Phytopathogenic Fungicidal Activity and Mechanism Approach of Three Kinds of Triphenylphosphonium Salts
Abstract
:1. Introduction
2. Experimental
2.1. Equipment and Materials
2.2. Synthetic Procedures
3. Results and Discussion
3.1. Chemistry
3.2. In Vitro Fungicidal Activity
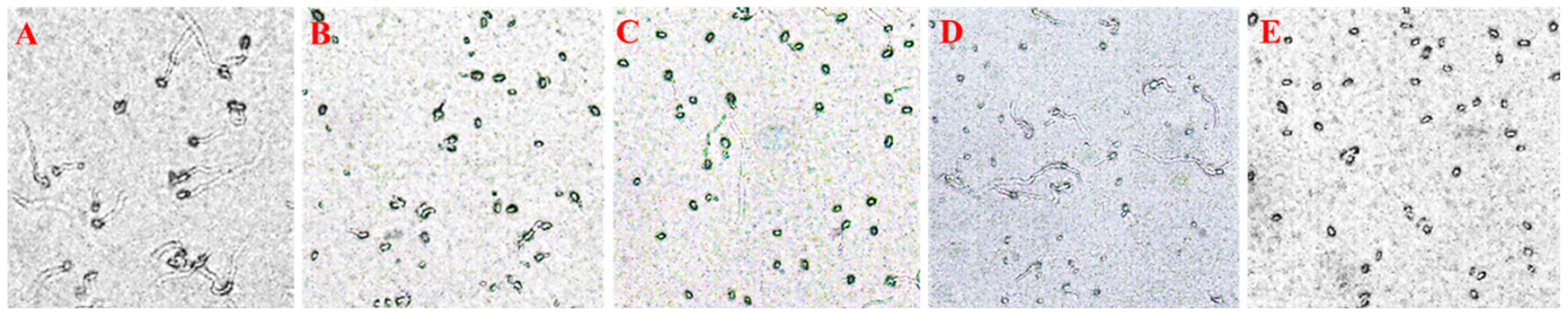
3.3. Spore Germination Inhibition Activity
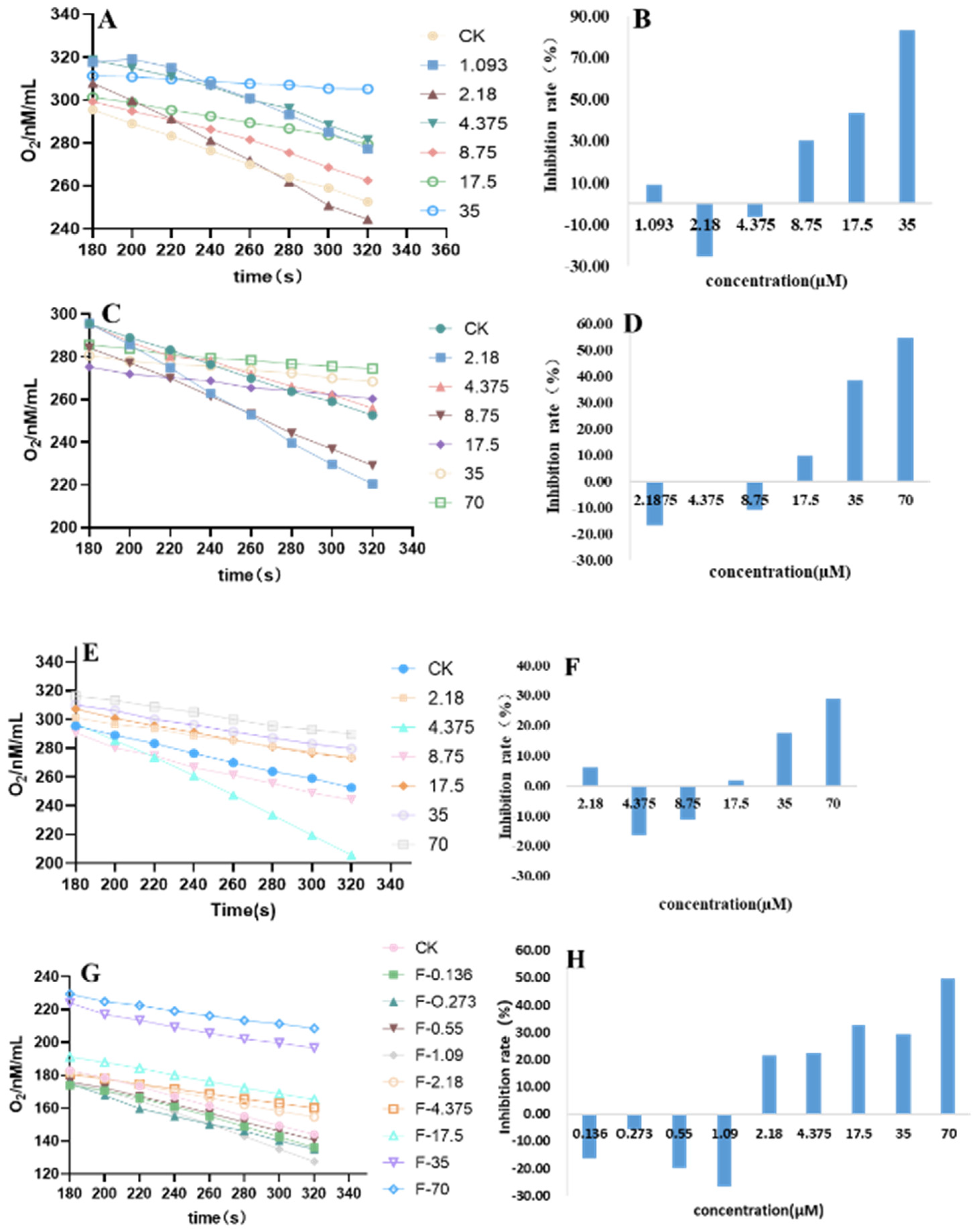
3.4. Influence on the Respiratory of P. capsici Mycelia
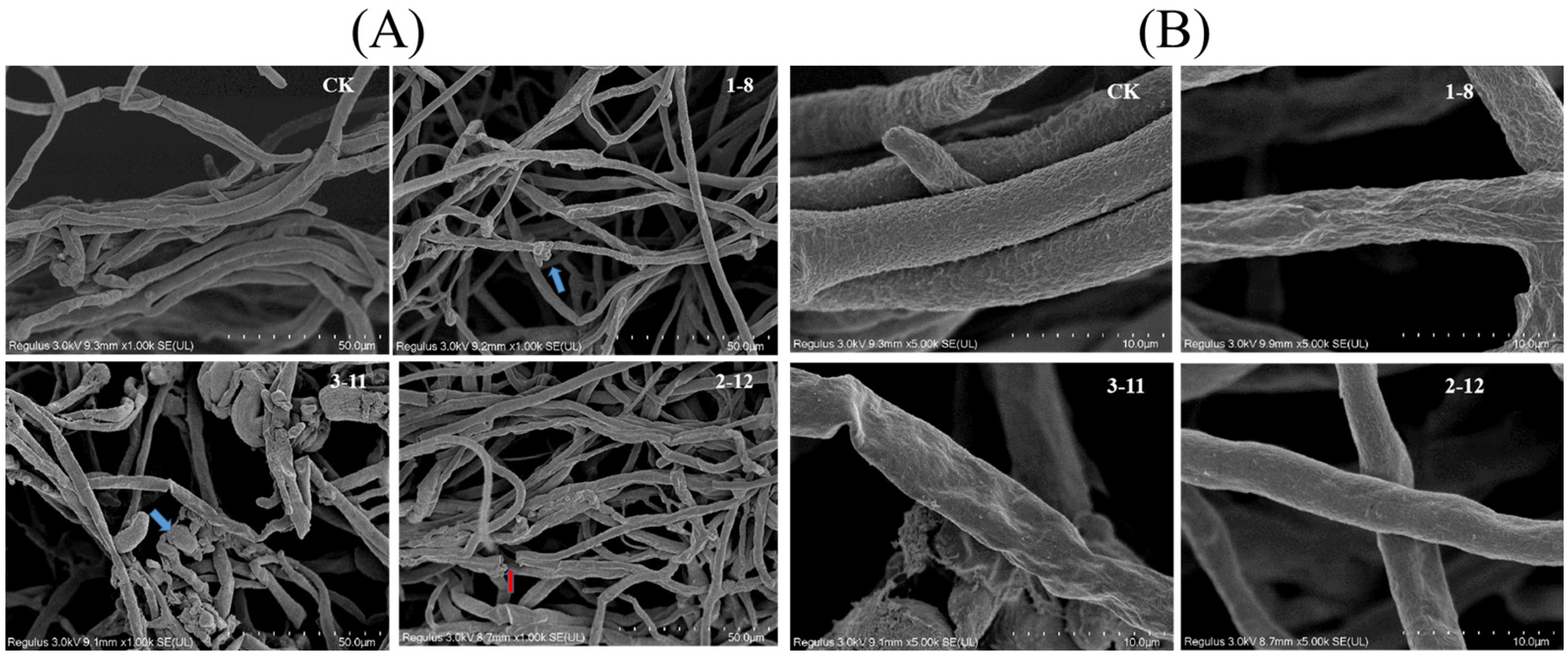
3.5. Mycelium Morphology of P. capsici
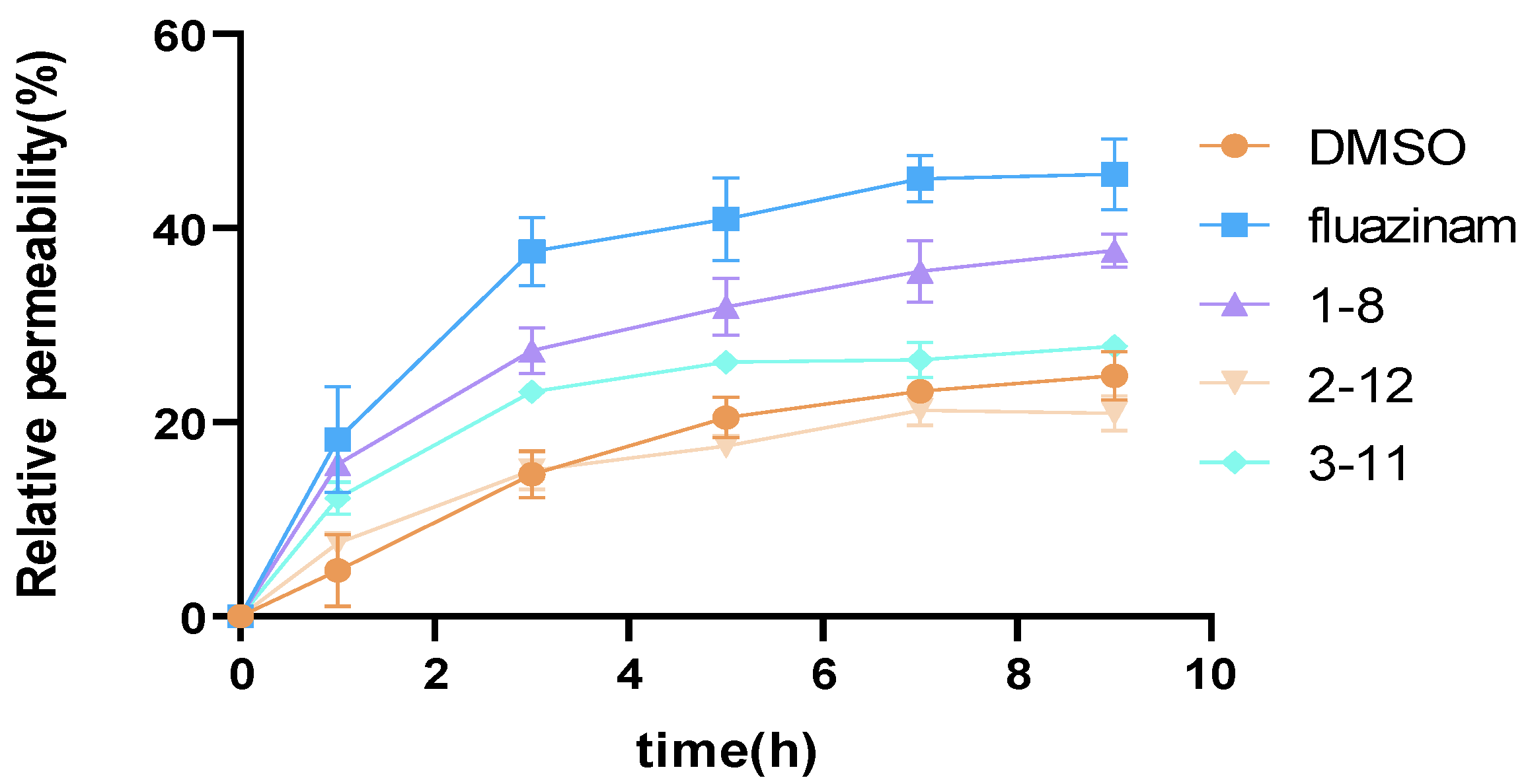
3.6. Influence of Compounds on Relative Permeability of P. capsici Mycelia
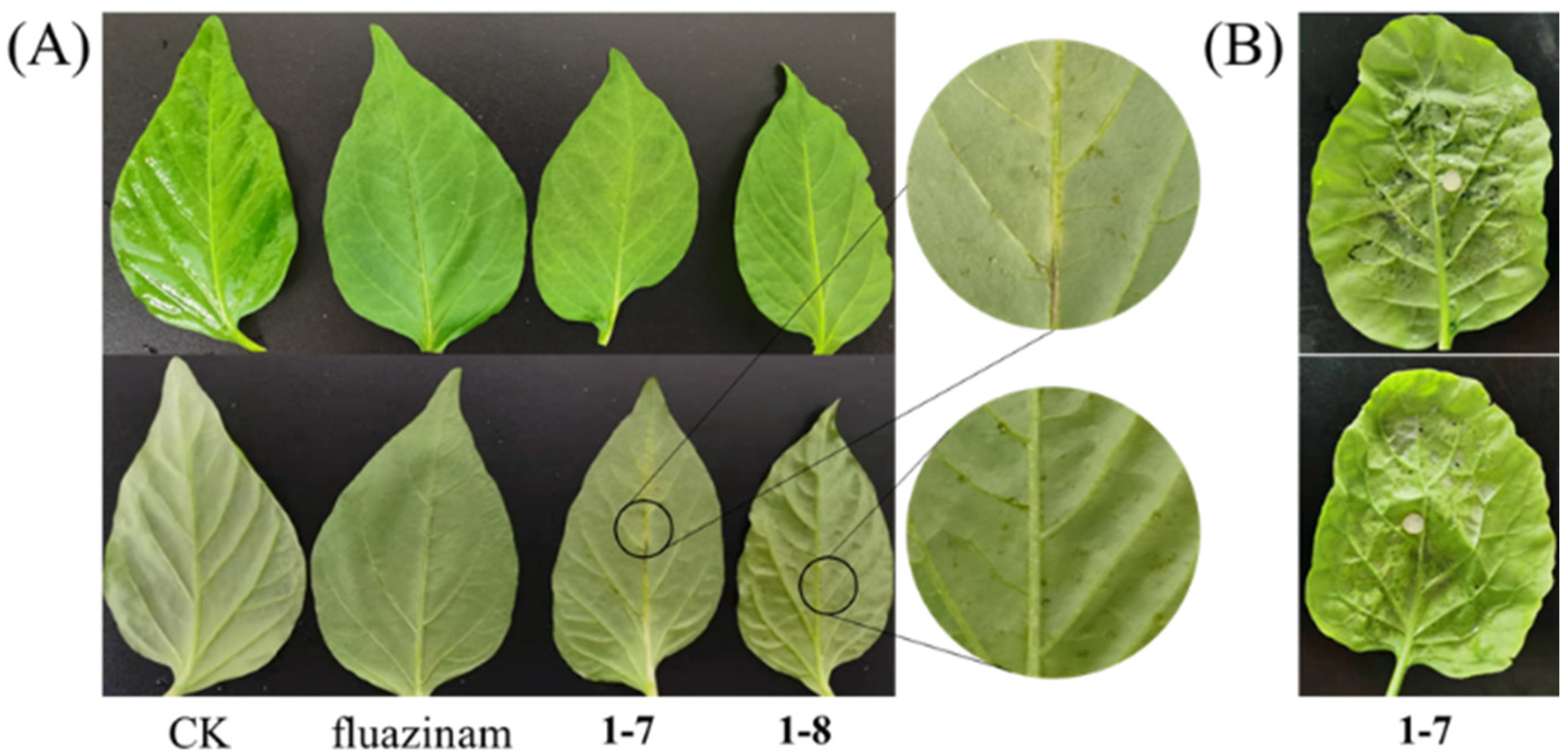
3.7. Phytotoxicity of Compounds 1–7 and 1–8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102, 3667–3691. [Google Scholar] [CrossRef] [PubMed]
- Batheja, S.; Gupta, S.; Tejavath, K.K.; Gupta, U. TPP-based conjugates: Potential targeting ligands. Drug Discov. Today 2024, 29, 103983. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.F.; Kelso, G.F.; Blaikie, F.H.; James, A.M.; Cocheme, H.M.; Filipovska, A.; Ros, T.; Hurd, T.R.; Smith, R.A.J.; Murphy, M.P. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry 2005, 70, 222–230. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Cocheme, H.M.; Smith, R.A.J.; Murphy, M.P. Interactions of Mitochondria-targeted and Untargeted Ubiquinones with the Mitochondrial Respiratory Chain and Reactive Oxygen Species: Implications for the use of exogenous ubiquinones as therapies and experimental tools. J. Biol. Chem. 2005, 280, 21295–21312. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.-H.; Hansen, B.S.; Olsen, P.H.; Tullin, S.; Murphy, M.P.; Brand, M.D. Mitochondrial uncouplers with an extraordinary dynamic range. Biochem. J. 2007, 407, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.A.; Fink, B.D.; Gibbs, B.E.; Chheda, P.R.; Wu, M.; Sivitz, W.I.; Kerns, R.J. A Novel Triphenylphosphonium Carrier to Target Mitochondria without Uncoupling Oxidative Phosphorylation. J. Med. Chem. 2021, 64, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhang, X.; Du, S.; Han, X.; Li, J.-Q.; Xiao, Y.; Xu, Z.; Wu, Q.; Xu, L.; et al. Fungicidal Action of the Triphenylphosphonium-Driven Succinate Dehydrogenase Inhibitors Is Mediated by Reactive Oxygen Species and Suggests an Effective Resistance Management Strategy. J. Agric. Food Chem. 2022, 70, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, D.; Yin, F.; Wang, J.; Zhang, X.; Xiao, Y.; Li, J.