Physicochemical Properties of Different Sulfated Polysaccharide Components from Laetiporus sulphureus and Their Anti-Proliferative Effects on MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Sulfated Polysaccharides (SPS)
2.3. Preparation of F1, F2, and F3 from SPS
2.4. Analysis of Carbohydrate and Protein Contents
2.5. Estimation of Uronic Acid Content
2.6. Determination of Sulfate Content
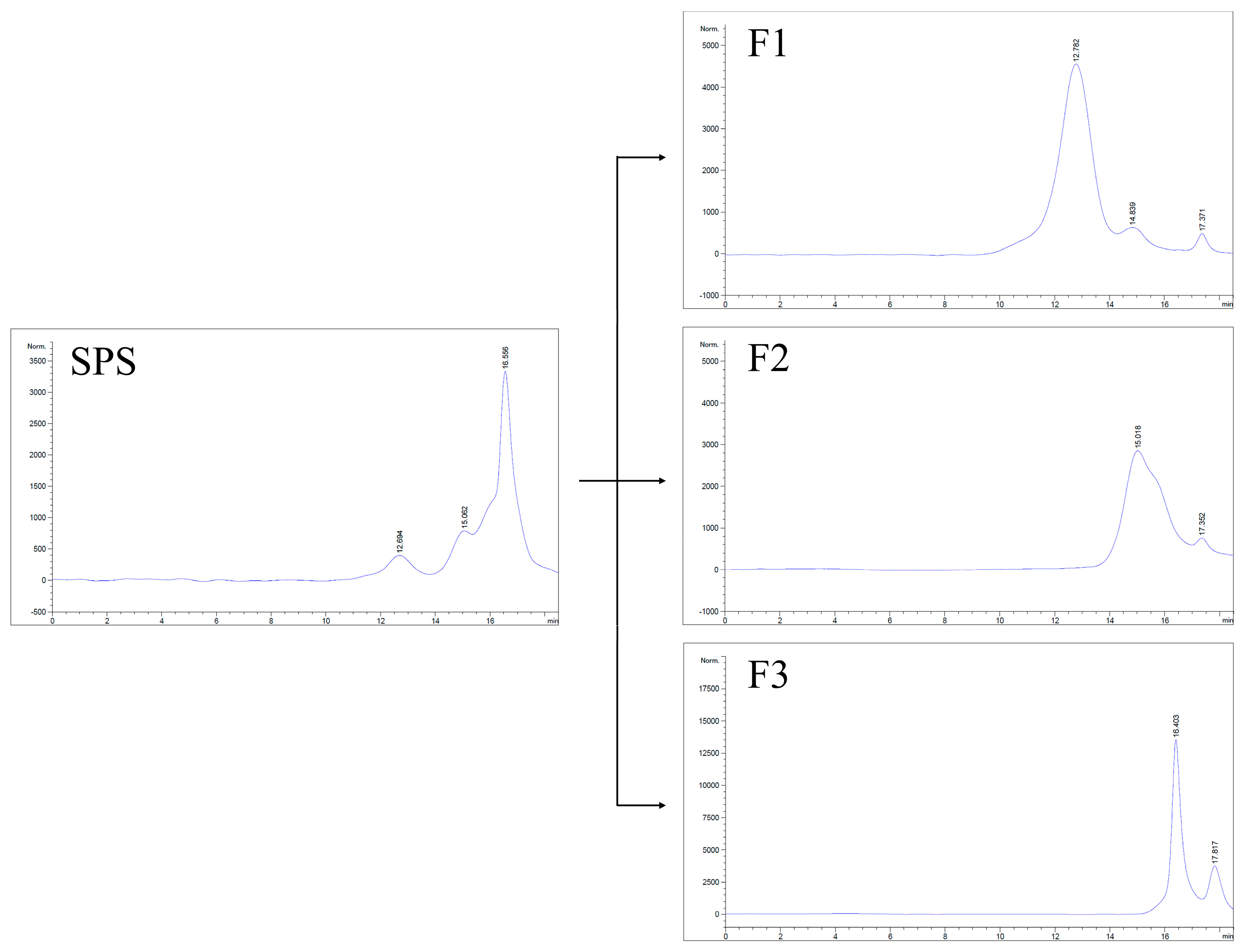
2.7. Analysis of Homogeneity and Molecular Weight
2.8. Determination of Monosaccharide Composition
2.9. Cell Culture
2.10. Cytotoxicity Assay
2.11. Cell Cycle Analysis
2.12. Cell Apoptosis Analysis
2.13. Protein Microarray Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Yield of SPS, F1, F2, and F3
3.2. Chemical Composition
3.3. Monosaccharide Composition
3.4. Molecular Weight
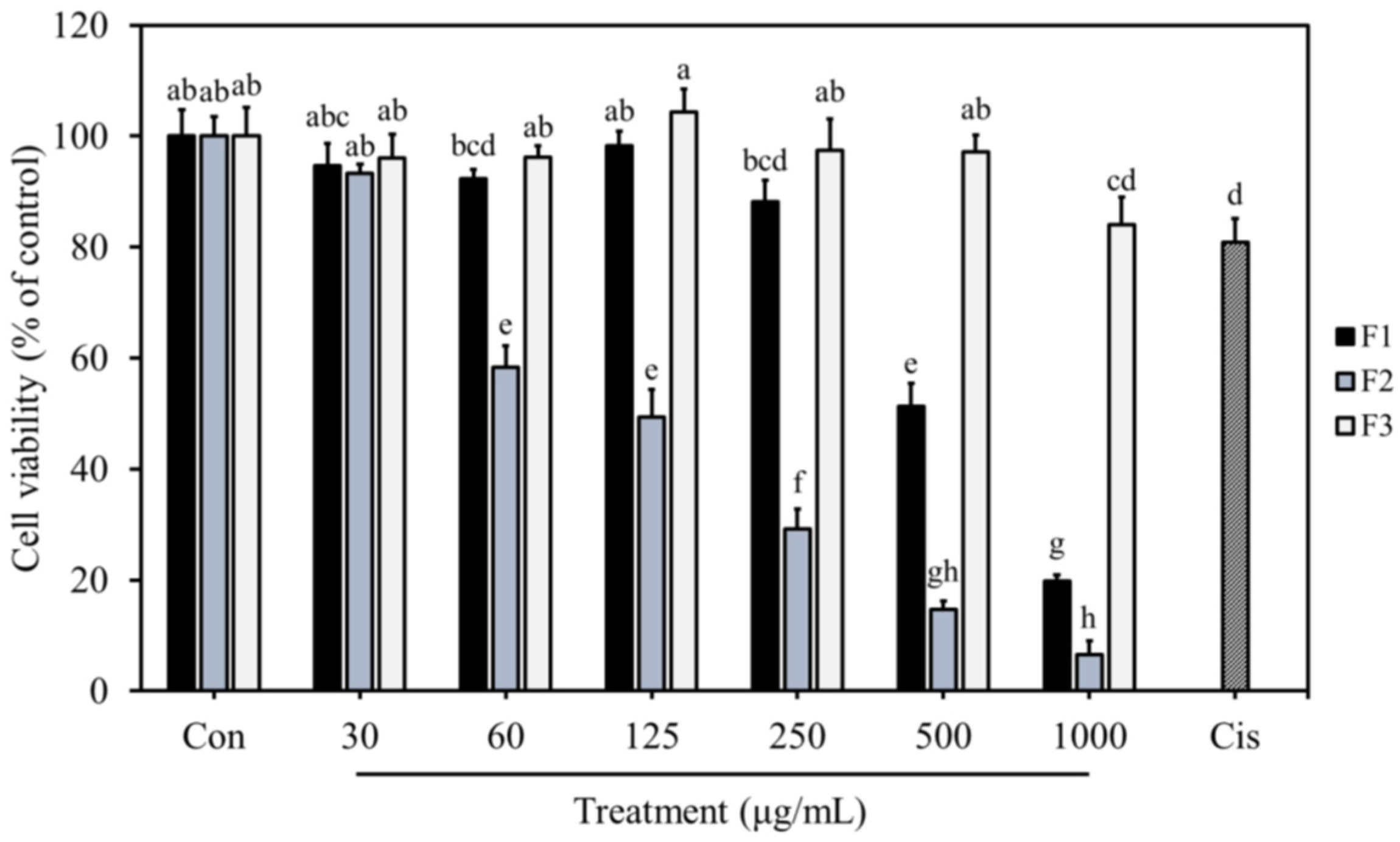
3.5. Anti-Proliferative Effects of F1, F2, and F3 on MDA-MB-231 Cells
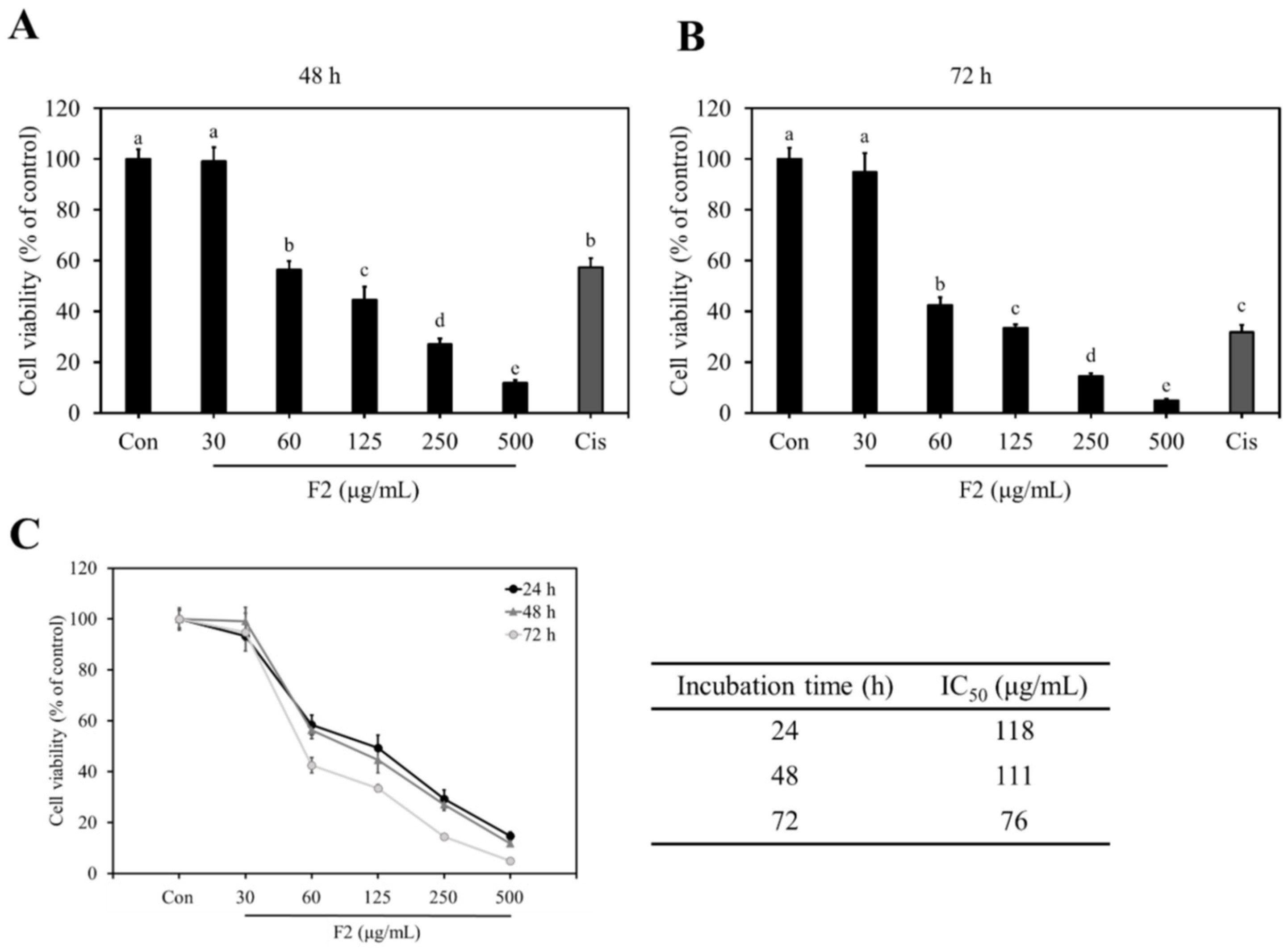
3.6. Effects of F1, F2, and F3 on Normal Cell Proliferation
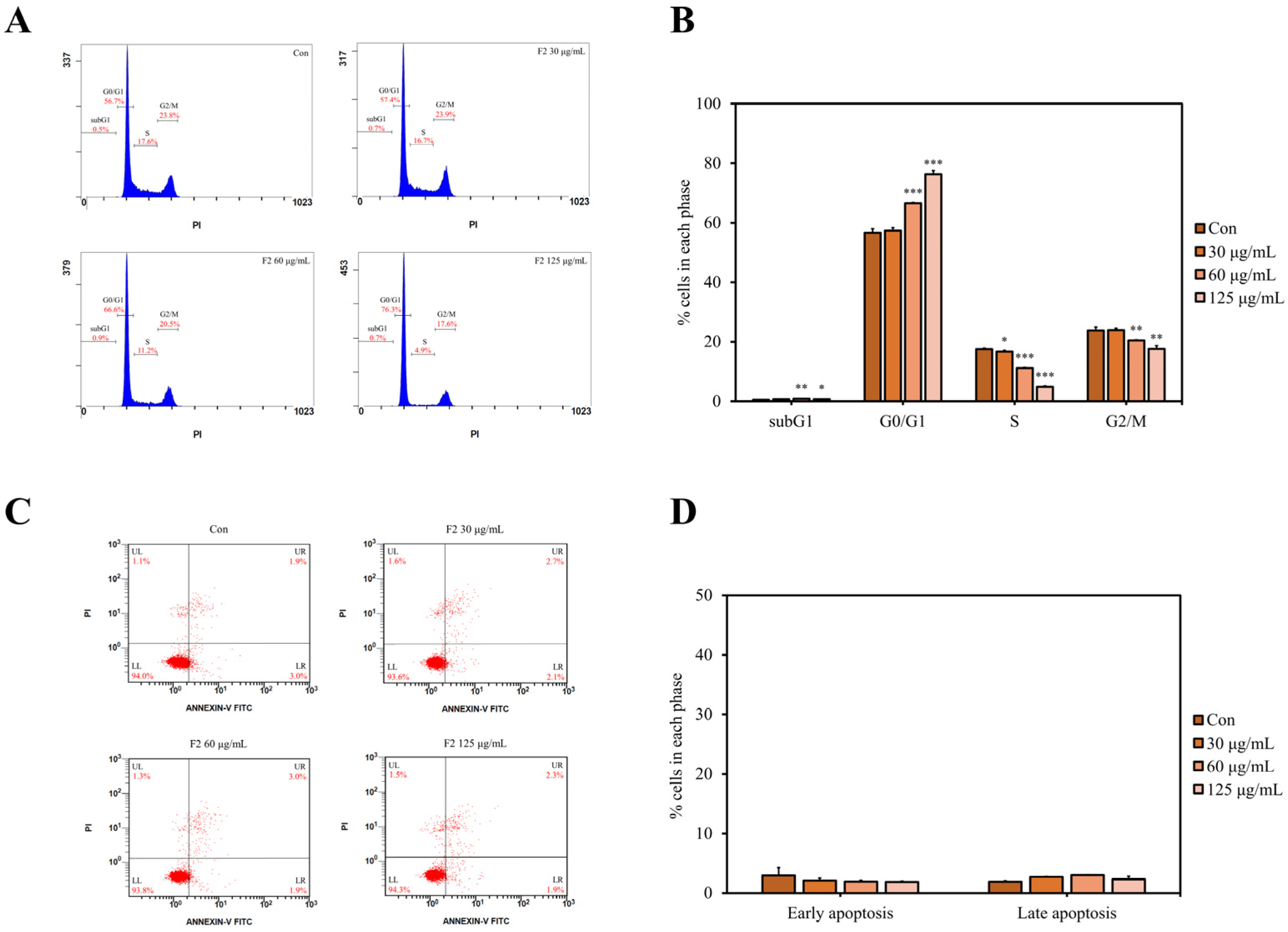
3.7. F2 Induced Cell Cycle Arrest but Not Apoptosis
3.8. Mechanistic Effects of F2 on Proteins Related to the Cell Cycle Arrest Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An overview on mushroom polysaccharides: Health-promoting properties, prebiotic and gut microbiota modulation effects and structure-function correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Su, C.H.; Lu, M.K.; Lu, T.J.; Lai, M.N.; Ng, L.T. A (1→6)-branched (1→4)-β-d-glucan from Grifola frondosa inhibits lipopolysaccharide-induced cytokine production in RAW264.7 macrophages by binding to TLR2 rather than dectin-1 or CR3 receptors. J. Nat. Prod. 2020, 83, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.I.; Su, C.H.; Lu, M.K.; Lai, M.N.; Ng, L.T. Synergistic anti-inflammatory effects of different polysaccharide components from Xylaria nigripes. J. Food Biochem. 2021, 45, e13694. