Alternaria alternata Pathogen from Cuscuta japonica Could Serve as a Potential Bioherbicide
Abstract
1. Introduction
2. Materials and Methods
2.1. Diseased Sample Collection and Pathogen Enrichment Culture
2.2. Pathogen Isolation and Pathogenicity Determination
2.3. Determination of Differences in the Pathogenicity of Dodder Pathogens
2.4. Physiological Characterization of Dodder Pathogens
2.5. Determination of the Pathogenic Range of Dodder Pathogens
2.6. Determination of the Efficacy of the Pathogen against Dodder in the Field
2.7. Identification of Dodder Pathogens
2.8. Data Statistics
3. Results
3.1. Dodder Pathogen Enrichment Culture
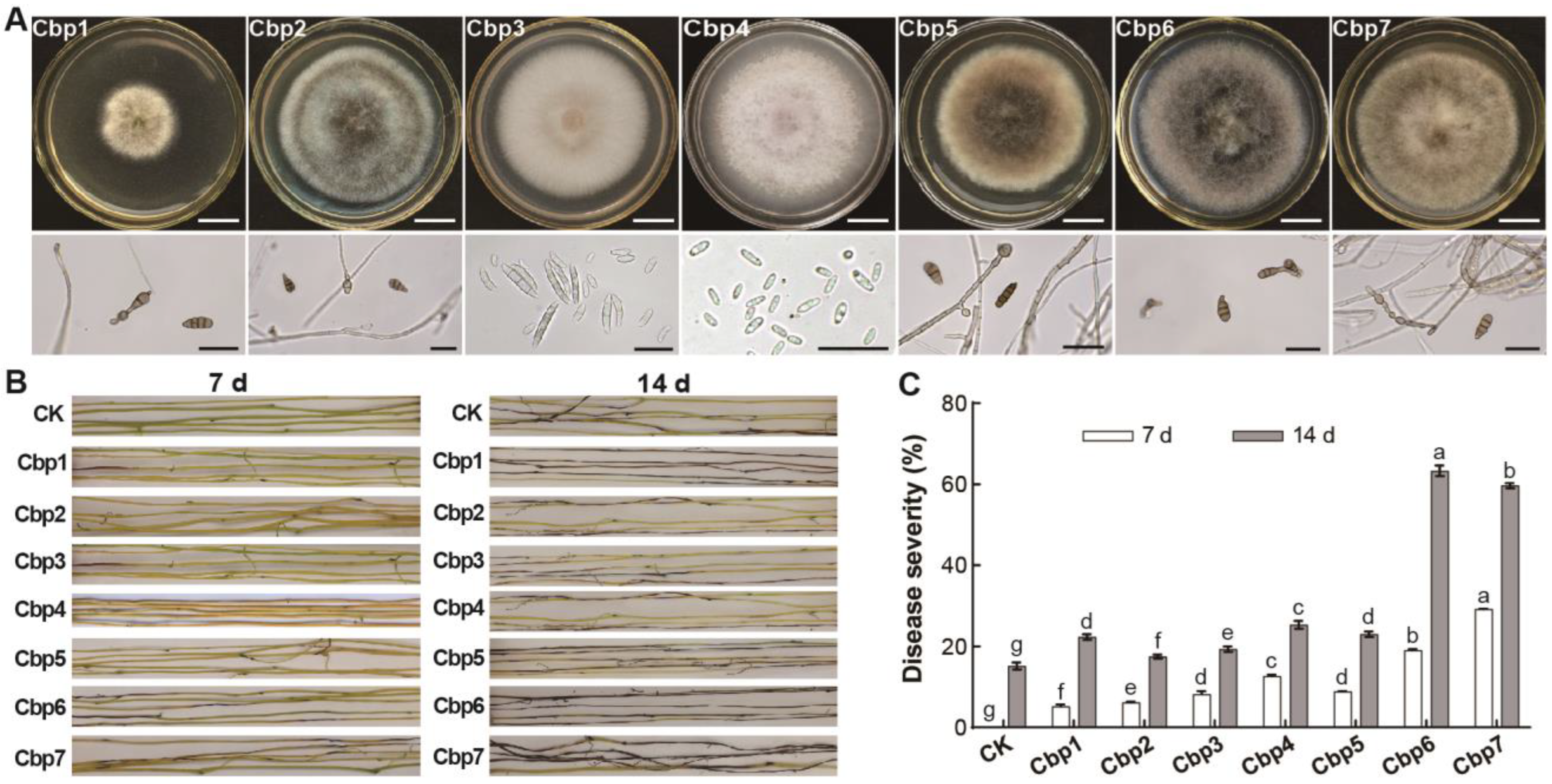
3.2. Pathogens of Dodder and Their Pathogenicity
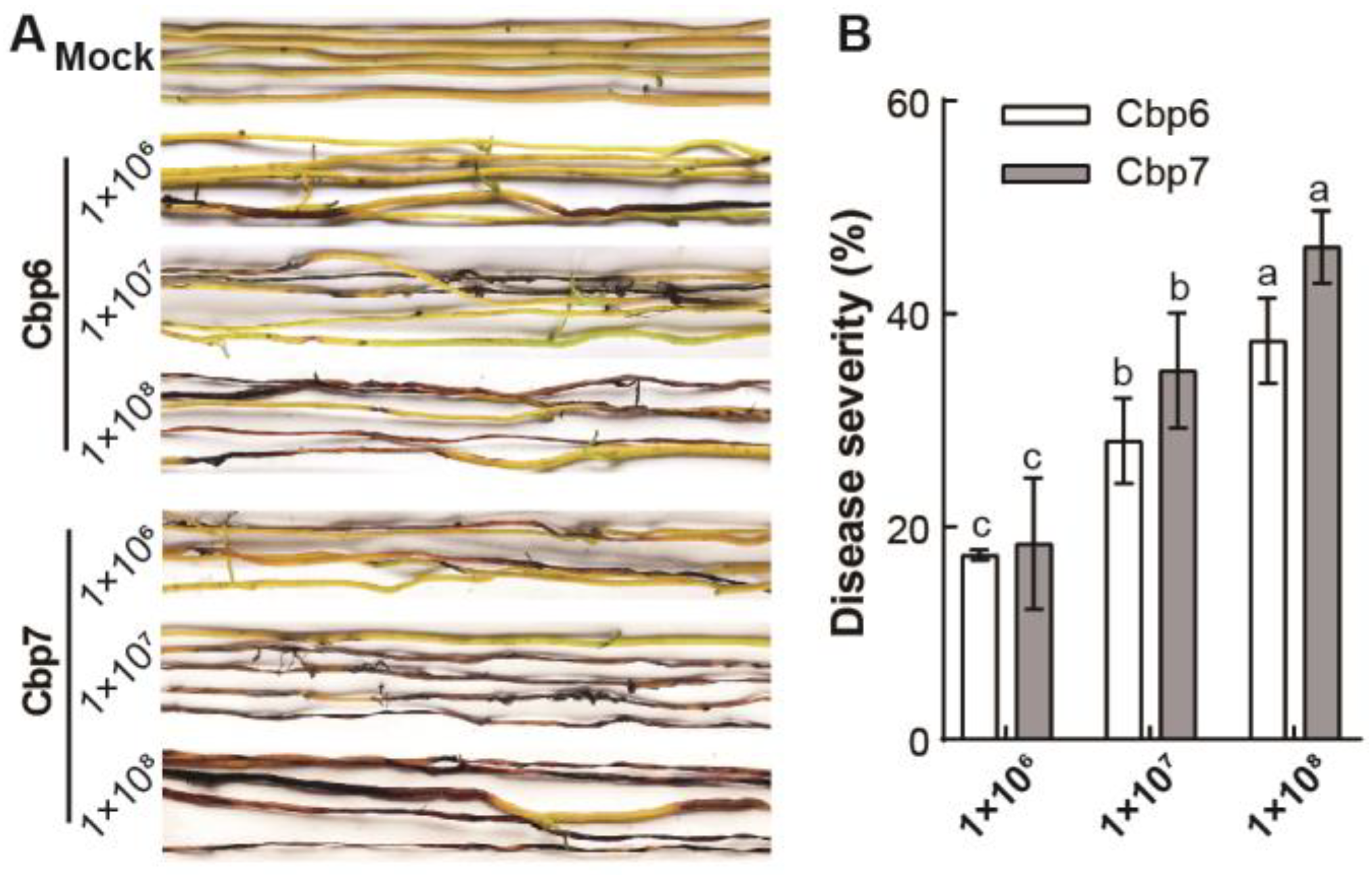
3.3. Differences in the Pathogenicity of Dodder Pathogens
3.4. Physiological Characterization of Dodder Pathogens
3.5. Pathogenic Range of Dodder Pathogens
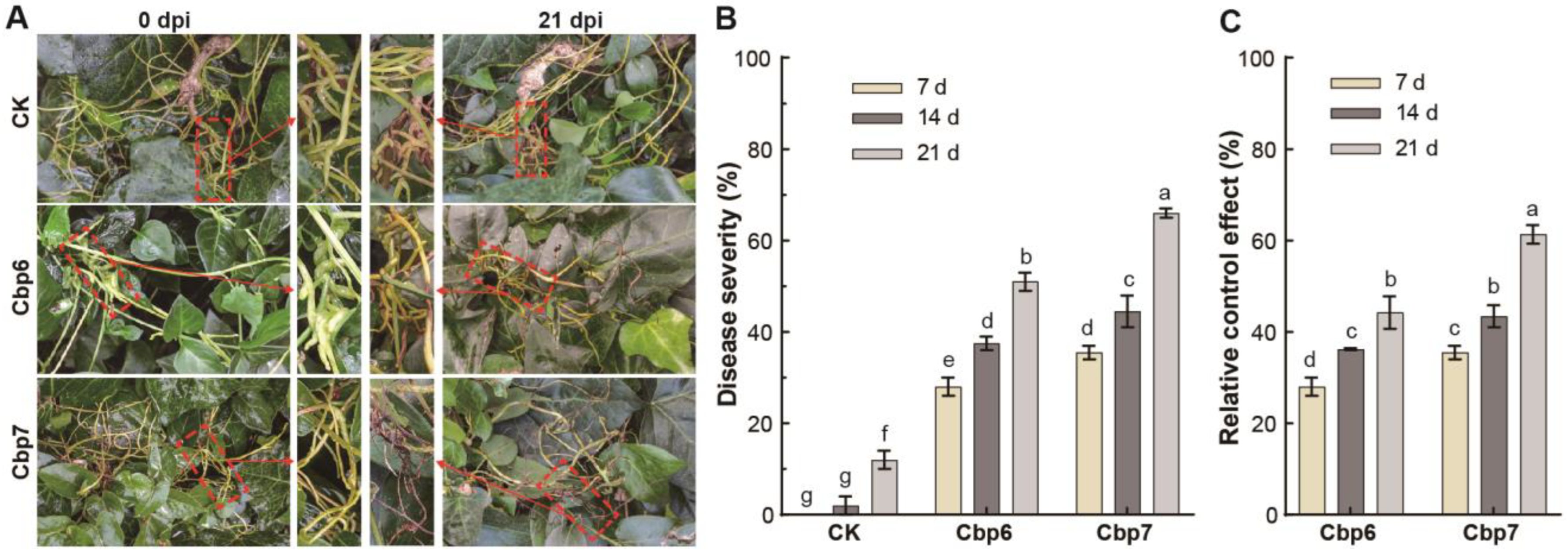
3.6. Efficacy of Pathogens against Dodder in the Field
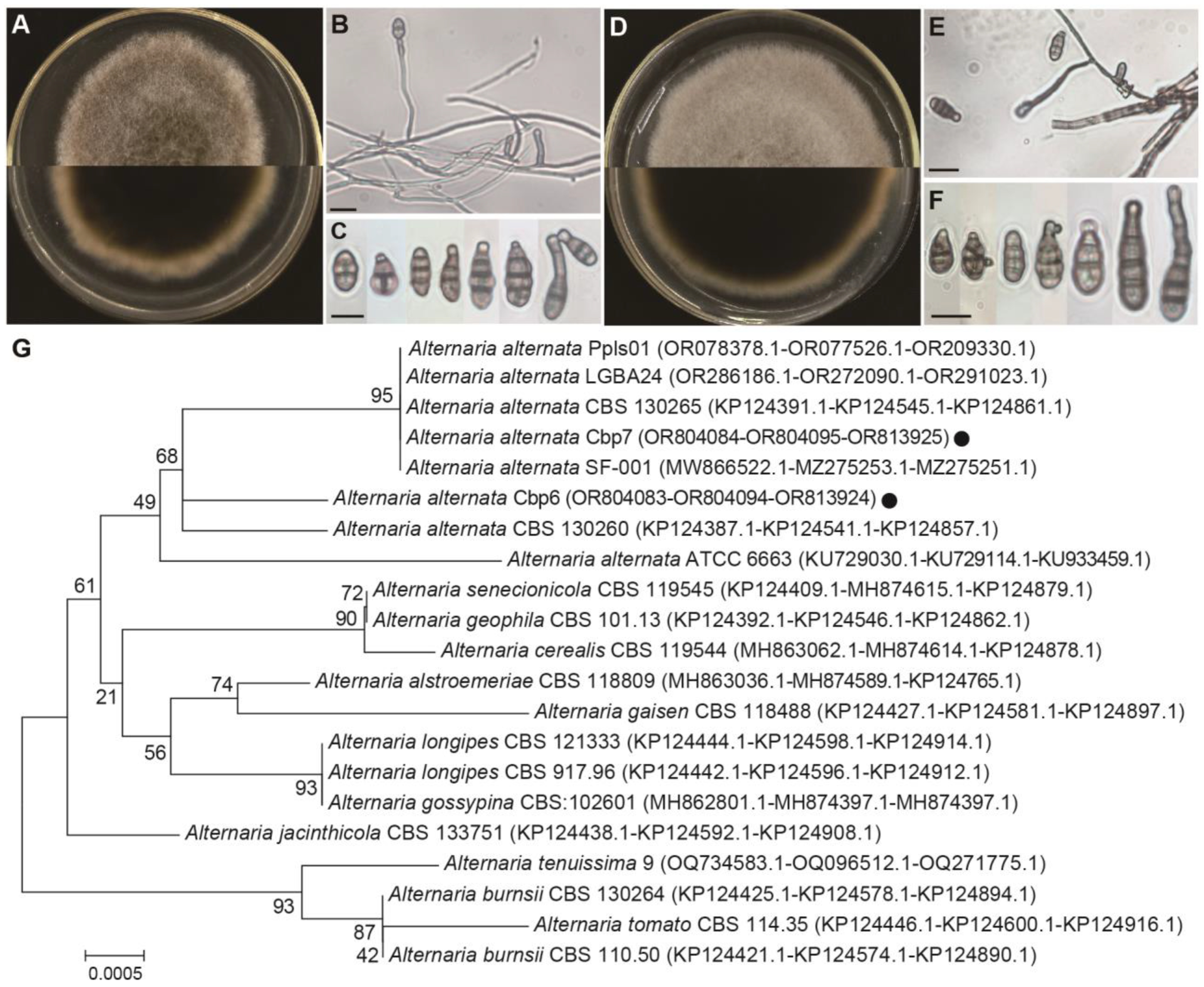
3.7. Identification of Dodder Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, G.; Liu, N.; Zhang, J.; Xu, Y.; Baldwin, I.T.; Wu, J. Cuscuta australis (dodder) parasite eavesdrops on the host plants’ FT signals to flower. Proc. Natl. Acad. Sci. USA 2020, 117, 23125–23130. [Google Scholar] [CrossRef]