-Q.; Qin, Z. Mitochondrion-Targeted Triphenylphosphonium-Based Kresoxim-Methyl Analogues: Synthesis, Fungicidal Activity, and Action Mechanism Approach. J. Agric. Food Chem. 2022, 70, 13563–13573. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Xu, Y.; Fu, B.; Zhang, X.; Xiao, Y.; Li, J.; Qin, Z. Triphenylphosphonium-Driven Targeting of Pyrimorph Fragment Derivatives Greatly Improved Its Action on Phytopathogen Mitochondria. J. Agric. Food Chem. 2023, 71, 2842–2852. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Yin, F.; Xu, Y.; Fu, B.; Li, J.; Qin, Z. Triphenylphosphonium (TPP)-Conjugated Quinolone Analogs Displayed Significantly Enhanced Fungicidal Activity Superior to Its Parent Molecule. J. Fungi 2023, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Jiang, J.; Liu, X.; Zhou, T.; Li, J.-Q.; Xiao, Y.; Qin, Z. Discovery of triphenylphosphonium (TPP)-conjugated N-(1,1′-biphenyl)-2-yl aliphatic amides as excellent fungicidal candidates. Pest Manag. Sci. 2023, 79, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
- Trnka, J.; Elkalaf, M.; Andel, M. Lipophilic triphenylphosphonium cations inhibit mitochondrial electron transport chain and induce mitochondrial proton leak. PLoS ONE 2015, 10, e0121837/0121831. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yang, M.-S.; Liang, M. The synthesis, characterization and antibacterial activity of quaternized poly(2,6-dimethyl-1,4-phenylene oxide)s modified with ammonium and phosphonium salts. React. Funct. Polym. 2010, 70, 944–950. [Google Scholar] [CrossRef]
- Taladriz, A.; Healy, A.; Flores Perez, E.J.; Herrero Garcia, V.; Rios Martinez, C.; Alkhaldi, A.A.M.; Eze, A.A.; Kaiser, M.; de Koning, H.P.; Chana, A.; et al. Synthesis and structure-activity analysis of new phosphonium salts with potent activity against african trypanosomes. J. Med. Chem. 2012, 55, 2606–2622. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, K.L.; Murphy, E.L.; Majofodun, O.; Munoz, L.D.; Williams, J.C., Jr.; Almeida, K.H. Arylphosphonium salts interact with DNA to modulate cytotoxicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 673, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kalinovich, A.V.; Mattsson, C.L.; Youssef, M.R.; Petrovic, N.; Ost, M.; Skulachev, V.P.; Shabalina, I.G. Mitochondria-targeted dodecyltriphenylphosphonium (C12TPP) combats high-fat-diet-induced obesity in mice. Int. J. Obes. 2016, 40, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, F.; Ueda, S.; Taguri, T.; Kawachi, S.; Abe, H. Antimicrobial activity and thermostability of silver 6-benzylaminopurine montmorillonite. Appl. Clay Sci. 2009, 46, 296–299. [Google Scholar] [CrossRef]