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Sulfated polysaccharides of some seaweeds exhibit neuroprotection via mitigation of oxidative stress, cholinergic dysfunction and inhibition of Zn–induced neuronal damage in HT-22 cells. BMC Complement. Altern. Med. 2020, 20, 251. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Lu, M.K.; Jen, C.I.; Chao, C.H.; Hsu, Y.C.; Ng, L.T. SPS, a sulfated galactoglucan of Laetiporus sulphureus, exhibited anti-inflammatory activities. Int. J. Biol. Macromol. 2023, 226, 1236–1247. [Google Scholar] [CrossRef]
- Cheng, J.J.; Chao, C.H.; Chang, P.C.; Lu, M.K. Studies on anti-inflammatory activity of sulfated polysaccharides from cultivated fungi Antrodia cinnamomea. Food Hydrocoll. 2016, 53, 37–45. [Google Scholar] [CrossRef]
- Hogwood, J.; Naggi, A.; Torri, G.; Page, C.; Rigsby, P.; Mulloy, B.; Gray, E. The effect of increasing the sulfation level of chondroitin sulfate on anticoagulant specific activity and activation of the kinin system. PLoS ONE 2018, 13, e0193482. [Google Scholar] [CrossRef] [PubMed]
- Surayot, U.; Wangtueai, S.; You, S.; Techapun, C.; Phimolsiripol, Y.; Leksawasdi, N.; Krusong, W.; Barba, F.J.; Seesuriyachan, P. Sulphation and hydrolysis improvements of bioactivities, and immuno-modulatory properties of edible Amanita hemibapha subspecies javanica (Corner and Bas) mucilage polysaccharide as a potential in personalized functional foods. J. Fungi 2021, 7, 847. [Google Scholar] [CrossRef] [PubMed]
- Rizkyana, A.D.; Ho, T.C.; Roy, V.C.; Park, J.S.; Kiddane, A.T.; Kim, G.D.; Chun, B.S. Sulfation and characterization of polysaccharides from oyster mushroom (Pleurotus ostreatus) extracted using subcritical water. J. Supercrit. Fluids 2022, 179, 105412. [Google Scholar] [CrossRef]
