Plant Growth Promotion and Biological Control against Rhizoctonia solani in Thai Local Rice Variety “Chor Khing” Using Trichoderma breve Z2-03
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Plant, Trichoderma, and Pathogen
2.2. Screening of a Total Indole Compounds Production
2.3. HPLC Analysis of Fungal IAA
2.4. Ability of Cell-Free Culture Filtrate to Impact Seed Germination and Plant Growth
2.5. Effect of Spore Suspension on Growth of Rice Seedlings
2.6. In Vitro Antifungal Test
2.7. Siderophore Production Test
2.8. Induction of Defense Responses in Rice by Selected Trichoderma Isolate
2.9. Biological Control of Rhizoctonia solani in Pot Experiment
2.10. Identification of Selected Trichoderma Isolate
2.11. Statistical Analysis
3. Results
3.1. Indole Compound-Producing Trichoderma Isolates and HPLC Analysis of Fungal IAA
3.2. HPLC Analysis of Fungal IAA
3.3. Effect of Cell Free-CF on Seed Germination
3.4. Increase in Rice Seedling Growth Caused by Trichoderma Isolate Z2-03
3.5. Antifungal Ability of Trichoderma Isolate Z2-03
3.6. Siderophore Production of Trichoderma Isolate Z2-03
3.7. Induction of Defense Responses in Rice by Trichoderma Isolate Z2-03
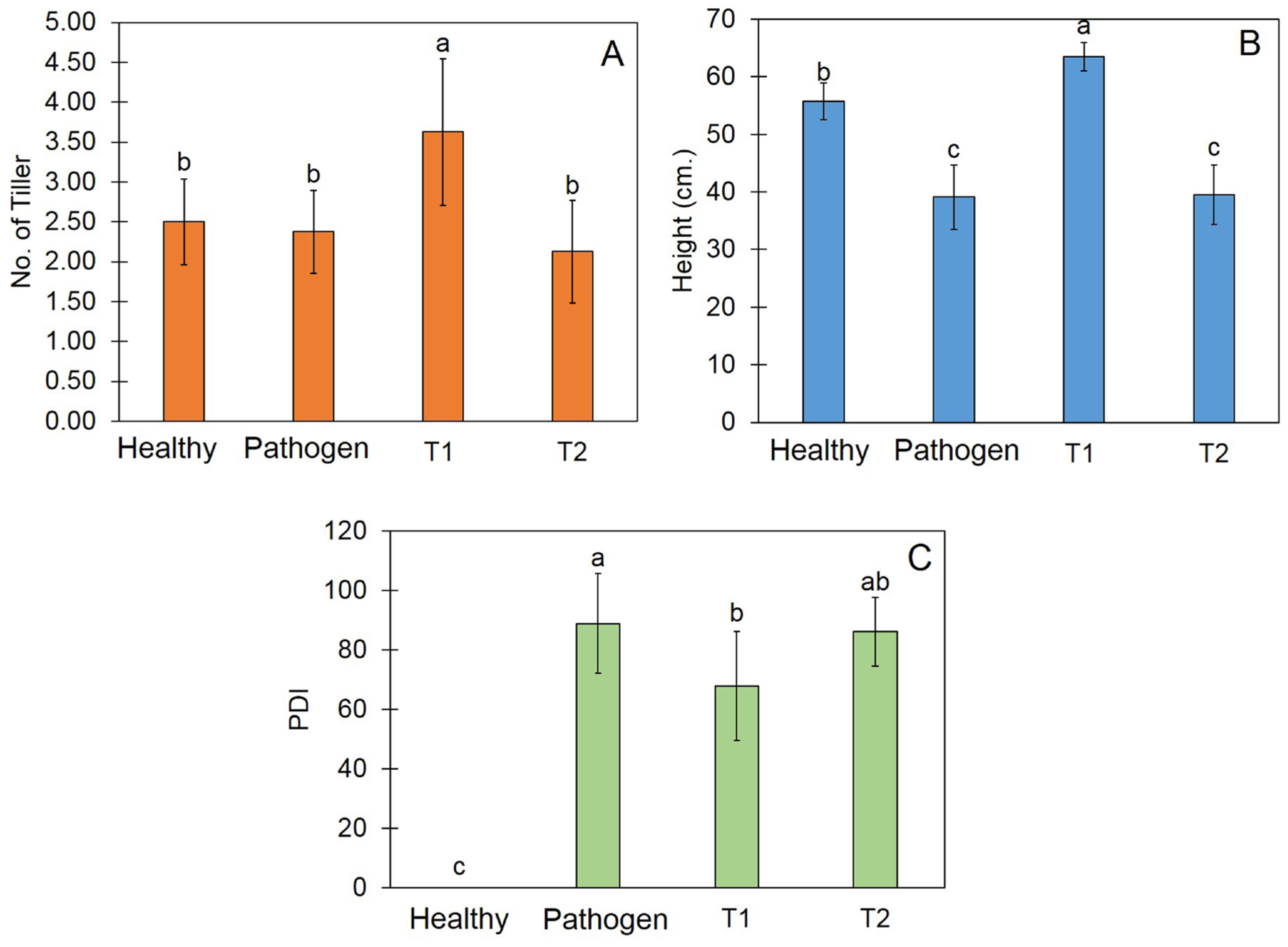
3.8. Trichoderma Promote Plant Growth and Reduce Disease Incidence
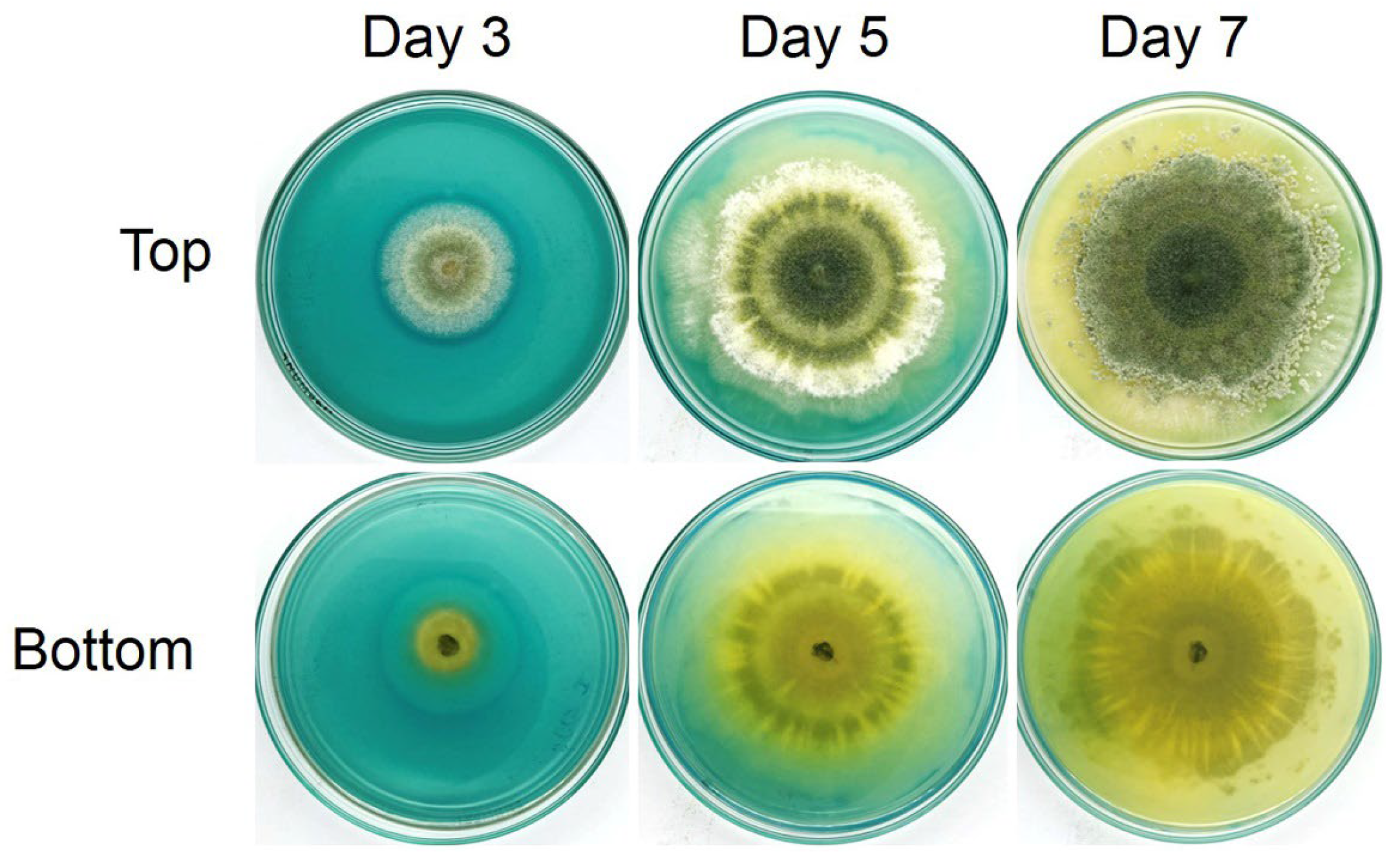
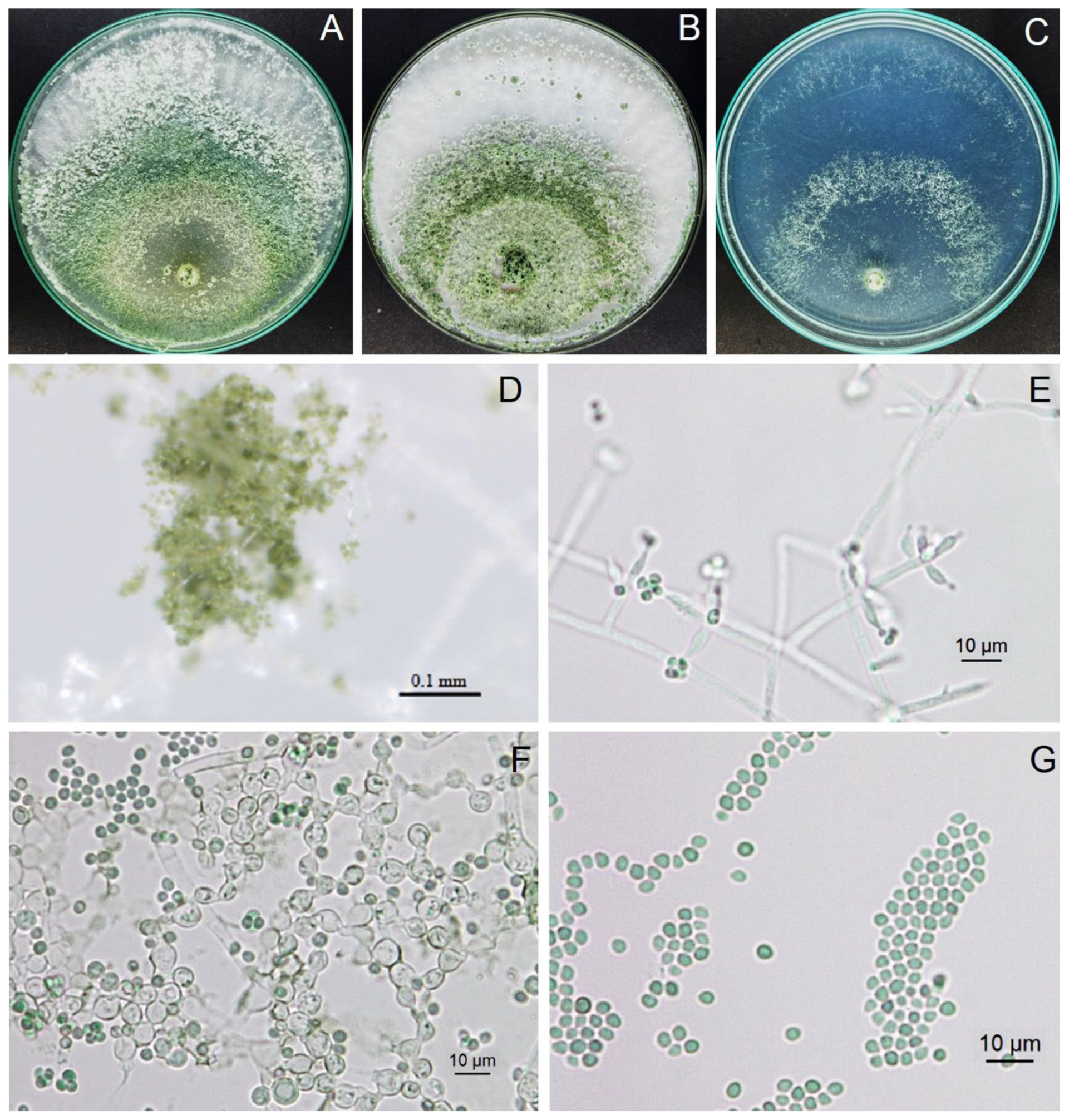
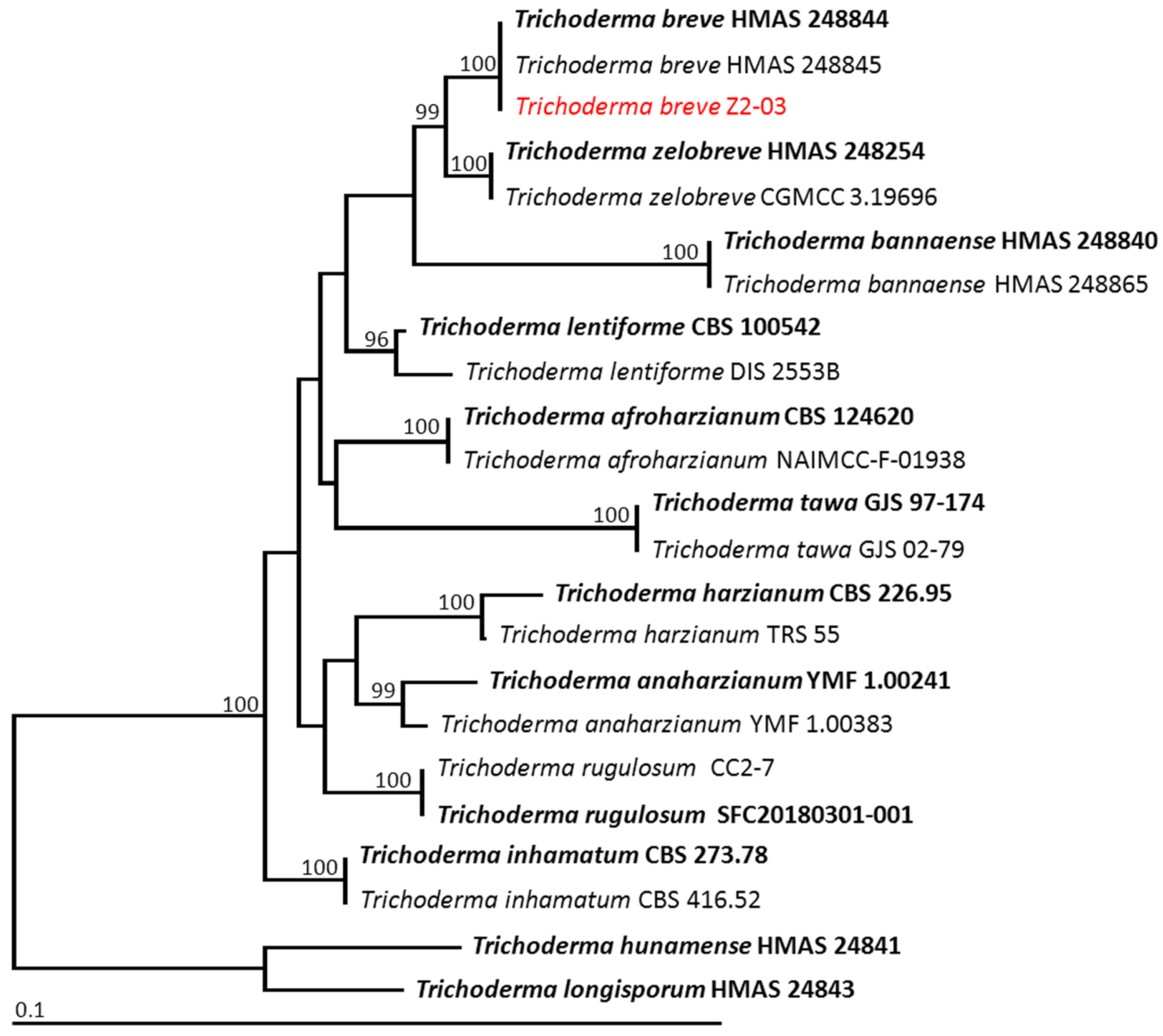
3.9. Identification of Trichoderma Isolate Z2-03
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Zhu, J.; Zhang, D.; Cheng, H.; Hao, B.; Cao, A.; Yan, D.; Wang, Q.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS ONE 2022, 17, e0266347. [Google Scholar] [CrossRef]
- Daryaei, A.; Jones, E.E.; Ghazalibiglar, H.; Glare, T.R.; Falloon, R.E. Effects of temperature, light and incubation period on production, germination and bioactivity of Trichoderma atroviride. J. Appl. Microbiol. 2016, 120, 999–1009. [Google Scholar] [CrossRef]
- Intana, W.; Kumla, J.; Suwannarach, N.; Sunpapao, A. Biological control potential of a soil fungus Trichoderma asperellum K1-02 against Neoscytalidium dimidiatum causing stem canker of dragon fruit. Physiol. Molec. Plant Pathol. 2023, 128, e102151. [Google Scholar] [CrossRef]