- Chen, J.Y.; Tian, R.L.; Leng, Y.X.; Yang, P.; Wang, J.; Wan, G.J.; Zhao, A.S.; Sun, H.; Huang, N. Effect of Ar plasma etching of Ti-O film surfaces on biological behavior of endothelial cell. Surf. Coat. Technol. 2007, 201, 6901–6905. [Google Scholar] [CrossRef]
- Liu, X.; Yang, D.; Yin, F.; Li, J.-Q.; Xiao, Y.; Fu, B.; Qin, Z. The application of “plug-in molecules” method in novel strobilurin fungicides screening. RSC Adv. 2020, 10, 42804–42809. [Google Scholar] [CrossRef]
- Hughes, J.K.; Hodge, A.; Fitter, A.H.; Atkin, O.K. Mycorrhizal respiration: Implications for global scaling relationships. Trends Plant Sci. 2008, 13, 583–588. [Google Scholar] [CrossRef]
- Cai, G.; Yu, W.; Song, D.; Zhang, W.; Guo, J.; Zhu, J.; Ren, Y.; Kong, L. Discovery of fluorescent coumarin-benzo[b]thiophene 1, 1-dioxide conjugates as mitochondria-targeting antitumor STAT3 inhibitors. Eur. J. Med. Chem. 2019, 174, 236–251. [Google Scholar] [CrossRef]
- Yin, X.-D.; Ma, K.-Y.; Wang, Y.-L.; Sun, Y.; Shang, X.-F.; Zhao, Z.-M.; Wang, R.-X.; Chen, Y.-J.; Zhu, J.-K.; Liu, Y.-Q. Design, Synthesis, and Antifungal Evaluation of 8-Hydroxyquinoline Metal Complexes against Phytopathogenic Fungi. J. Agric. Food Chem. 2020, 68, 11096–11104. [Google Scholar] [CrossRef]
- Devinsky, F.; Kopecka-Leitmanova, A.; Sersen, F.; Balgavy, P. Interaction of surfactants with model and biological membranes. Part VIII. Cut-off effect in antimicrobial activity and in membrane perturbation efficiency of the homologous series of N,N-dimethylalkylamine oxides. J. Pharm. Pharmacol. 1990, 42, 790. [Google Scholar] [CrossRef] [PubMed]
- Sersen, F.; Gabunia, G.; Krejcirova, E.; Kralova, K. The relationship between lipophilicity of N-alkyl-N,N-dimethylamine oxides and their effects on the thylakoid membranes of chloroplasts. Photosynthetica 1992, 26, 205. [Google Scholar]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharmacol. 2013, 67, 456–467. [Google Scholar] [CrossRef]
- Chen, F.-J.; Long, X.-H.; Li, E.-Z. Evaluation of antifungal phenolics from Helianthus tuberosus L. leaves against Phytophthora capsici Leonian by chemometric analysis. Molecules 2019, 24, 4300. [Google Scholar] [CrossRef] [PubMed]