- Lu, M.K.; Lee, M.H.; Chao, C.H.; Hsu, Y.C. Sodium sulfate addition increases the bioresource of biologically active sulfated polysaccharides from Antrodia cinnamomea. Int. J. Biol. Macromol. 2024, 257, 128699. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.W. Mushroom polysaccharide-assisted anticarcinogenic mycotherapy: Reviewing its clinical trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, B.; Semkova, S.; Tsoneva, I.; Antov, G.; Ivanova, J.; Vasileva, I.; Kardaleva, P.; Stoineva, I.; Christova, N.; Nacheva, L. Characterization and potential antitumor effect of a heteropolysaccharide produced by the red alga Porphyridium sordidum. Eng. Life Sci. 2019, 19, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Granja, S.; Neves, N.M.; Reis, R.L.; Baltazar, F.; Silva, T.H.; Martins, A. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr. Polym. 2019, 223, 115034. [Google Scholar] [CrossRef]
- Vaikundamoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Structural characterization and anticancer activity (MCF7 and MDA-MB-231) of polysaccharides fractionated from brown seaweed Sargassum wightii. Int. J. Biol. Macromol. 2018, 111, 1229–1237. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Ćirić, A.; Nikolić, M.; Bukvički, D.; Guerzoni, M.E.; Soković, M.D. Laetiporus sulphureus, edible mushroom from Serbia: Investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem. Toxicol. 2013, 59, 297–302. [Google Scholar] [CrossRef]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007, 101, 267–273. [Google Scholar] [CrossRef]
- Duan, Y.; Qi, J.; Gao, J.; Liu, C. Bioactive components of Laetiporus species and their pharmacological effects. Appl. Microbiol. Biotechnol. 2022, 106, 5929–5944. [Google Scholar] [CrossRef]
- Jen, C.I.; Lu, M.K.; Lai, M.N.; Ng, L.T. Sulfated polysaccharides of Laetiporus sulphureus fruiting bodies exhibit anti-breast cancer activity through cell cycle arrest, apoptosis induction, and inhibiting cell migration. J. Ethnopharmacol. 2024, 321, 117546. [Google Scholar] [CrossRef]