- Abdenaceur, R.; Farida, B.T.; Mourad, D.; Rima, H.; Zahia, O.; Fatma, S.H. Effective biofertilizer Trichoderma spp. isolates with enzymatic activity and metabolites enhancing plant growth. Int. Microbiol. 2022, 25, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Wonglom, P.; Daengsuwan, W.; Ito, S.; Sunpapao, A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76- 12/2 and the mechanism involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- Athinuwat, D.; Ruangwong, O.U.; Harishchandra, D.L.; Pitija, K.; Sunpapao, A. Biological control activities of rhizosphere fungus Trichoderma virens T1-02 in suppressing flower blight of flamingo flower (Anthurium andraeanum Lind.). J. Fungi 2024, 10, e66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-I.; Zhang, B.; Yang, X.-Y.; Naicker, O.; Zhao, L. Brown rot disease caused by Trichoderma hamatum on the edible lily, Lilium leichtlinii var. maximowiczii. Chiang Mai J. Sci. 2022, 49, 1500–1508. [Google Scholar] [CrossRef]
- Campanoni, P.; Nick, P. Auxin-dependent cell division and cell elongation. 1-Naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol. 2005, 137, 939–948. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ. Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, e991. [Google Scholar] [CrossRef]
- Jamil, A. Antifungal and plant growth promoting activity of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici colonizing tomato. J. Plant Prot. Res. 2021, 61, 243–253. [Google Scholar]
- Zhang, X.; Harvey, P.R.; Stummer, B.E.; Warren, R.A.; Zhang, G.; Guo, K.; Li, J.; Yang, H. Antibiosis functions during interactions of Trichoderma afroharzianum and Trichoderma gamsii with plant pathogenic Rhizoctonia and Pythium. Funct. Integr. Genom. 2015, 15, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Esteban, P.; Vicente, I.; Bernardi, R.; Plainchamp, T.; Domenichini, S.; Puntoni, G.; Baroncelli, R.; Vannacci, G.; Dufresne, M. Straw competition and wheat root endophytism of Trichoderma gamsii T6085 as useful traits in the biological control of Fusarium head blight. Phytopathology 2021, 111, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Molec. Plant Pathol. 2021, 115, e101663. [Google Scholar] [CrossRef]
- Pratap Singh, S.; Keswani, C.; Pratap Singh, S.; Sansinenea, E.; Xuan Hoat, T. Trichoderma spp. mediated induction of systemic defense response in brinjal against Sclerotinia sclerotiorum. Curr. Res. Microb. Sci. 2021, 2, e100051. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.L.; Ni, H.; Wang, W.Y.; Wu, X.Q. Antifungal effects of volatile organic compounds produced by Trichoderma koningiopsis T2 against Verticillium dahliae. Front. Microbiol. 2022, 13, e1013468. [Google Scholar] [CrossRef] [PubMed]
- Ruangwong, O.-U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol mechanisms of Trichoderma koningiopsis PSU3-2 against postharvest anthracnose of chili pepper. J. Fungi. 2021, 7, e276. [Google Scholar] [CrossRef] [PubMed]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma asperelloides PSU-P1 induced expression of pathogenesis-related protein genes against gummy stem blight of muskmelon (Cucumis melo) in field evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Statista. Principal Rice Exporting Countries Worldwide in 2022/2023. Available online: https://www.statista.com/statistics/255947/top-rice-exporting-countries-worldwide-2011/ (accessed on 8 March 2024).