- Khailova, L.S.; Nazarov, P.A.; Sumbatyan, N.V.; Korshunova, G.A.; Rokitskaya, T.I.; Dedukhova, V.I.; Antonenko, Y.N.; Skulachev, V.P. Uncoupling and toxic action of alkyltriphenylphosphonium cations on mitochondria and the bacterium Bacillus subtilis as a function of alkyl chain length. Biochemistry 2015, 80, 1589–1597. [Google Scholar] [CrossRef]
- Severin, F.F.; Severina, I.I.; Antonenko, Y.N.; Rokitskaya, T.I.; Cherepanov, D.A.; Mokhova, E.N.; Vyssokikh, M.Y.; Pustovidko, A.V.; Markova, O.V.; Yaguzhinsky, L.S.; et al. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc. Natl. Acad. Sci. USA 2010, 107, 663. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, P.; Strom, R.; Giordano, M.G. Reactivation of succinic dehydrogenase by phospholipids. Biochem. Biophys. Res. Commun. 1965, 18, 259. [Google Scholar] [CrossRef]
- Fry, M.; Green, D.E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J. Biol. Chem. 1981, 256, 1874. [Google Scholar] [CrossRef]
- Samovski, D.; Kalderon, B.; Yehuda-Shnaidman, E.; Bar-Tana, J. Gating of the Mitochondrial Permeability Transition Pore by Long Chain Fatty Acyl Analogs In Vivo. J. Biol. Chem. 2010, 285, 6879–6890. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Penzo, D.; Wojtczak, L. Mitochondrial energy dissipation by fatty acids: Mechanisms and implications for cell death. Vitam. Horm. 2002, 65, 97–126. [Google Scholar] [CrossRef]
- Finichiu, P.G.; James, A.M.; Larsen, L.; Smith, R.A.J.; Murphy, M.P. Mitochondrial accumulation of a lipophilic cation conjugated to an ionisable group depends on membrane potential, pH gradient and pK a: Implications for the design of mitochondrial probes and therapies. J. Bioenerg. Biomembr. 2013, 45, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Griepernau, B.; Leis, S.; Schneider, M.F.; Sikor, M.; Steppich, D.; Boeckmann, R.A. 1-Alkanols and membranes: A story of attraction. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2899–2913. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Morin, P.A.; Sierra-Valdez, F.J.; Ruiz-Suarez, J.C. The cut-off effect of n-alcohols in lipid rafts: A lipid-dependent phenomenon. J. Mol. Graph. Modell. 2020, 101, 107732. [Google Scholar] [CrossRef]
- Hammond, D.G.; Kubo, I. Alkanols inhibit respiration of intact mitochondria and display cutoff similar to that measured in vivo. J. Pharmacol. Exp. Ther. 2000, 293, 822–828. [Google Scholar]