- Albano, R.M.; Mourão, P.A. Isolation, fractionation, and preliminary characterization of a novel class of sulfated glycans from the tunic of Styela plicata (Chordata Tunicata). J. Biol. Chem. 1986, 261, 758–765. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus versicolor: Physiological activity, uses, and production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.S. Methods for Quantification and Extraction of Fucoidan, and Quantification of the Release of Total Carbohydrate and Fucoidan from the Brown Algae Laminaria hyperborea. Master’s Thesis, Norwegian University of Science and Technology, Department of Biotechnology, Trondheim, Norway, 2015. [Google Scholar]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.; Sommer, S. Polysaccharides influence the results of polymeric pigment analysis in red wines. ACS Food Sci. Technol. 2021, 1, 1770–1775. [Google Scholar] [CrossRef]
- Wei, D.; Wei, Y.; Cheng, W.; Zhang, L. Sulfated modification, characterization and antitumor activities of Radix hedysari polysaccharide. Int. J. Biol. Macromol. 2012, 51, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Jin, X.; Wu, X.; Yang, X.; Lin, D.; Li, C.; Fu, Y.; Liu, Y.; Liu, X.; Lv, J.; et al. Structural characterisation and antitumor activity against non-small cell lung cancer of polysaccharides from Sanghuangporus vaninii. Carbohydr. Polym. 2022, 276, 118798. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, D.; Xiao, H.; Fu, X.; Kong, Q.; Zhu, C.; Han, Z.; Mou, H. Marine-derived uronic acid-containing polysaccharides: Structures, sources, production, and nutritional functions. Trends Food Sci. Technol. 2022, 122, 1–12. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Li, L. The relationship between charge intensity and bioactivities/processing characteristics of exopolysaccharides from lactic acid bacteria. LWT—Food Sci. Technol. 2022, 153, 112345. [Google Scholar] [CrossRef]
- Ray, B.; Schütz, M.; Mukherjee, S.; Jana, S.; Ray, S.; Marschall, M. Exploiting the amazing diversity of natural source-derived polysaccharides: Modern procedures of isolation, engineering, and optimization of antiviral activities. Polymers 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ghosh, K.; Hahn, F.; Wangen, C.; Strojan, H.; Müller, R.; Anand, N.; Ali, I.; Bera, K.; Ray, B. Chemically sulfated polysaccharides from natural sources: Assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int. J. Biol. Macromol. 