37 ± 0.08 | 31.33 ± 2.08 | 45.67 ± 4.16 | 23.00 ± 3.61 |
| 6a | 4.65 ± 0.07 | 0.23 ± 0.03 | 1.30 ± 0.06 | 46.67 ± 17.24 | 31.00 ± 7.94 | 22.33 ± 9.50 |
| 7a | 4.71 ± 0.33 | 0.22 ± 0.07 | 1.42 ± 0.11 | 44.67 ± 9.02 | 34.33 ± 3.21 | 21.00 ± 6.24 |
| 9a | 4.55 ± 0.07 | 0.23 ± 0.03 | 1.32 ± 0.11 | 45.00 ± 5.57 | 33.67 ± 8.02 | 21.33 ± 2.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Mo, X.-Y.; Liu, L.-T.; Lai, G.-Z.; Qiu, G.-W. Changes in the Arbuscular Mycorrhizal Fungal Community in the Roots of Eucalyptus grandis Plantations at Different Ages in Southern Jiangxi, China. J. Fungi 2024, 10, 404. https://doi.org/10.3390/jof10060404
Jiang Y, Mo X-Y, Liu L-T, Lai G-Z, Qiu G-W. Changes in the Arbuscular Mycorrhizal Fungal Community in the Roots of Eucalyptus grandis Plantations at Different Ages in Southern Jiangxi, China. Journal of Fungi. 2024; 10(6):404. https://doi.org/10.3390/jof10060404
Chicago/Turabian StyleJiang, Yao, Xiao-Yong Mo, Li-Ting Liu, Guo-Zhen Lai, and Guo-Wei Qiu. 2024. "Changes in the Arbuscular Mycorrhizal Fungal Community in the Roots of Eucalyptus grandis Plantations at Different Ages in Southern Jiangxi, China" Journal of Fungi 10, no. 6: 404. https://doi.org/10.3390/jof10060404
APA StyleJiang, Y., Mo, X.-Y., Liu, L.-T., Lai, G.-Z., & Qiu, G.-W. (2024). Changes in the Arbuscular Mycorrhizal Fungal Community in the Roots of Eucalyptus grandis Plantations at Different Ages in Southern Jiangxi, China. Journal of Fungi, 10(6), 404. https://doi.org/10.3390/jof10060404