- Wang, B.; He, B.; Chen, T.; Li, H.; Chen, L.; Chen, Y.; Tian, K.; Yang, K.; Shen, D.; Yan, W.; et al. Discovery of Tropolone Stipitaldehyde as a Potential Agent for Controlling Phytophthora Blight and Its Action Mechanism Research. J. Agric. Food Chem. 2022, 70, 8693–8703. [Google Scholar] [CrossRef]
- Strobykina, I.Y.; Voloshina, A.D.; Andreeva, O.V.; Sapunova, A.S.; Lyubina, A.P.; Amerhanova, S.K.; Belenok, M.G.; Saifina, L.F.; Semenov, V.E.; Kataev, V.E. Synthesis, antimicrobial activity and cytotoxicity of triphenylphosphonium (TPP) conjugates of 1,2,3-triazolyl nucleoside analogues. Bioorg. Chem. 2021, 116, 105328. [Google Scholar] [CrossRef]
- Kang, Z.; Gu, B.G. Standard Operating Practice for Pesticide Biological Activity Test-Fungicide Roll; Chemical Industry Press: Beijing, China, 2016; pp. 1–206. [Google Scholar]


| Compd. | Inhibition Rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA | RS | SS | FO | PO | CG | PC | BC | GZ | Mean | |
| 1-1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.71 ± 1.27 | 5.79 ± 1.77 | −2.45 ± 2.04 | 14.67 ± 1.09 | 11.71 ± 1.27 | 4.17 ± 1.18 | 5.07 |
| 1-2 | 36.25 ± 1.02 | 5.42 ± 1.18 | 10.42 ± 0.59 | 31.53 ± 0.64 | 5.79 ± 0.00 | 11.01 ± 1.36 | 61.11 ± 1.75 | 31.53 ± 0.64 | 16.25 ± 0.00 | 23.26 |
| 1-3 | 99.17 ± 1.18 | 50.42 ± 0.59 | 48.96 ± 0.78 | 45.05 ± 3.19 | 11.17 ± 1.02 | 38.43 ± 2.97 | 79.33 ± 0.94 | 45.05 ± 3.19 | 36.04 ± 4.63 | 50.40 |
| 1-4 | 99.58 ± 0.59 | 62.92 ± 1.18 | 84.17 ± 0.59 | 55.41 ± 0.96 | 30.91 ± 0.59 | 58.39 ± 2.91 | 95.56 ± 1.26 | 55.41 ± 0.96 | 23.33 ± 2.62 | 62.85 |
| 1-5 | 99.58 ± 0.59 | 64.58 ± 1.56 | 93.75 ± 0.00 | 65.20 ± 0.34 | 62.99 ± 0.00 | 71.14 ± 2.04 | 98.89 ± 0.83 | 65.20 ± 0.34 | 33.96 ± 4.85 | 72.81 |
| 1-6 | 100 ± 0.00 | 98.13 ± 1.35 | 97.29 ± 1.06 | 87.39 ± 0.64 | 76.22 ± 0.78 | 75.71 ± 0.34 | 99.33 ± 0.54 | 87.39 ± 0.64 | 40.63 ± 0.88 | 84.68 |
| 1-7 | 100 ± 0.00 | 84.38 ± 2.22 | 100 ± 0.00 | 90.99 ± 0.32 | 79.14 ± 0.51 | 85.09 ± 0.68 | 100 ± 0.00 | 90.99 ± 0.32 | 39.17 ± 7.24 | 85.53 |
| 1-8 | 100 ± 0.00 | 78.75 ± 2.04 | 97.29 ± 0.29 | 71.62 ± 0.00 | 74.88 ± 0.29 | 77.39 ± 0.68 | 89.11 ± 0.31 | 71.62 ± 0.00 | 30.21 ± 1.56 | 76.76 |
| 1-9 | 90.21 ± 0.29 | 63.75 ± 2.04 | 97.71 ± 0.59 | 45.05 ± 1.27 | 65.68 ± 0.00 | 51.42 ± 1.80 | 88.89 ± 0.63 | 45.05 ± 1.27 | 17.29 ± 1.93 | 62.78 |
| 1-10 | 70.42 ± 2.12 | 20.00 ± 1.02 | 96.46 ± 0.29 | 14.41 ± 0.64 | 38.76 ± 0.51 | 0.43 ± 1.18 | 68.00 ± 1.09 | 14.41 ± 0.64 | 18.33 ± 2.52 | 37.91 |