2019, 136, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Yue, G.L.; Yue, R.Q.; Dong, C.X.; Chan, C.L.; Ko, C.H.; Cheung, W.S.; Luo, K.W.; Dai, H.; Wong, C.K. Structure elucidation and immunomodulatory activity of a beta-glucan from the fruiting bodies of Ganoderma sinense. PLoS ONE 2014, 9, e100380. [Google Scholar] [CrossRef]
- Chari, R.V.J. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008, 41, 98–107. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Liao, S.F.; Liang, C.H.; Ho, M.Y.; Hsu, T.L.; Tsai, T.I.; Hsieh, Y.S.Y.; Tsai, C.M.; Li, S.T.; Cheng, Y.Y.; Tsao, S.M. Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc. Natl. Acad. Sci. USA 2013, 110, 13809–13814. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.X.; Wang, J.; Hu, J.; Lou, G.H.; Xiong, H.J.; Peng, C.Y.; Huang, Q.W. Modulation of apoptosis by plant polysaccharides for exerting anti-cancer effects: A review. Front. Pharmacol. 2020, 11, 792. [Google Scholar] [CrossRef]
- Yao, W.Z.; Veeraperumal, S.; Qiu, H.M.; Chen, X.Q.; Cheong, K.L. Anti-cancer effects of Porphyra haitanensis polysaccharides on human colon cancer cells via cell cycle arrest and apoptosis without causing adverse effects in vitro. 3 Biotech 2020, 10, 386. [Google Scholar] [CrossRef]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Khoo, K.H.; Chen, S.T.; Lin, C.C.; Wong, C.H.; Lin, C.H. Studies on the immune-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg. Med. Chem. 2002, 10, 1057–1062. [Google Scholar] [CrossRef]
- Al-Tuwaijri, M.M.; Basal, W.T.; Sabry, N.M.; Moussa, T.A.; Das, B.; Eid, J.I. Inonotus obliquus polysaccharides inhibited cellular growth of NCI-H23 and A549 lung cancer cells through G0/G1 cell cycle arrest and ROS mediated cell death. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2021, 13, 27–40. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising anticancer activity of polysaccharides and other macromolecules derived from oyster mushroom (Pleurotus sp.): An updated review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971. [Google Scholar] [CrossRef]