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of volatiles from the endophytic fungus Trichoderma asperelloides PSU-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic pathway of indole-3-acetic acid in ectomycorrhizal fungi collected from northern Thailand. PLoS ONE 2020, 15, e0227478. [Google Scholar] [CrossRef] [PubMed]
- Palta, J.P. Leaf chlorophyll content. Rem. Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Schwyn, B.; Neiland, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Nagle, N.E.; Haard, N.F. Fractionation and characteri-zation of peroxidase from ripe banana fruit. J. Food Sci. 1975, 40, 576–579. [Google Scholar] [CrossRef]
- Luh, B.S.; Phithakpol, B. Characteristic of polyphenol-oxidase related to browning in cling peaches. J. Food Sci. 1972, 37, 18–25. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System for Rice, 4th ed.; INGER Genetic Resource Centre: Manila, Philippines, 1996. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Tayler, J. Amplification and direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, A.M., Gelfelfard, D.H., Snindky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from and RNA polymerase II subunit. Molec. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Hall, T. Bioedit Version 6.0.7. 2004. Available online: http://www.mbio.ncsu.edu/bioedit/bioedit.html (accessed on 25 March 2024).
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree Tree Figure Drawing Tool Version 131, Institute of Evolutionary Biology, University of Edinburgh. Available online: http://treebioedacuk/software/figtree/ (accessed on 25 March 2024).
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, M.; Voll, L.M.; Ding, Y.; Hofmann, J.; Sharma, M.; Zuccaro, A. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012, 196, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.I.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Saber, W.I.A.; Ghoneem, K.M.; Rashad, Y.M.; Al-Askar, A.A. Trichoderma harzianum WKY1: An indole acetic acid producer for growth improvement and anthracnose disease control in sorghum. Biocontrol Sci. Technol. 2017, 27, 654–676. [Google Scholar] [CrossRef]
- Mahmoodian, S.; Kowsari, M.; Zaman, M.; Jahromi, Z.M. Effect of improved Trichoderma harzianum on growth and resistance promotion in bean plant. Braz. Arch. Biol. Technol. 2022, 65, e22210671. [Google Scholar] [CrossRef]
- Güçlü, T.; Özer, N. Trichoderma harzianum antagonistic activity and competition for seed colonization against seedborne pathogenic fungi of sunflower. Lett. Appl. Microbiol. 2022, 74, 1027–1035. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar]
- Baiyee, B.; Ito, S.; Sunpapao, A. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol. Mol. Plant. Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Rathmell, W.G.; Sequeira, L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974, 53, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, F.; Peragón, J. The response of phenylalanine ammonia-lyase, polyphenol oxidase and phenols to cold stress in the olive tree (Olea europaea, L. cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Chen, C.; Belanger, R.R.; Benhamou, N.; Paulitz, T.C. Defense enzymes induced incucumber roots by treatment with plant-growth promoting rhizobacteria (PGPR). Physiol. Mol. Plant Pathol. 2000, 56, 13–23. [Google Scholar] [CrossRef]