| 1A-1 | 98.75 ± 1.08 | 83.49 ± 0.38 | 100 ± 0.00 | 87.18 ± 0.36 | 74.31 ± 1.04 | 73.96 ± 0.92 | 93.36 ± 5.85 | 90.42 ± 2.6 | 75.35 ± 9.32 | 86.31 |
| 1A-2 | 87.71 ± 2.82 | 79.14 ± 0.00 | 97.29 ± 0.95 | 74.78 ± 1.26 | 66.80 ± 0.79 | 70.42 ± 0.68 | 88.94 ± 1.28 | 92.92 ± 0.72 | 19.72 ± 0.00 | 75.30 |
| 1B-1 | 96.88 ± 2.25 | 84.57 ± 0.38 | 98.75 ± 1.25 | 94.12 ± 0.73 | 80.90 ± 0.68 | 80.00 ± 0.00 | 99.26 ± 0.00 | 90.63 ± 0.63 | 68.08 ± 0.81 | 88.13 |
| 1B-2 | 81.67 ± 0.72 | 86.09 ± 1.51 | 90.00 ± 1.65 | 77.93 ± 0.63 | 72.94 ± 0.39 | 60.00 ± 0.88 | 87.95 ± 1.54 | 85.42 ± 1.80 | 50.23 ± 2.93 | 76.91 |
| 1C-1 | 89.58 ± 3.61 | 71.97 ± 1.72 | 53.13 ± 2.72 | 65.32 ± 0.63 | 62.94 ± 0.39 | 53.33 ± 0.51 | 85.25 ± 0.00 | 62.92 ± 0.72 | 21.36 ± 2.47 | 62.87 |
| 1C-2 | 74.79 ± 1.57 | 52.74 ± 2.30 | 30.42 ± 1.44 | 45.57 ± 0.36 | 44.52 ± 1.97 | 26.67 ± 0.51 | 69.27 ± 2.13 | 40.00 ± 0.00 | 13.15 ± 2.15 | 44.13 |
| 2-1 | 1.29 ± 0.72 | 17.50 ± 4.42 | 15.79 ± 4.96 | 6.82 ± 2.41 | 5.09 ± 4.59 | 5.57 ± 3.00 | −1.50 ± 1.19 | 2.07 ± 0.80 | 5.62 ± 1.83 | 6.47 |
| 2-2 | 1.50 ± 1.08 | 6.25 ± 0.00 | 15.35 ± 1.86 | 4.08 ± 2.41 | 4.22 ± 2.24 | 2.57 ± 1.23 | −2.21 ± 0.52 | 0.64 ± 0.92 | 7.55 ± 1.88 | 4.44 |
| 2-3 | −0.37 ± 0.00 | 7.50 ± 1.88 | 23.25 ± 1.86 | 0.18 ± 2.60 | 5.81 ± 3.28 | 2.44 ± 0.83 | −2.33 ± 1.55 | 2.07 ± 2.25 | 4.77 ± 2.66 | 4.81 |
| 2-4 | −0.17 ± 0.36 | 5.42 ± 4.07 | 21.93 ± 4.96 | 6.94 ± 2.43 | 8.67 ± 2.59 | −0.29 ± 0.92 | −3.04 ± 1.79 | −4.30 ± 2.39 | −0.13 ± 1.78 | 3.89 |
| 2-5 | 0.25 ± 0.62 | 12.19 ± 3.98 | 27.63 ± 0.62 | 9.34 ± 0.00 | 5.30 ± 5.42 | 6.11 ± 0.12 | 5.74 ± 0.64 | 1.27 ± 0.80 | 1.88 ± 1.51 | 7.75 |
| 2-6 | 1.50 ± 3.47 | 3.75 ± 3.80 | 22.81 ± 2.48 | 5.68 ± 2.78 | 1.36 ± 3.93 | 3.69 ± 1.25 | 0.22 ± 2.64 | −0.85 ± 2.30 | 4.79 ± 1.03 | 4.77 |
| 2-7 | 1.91 ± 1.44 | 0.83 ± 3.08 | 28.95 ± 2.48 | 18.73 ± 2.10 | −1.83 ± 4.41 | 4.50 ± 2.23 | −3.36 ± 2.68 | 4.46 ± 2.87 | 9.48 ± 0.91 | 7.07 |
| 2-8 | 5.65 ± 3.43 | 2.29 ± 2.01 | 16.84 ± 2.73 | 28.11 ± 1.05 | −4.27 ± 2.52 | 2.08 ± 2.58 | 3.57 ± 1.30 | 14.81 ± 3.18 | 28.83 ± 3.52 | 10.88 |
| 2-9 | 28.10 ± 2.88 | 6.25 ± 3.80 | 41.67 ± 4.34 | 23.31 ± 2.10 | 1.34 ± 3.19 | 5.12 ± 1.40 | 61.71 ± 1.38 | 43.47 ± 4.21 | 43.03 ± 2.41 | 28.22 |
| 2-10 | 50.12 ± 1.87 | 5.00 ± 4.42 | 63.74 ± 1.34 | 37.96 ± 1.05 | −0.74 ± 5.44 | 10.58 ± 0.96 | 83.36 ± 2.94 | 51.7 ± 3.59 | 47.31 ± 5.17 | 38.78 |
| 2-11 | 53.24 ± 3.79 | 3.13 ± 1.88 | 64.04 ± 1.24 | 33.38 ± 1.82 | 10.69 ± 1.80 | 9.55 ± 0.95 | 69.59 ± 0.52 | 64.97 ± 2.25 | 50.96 ± 3.69 | 39.95 |