- Hsu, W.H.; Qiu, W.L.; Tsao, S.M.; Tseng, A.J.; Lu, M.K.; Hua, W.J.; Cheng, H.C.; Hsu, H.Y.; Lin, T.Y. Effects of WSG, a polysaccharide from Ganoderma lucidum, on suppressing cell growth and mobility of lung cancer. Int. J. Biol. Macromol. 2020, 165, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Pruitt, S.C.; Hershberger, P.A.; Witkiewicz, A.K.; Goodrich, D.W. Cell cycle and beyond: Exploiting new RB1 controlled mechanisms for cancer therapy. Trends Cancer 2019, 5, 308–324. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-CDK4,6 drives cell-cycle progression via the retinoblastoma protein’s C-terminal helix. Mol. Cell 2019, 74, 758–770.E4. [Google Scholar] [CrossRef]
- Song, X.; Fang, C.; Dai, Y.; Sun, Y.; Qiu, C.; Lin, X.; Xu, R. Cyclin-dependent kinase 7 (CDK7) inhibitors as a novel therapeutic strategy for different molecular types of breast cancer. Br. J. Cancer 2024, 130, 1239–1248. [Google Scholar] [CrossRef]
- Wang, P.; Zou, F.; Zhang, X.; Li, H.; Dulak, A.; Jr Tomko, R.J.; Lazo, J.S.; Wang, Z.; Zhang, L.; Yu, J. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009, 69, 8157–8165. [Google Scholar] [CrossRef]
- Han, X.; Liu, J. Cell cycle-independent roles of p19INK4d in human terminal erythropoiesis. Chin. J. Cancer 2017, 36, 22. [Google Scholar] [CrossRef]
- Guillamot, M.; Manchado, E.; Chiesa, M.; Gómez-López, G.; Pisano, D.G.; Sacristán, M.P.; Malumbres, M. Cdc14b regulates mammalian RNA polymerase II and represses cell cycle transcription. Sci. Rep. 2011, 1, 189. [Google Scholar] [CrossRef]