- Nandakumar, R.; Babu, S.; Viswanathan, R.; Raguchander, T.; Samiyappan, R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol. Biochem. 2001, 33, 603–612. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Raguchander, T.; Samiyappan, R. Enhancing resistance of tomato and hot pepper to Pythium diseases by seed treatment with fluorescent pseudomonads. Eur. J. Plant Pathol. 2002, 108, 429–441. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Radhika, K.; Mathiyazhagan, S.; Bhaskaran, R.; Samiyappan, R.; Velazhahan, R. Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agents. Braz. J. Plant Physiol. 2006, 18, 367–377. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Che Mohd Zain, C.R.; Yusoff, W.M.W. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Expr. 2014, 4, e45. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Isahak, A.; Fathurrahman, F.; Yusoff, W.M.W. Rice plants’ resistance to sheath blight infection is increased by the synergistic effects of Trichoderma inoculation with SRI management. Agronomy 2023, 13, e711. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, D.; Tu, D.; Ma, F.; Liu, C. Overexpression of auxin/indole-3-acetic acid gene MdIAA24 enhance Glomerella leaf spot resistance in apple (Malvus domestica). Hort. Plant J. 2024, 10, 15–24. [Google Scholar] [CrossRef]
- Sharaf, E.F.; Farrag, A.A. Induced resistance in tomato plants by IAA against Fusarium oxysporum lycopersici. Pol. J. Microbiol. 2004, 53, 111–116. [Google Scholar] [PubMed]
- Buysens, S.; Heungens, K.; Poppe, J.; Hofte, M. Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl. Environ. Microbiol. 1996, 62, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhuang, W.Y. Discovery from a large-scaled survey of Trichoderma in soil of China. Sci. Rep. 2017, 7, 9090. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Jalk, A.; Leiva, S.; Bobadilla, L.G.; Vigo, C.N.; Arce, M.; Oliva-Cruz, M. Effect of endophytic Trichoderma sp. strains on the agronomic characteristics of ecotypes of Theobroma cacao L. under nursery conditions in Peru. Int. J. Agron 2022, 2022, 297706. [Google Scholar] [CrossRef]
- Surawut, S.; Kunsook, C.; Chaikul, S.; Kulchawa, W.; Phophuek, K.; Yasawong, M.; Kanjanavas, P. Vermicompost contained fungi act as biocontrol agents against fungal pathogens of chili. Sci. Technol. Asia 2023, 28, 264–272. [Google Scholar]
- Intana, W.; Wonglom, P.; Dy, K.S.; Sunpapao, A. Development of a novel emulsion formulation of Trichoderma asperelloides PSU-P1 conidia against stem canker on dragon fruit caused by Neoscytalidium dimidiatum. Microbiol. Res. 2023, 14, 1139–1149. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intana, W.; Suwannarach, N.; Kumla, J.; Wonglom, P.; Sunpapao, A. Plant Growth Promotion and Biological Control against Rhizoctonia solani in Thai Local Rice Variety “Chor Khing” Using Trichoderma breve Z2-03. J. Fungi 2024, 10, 417. https://doi.org/10.3390/jof10060417
Intana W, Suwannarach N, Kumla J, Wonglom P, Sunpapao A. Plant Growth Promotion and Biological Control against Rhizoctonia solani in Thai Local Rice Variety “Chor Khing” Using Trichoderma breve Z2-03. Journal of Fungi. 2024; 10(6):417. https://doi.org/10.3390/jof10060417
Chicago/Turabian StyleIntana, Warin, Nakarin Suwannarach, Jaturong Kumla, Prisana Wonglom, and Anurag Sunpapao. 2024. "Plant Growth Promotion and Biological Control against Rhizoctonia solani in Thai Local Rice Variety “Chor Khing” Using Trichoderma breve Z2-03" Journal of Fungi 10, no. 6: 417. https://doi.org/10.3390/jof10060417