| 2-12 | 76.72 ± 0.36 | 70.83 ± 1.91 | 86.55 ± 1.83 | 69.09 ± 4.86 | 35.75 ± 2.51 | 37.29 ± 3.66 | 71.53 ± 0.89 | 62.05 ± 2.00 | 68.32 ± 1.47 | 64.24 |
| 2-13 | 62.93 ± 0.28 | 75.42 ± 1.57 | 93.27 ± 4.42 | 65.89 ± 0.40 | 37.15 ± 2.10 | 34.38 ± 1.48 | 58.05 ± 1.77 | 53.03 ± 2.25 | 67.67 ± 1.45 | 60.87 |
| 2-14 | 52.41 ± 2.00 | 63.13 ± 0.00 | 78.07 ± 3.51 | 50.09 ± 2.60 | 16.53 ± 3.04 | 20.45 ± 0.45 | 44.55 ± 1.63 | 45.86 ± 0.80 | 57.26 ± 2.05 | 47.59 |
| 2-15 | 0.04 ± 0.72 | −1.46 ± 0.95 | 10.53 ± 3.72 | 2.70 ± 2.86 | −0.56 ± 4.37 | 3.20 ± 0.39 | 3.41 ± 1.20 | −0.58 ± 4.60 | 8.98 ± 1.89 | 2.92 |
| 2-12′ | / | 72.52 ± 1.89 | 83.36 ± 0.80 | 35.88 ± 0.11 | 10.02 ± 0.22 | 15.04 ± 0.70 | 63.59 ± 0.28 | 68.90 ± 0.11 | 25.48 ± 0.15 | 46.85 a |
| 3-1 | −4.56 ± 1.02 | −4.74 ± 1.66 | 21.50 ± 0.21 | 1.76 ± 1.51 | −1.70 ± 0.35 | 1.93 ± 1.17 | 14.36 ± 0.00 | −0.95 ± 1.41 | −1.68 ± 1.26 | 2.88 |
| 3-2 | −0.48 ± 1.01 | −1.34 ± 0.92 | 11.97 ± 0.22 | 6.38 ± 0.68 | 10.02 ± 0.71 | 1.24 ± 1.14 | 8.45 ± 3.96 | −1.81 ± 1.57 | 0.87 ± 1.93 | 3.92 |
| 3-3 | 0.96 ± 0.43 | −5.39 ± 0.21 | 7.23 ± 0.65 | 7.64 ± 0.66 | −0.81 ± 0.44 | 6.06 ± 0.50 | 11.69 ± 3.79 | 0.82 ± 1.25 | 11.32 ± 1.11 | 4.39 |
| 3-4 | −1.68 ± 0.40 | 4.08 ± 1.84 | −1.36 ± 0.35 | 28.04 ± 0.10 | −1.70 ± 4.92 | 2.20 ± 0.48 | 10.69 ± 4.32 | −1.50 ± 0.82 | −1.31 ± 0.73 | 4.16 |
| 3-5 | −0.89 ± 1.27 | 4.47 ± 4.43 | −1.08 ± 0.76 | 1.29 ± 2.26 | −2.41 ± 0.94 | 1.00 ± 1.97 | 13.69 ± 4.36 | 1.22 ± 1.52 | 0.27 ± 2.07 | 1.95 |
| 3-6 | 0.86 ± 2.44 | −2.21 ± 1.47 | 9.64 ± 0.86 | 0.14 ± 0.21 | −1.70 ± 1.33 | 0.86 ± 2.88 | 13.31 ± 4.53 | −0.61 ± 0.58 | 3.79 ± 1.09 | 2.68 |
| 3-7 | 2.81 ± 0.44 | 5.03 ± 2.82 | 5.76 ± 0.51 | −0.30 ± 0.58 | 0.77 ± 2.06 | 0.50 ± 0.88 | 21.21 ± 4.78 | 20.05 ± 1.98 | 9.26 ± 4.37 | 7.23 |
| 3-8 | 8.20 ± 1.62 | −0.15 ± 0.88 | 1.20 ± 2.13 | 1.63 ± 1.21 | 9.09 ± 0.56 | 6.52 ± 0.47 | 40.36 ± 4.27 | 42.93 ± 0.56 | 11.69 ± 1.38 | 13.50 |
| 3-9 | 41.18 ± 2.10 | 7.92 ± 3.48 | 52.10 ± 1.00 | 9.78 ± 1.00 | 26.96 ± 1.32 | 3.87 ± 0.68 | 66.71 ± 4.17 | 46.69 ± 2.20 | 24.44 ± 0.42 | 31.07 |
| 3-10 | 70.82 ± 1.51 | 34.01 ± 0.84 | 70.51 ± 0.54 | 14.23 ± 0.90 | 53.45 ± 0.84 | 5.66 ± 0.73 | 84.52 ± 3.94 | 47.10 ± 1.80 | 42.42 ± 0.52 | 46.97 |
| 3-11 | 88.42 ± 1.49 | 68.00 ± 2.89 | 83.36 ± 1.80 | 28.08 ± 0.20 | 68.55 ± 2.76 | 25.21 ± 5.27 | 92.17 ± 4.24 | 47.76 ± 2.24 | 49.34 ± 0.52 | 61.21 |