- Schrock, M.S.; Stromberg, B.R.; Scarberry, L.; Summers, M.K. APC/C ubiquitin ligase: Functions and mechanisms in tumorigenesis. Semin. Cancer Biol. 2020, 67, 80–91. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Donovan, J.; Slingerland, J. Transforming growth factor-β and breast cancer: Cell cycle arrest by transforming growth factor-β and its disruption in cancer. Breast Cancer Res. 2000, 2, 116. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, H.; Lu, Y.; Wu, T. Role and clinical significance of TGF-β1 and TGF-βR1 in malignant tumors. Int. J. Mol. Med. 2021, 47, 55. [Google Scholar] [CrossRef]

| Sample | Chemical Composition (%) | |||
|---|---|---|---|---|
| Total Sugar | Total Protein | Uronic Acid | Sulfate | |
| F1 | 92.95 ± 0.10 | 5.12 ± 0.26 | 12.86 ± 2.10 | 0.24 ± 0.02 |
| F2 | 53.86 ± 3.62 | 15.57 ± 2.44 | 4.45 ± 0.42 | 1.99 ± 0.07 |
| F3 | 35.34 ± 9.00 | 1.55 ± 0.34 | 2.70 ± 0.89 | 2.24 ± 0.02 |
| Sample | Monosaccharide Composition (Molar %) | ||||||
|---|---|---|---|---|---|---|---|
| Man | Rib | Glc | Gal | Xyl | Ara | Fuc | |
| F1 | 4.31 ± 0.02 | ND | 84.92 ± 0.07 | 5.88 ± 0.03 | ND | ND | 4.89 ± 0.01 |
| F2 | 27.70 ± 3.61 | ND | 16.65 ± 2.22 | 31.58 ± 3.58 | ND | ND | 24.06 ± 2.43 |
| F3 | 38.73 ± 4.33 | 10.45 ± 1.26 | 25.07 ± 7.79 | 10.56 ± 0.56 | 6.44 ± 0.59 | 8.76 ± 1.09 | ND |
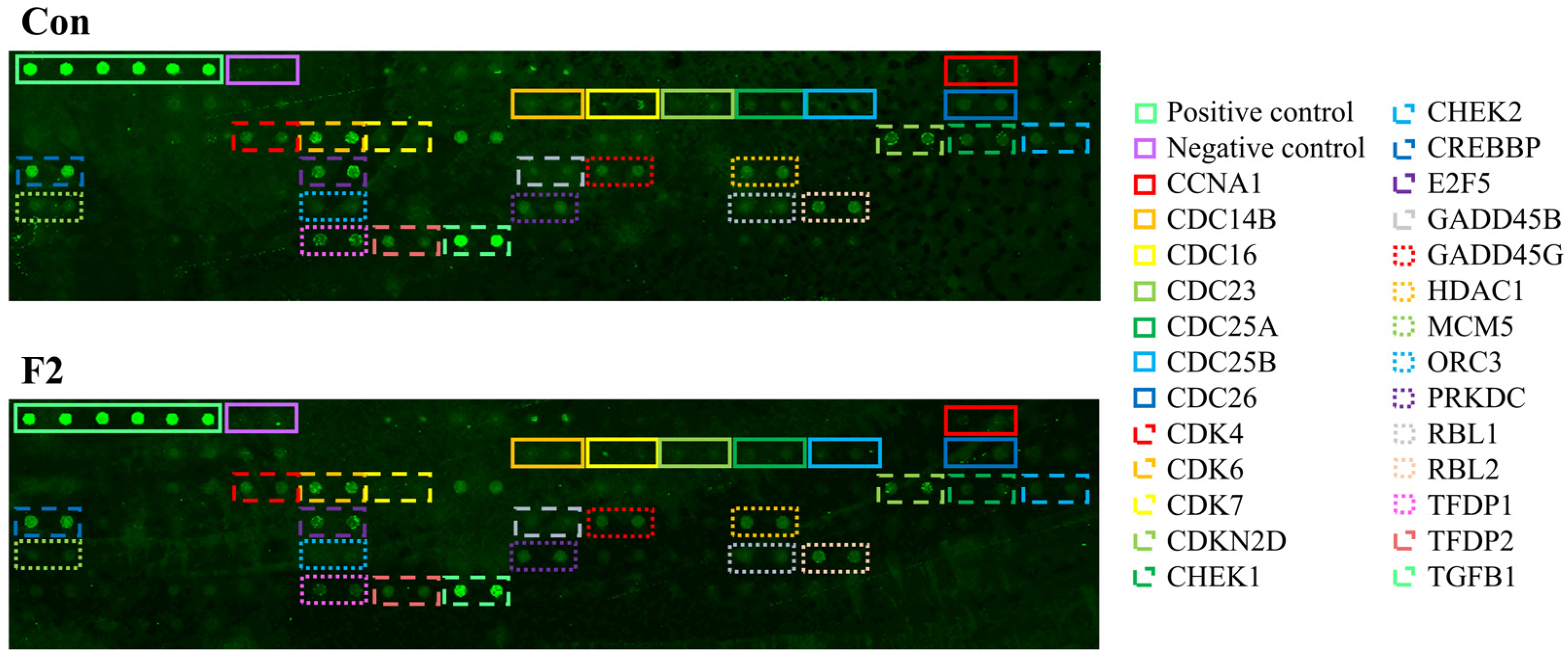
| Target Gene Name | Target Protein Name | Fluorescence | |
|---|---|---|---|
| Con | F2 | ||
| POS-Ave (positive control) | 26,477 | 26,477 | |
| Neg (negative control) | 775 | 642 | |
| CCNA1 | Cyclin A1 | 1186 | 863 |
| CDC14B | Dual specificity protein phosphatase | 1504 | 1095 |
| CDC16 | Cell division cycle protein 16 homolog | 2222 | 1225 |
| CDC23 | Cell division cycle protein 23 homolog | 1135 | 651 |
| CDC25A | Cell division cycle protein 25A | 1403 | 736 |
| CDC25B | Cell division cycle protein 25B | 1003 | 823 |
| CDC26 | Cell division cycle protein 26 homolog | 1424 | 1183 |
| CDK4 | Cyclin-dependent kinase 4 | 2882 | 2137 |
| CDK6 | Cyclin-dependent kinase 6 | 11,611 | 8464 |
| CDK7 | Cyclin-dependent kinase 7 | 1261 | 362 |
| CDKN2D | Cyclin-dependent kinase 4 inhibitor p19 | 4099 | 5613 |
| CHEK1 | Cell cycle checkpoint kinase 1 | 2247 | 1004 |
| CHEK2 | Cell cycle checkpoint kinase 2 | 1360 | 898 |
| CREBBP | Histone lysine acetyltransferase | 12,918 | 7627 |
| E2F5 | Transcription factor E2F5 | 7486 | 4780 |
| GADD45B | Myeloid differentiation primary response protein | 1352 | 909 |
| GADD45G | DNA damage-inducible transcript 2 protein | 2843 | 2397 |
| HDAC1 | Histone deacetylase 1 | 3731 | 3367 |
| MCM5 | DNA replication licensing factor MCM5 | 1675 | 1043 |
| ORC3 | Origin recognition complex subunit 3 | 1413 | 709 |
| PRKDC | DNA-dependent protein kinase catalytic subunit | 2996 | 2602 |
| RBL1 | Retinoblastoma-like protein 1 | 2010 | 1662 |
| RBL2 | Retinoblastoma-like protein 2 | 4160 | 2878 |
| TFDP1 | Transcription factor Dp-1 | 5082 | 1639 |
| TFDP2 | Transcription factor Dp-2 | 5290 | 2268 |
| TGFB1 | Transforming growth factor β1 | 32,173 | 19,476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jen, C.-I.; Ng, L.-T. Physicochemical Properties of Different Sulfated Polysaccharide Components from Laetiporus sulphureus and Their Anti-Proliferative Effects on MDA-MB-231 Breast Cancer Cells. J. Fungi 2024, 10, 457. https://doi.org/10.3390/jof10070457
Jen C-I, Ng L-T. Physicochemical Properties of Different Sulfated Polysaccharide Components from Laetiporus sulphureus and Their Anti-Proliferative Effects on MDA-MB-231 Breast Cancer Cells. Journal of Fungi. 2024; 10(7):457. https://doi.org/10.3390/jof10070457
Chicago/Turabian StyleJen, Chia-I, and Lean-Teik Ng. 2024. "Physicochemical Properties of Different Sulfated Polysaccharide Components from Laetiporus sulphureus and Their Anti-Proliferative Effects on MDA-MB-231 Breast Cancer Cells" Journal of Fungi 10, no. 7: 457. https://doi.org/10.3390/jof10070457
APA StyleJen, C.-I., & Ng, L.-T. (2024). Physicochemical Properties of Different Sulfated Polysaccharide Components from Laetiporus sulphureus and Their Anti-Proliferative Effects on MDA-MB-231 Breast Cancer Cells. Journal of Fungi, 10(7), 457. https://doi.org/10.3390/jof10070457