| 3-12 | 82.11 ± 1.38 | 78.21 ± 0.57 | 85.68 ± 0.75 | 36.03 ± 0.60 | 66.29 ± 0.24 | 47.04 ± 0.58 | 83.93 ± 4.06 | 59.23 ± 1.94 | 37.32 ± 2.06 | 63.98 |
| 3-13 | 76.28 ± 0.92 | 82.90 ± 1.95 | 82.88 ± 1.32 | 28.91 ± 2.54 | 18.18 ± 1.29 | 34.84 ± 2.03 | 63.29 ± 4.17 | 44.76 ± 0.65 | 37.20 ± 3.50 | 52.14 |
| 3-11′ | / | 80.1 ± 2.53 | 73.94 ± 0.72 | 42.95 ± 0.23 | 37.95 ± 0.08 | 5.65 ± 0.56 | 86.99 ± 0.28 | 80.59 ± 0.12 | 24.53 ± 0.32 | 54.09 a |
| Bs | 3.16 ± 1.30 | 81.67 ± 0.72 | 94.44 ± 1.01 | 29.72 ± 0.79 | 14.79 ± 5.01 | 24.52 ± 4.71 | 27.22 ± 1.00 | 93.10 ± 1.22 | 40.32 ± 2.66 | 45.44 |
| Km | 28.93 ± 2.65 | 62.92 ± 0.36 | 94.44 ± 2.82 | 50.09 ± 1.05 | 51.55 ± 0.80 | 53.60 ± 1.02 | 22.61 ± 1.21 | 37.90 ± 2.30 | 39.11 ± 0.88 | 49.02 |
| Flu | 93.14 ± 0.62 | 100 ± 0.00 | 95.91 ± 3.96 | 85.12 ± 3.91 | 78.57 ± 1.93 | 99.06 ± 0.28 | 83.53 ± 0.22 | 99.63 ± 0.12 | 99.89 ± 0.08 | 92.76 |
| Compound | EC50 | Regression Equation | R2 | 95% Confidence Interval |
|---|---|---|---|---|
| Fluazinam | 0.82 | y = 1.63x + 0.13 | 0.98 | 0.64–1.05 |
| 1–6 | 0.93 | y = 0.47x + 0.09 | 0.96 | 0.18–2.01 |
| 1–7 | 0.90 | y = 0.50x − 0.03 | 0.97 | 0.19–1.89 |
| 1–8 | 0.76 | y = 0.94x − 0.16 | 0.88 | 0.10–1.87 |
| 1–9 | 1.50 | y = 0.41x − 0.13 | 0.97 | 0.46–2.78 |
| 2–12 | 36.73 | y = 2.71x − 4.24 | 0.98 | 30.16–46.66 |
| 3–11 | 6.64 | y = 2.06x − 1.70 | 0.99 | 5.24–8.26 |
| Compound | IC50 (μM) | Regression Equation | R2 | 95% Confidence Interval |
|---|---|---|---|---|
| 1–8 | 19.27 | Y = −3.03 + 2.39X | 0.99 | 15.60–25.15 |
| 2–12 | 70.55 | Y = −2.85 + 1.55X | 0.99 | 47.76–136.56 |
| 3–11 | 23.42 | Y = −5.12 + 3.85X | 0.97 | 18.10–31.19 |
| Fluazinam | 1.17 | Y = −0.25 + 0.9X | 0.97 | 0.916–1.683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, H.; Yin, F.; Li, Y.; Jiang, J.; Xiao, Y.; Wu, Y.; Qin, Z. Phytopathogenic Fungicidal Activity and Mechanism Approach of Three Kinds of Triphenylphosphonium Salts. J. Fungi 2024, 10, 450. https://doi.org/10.3390/jof10070450
Liu X, Liu H, Yin F, Li Y, Jiang J, Xiao Y, Wu Y, Qin Z. Phytopathogenic Fungicidal Activity and Mechanism Approach of Three Kinds of Triphenylphosphonium Salts. Journal of Fungi. 2024; 10(7):450. https://doi.org/10.3390/jof10070450
Chicago/Turabian StyleLiu, Xuelian, Huihui Liu, Fahong Yin, Yiyi Li, Jiazhen Jiang, Yumei Xiao, Yanhua Wu, and Zhaohai Qin. 2024. "Phytopathogenic Fungicidal Activity and Mechanism Approach of Three Kinds of Triphenylphosphonium Salts" Journal of Fungi 10, no. 7: 450. https://doi.org/10.3390/jof10070450