- Sun, G.; Xu, Y.; Liu, H.; Sun, T.; Zhang, J.; Hettenhausen, C.; Shen, G.; Qi, J.; Qin, Y.; Li, J.; et al. Large-scale gene losses underlie the genome evolution of parasitic plant Cuscuta australis. Nat. Commun. 2018, 9, 2683. [Google Scholar] [CrossRef]
- Hartenstein, M.; Albert, M.; Krause, K.; Lunn, J. The plant vampire diaries: A historic perspective on Cuscuta research. J. Exp. Bot. 2023, 74, 2944–2955. [Google Scholar] [CrossRef] [PubMed]
- Jhu, M.-Y.; Sinha, N.R. Cuscuta species: Model organisms for haustorium development in stem holoparasitic plants. Front. Plant Sci. 2022, 13, 1086384. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule exchange in Cuscuta–host plant interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; dePamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, D.; Fang, L.; Zhou, Q.; Liu, W.; Liu, Z. Bidirectional lncRNA transfer between Cuscuta parasites and their host plant. Int. J. Mol. Sci. 2022, 23, 561. [Google Scholar] [CrossRef] [PubMed]
- Masanga, J.; Mwangi, B.N.; Kibet, W.; Sagero, P.; Wamalwa, M.; Oduor, R.; Ngugi, M.; Alakonya, A.; Ojola, P.; Bellis, E.S.; et al. Physiological and ecological warnings that dodders pose an exigent threat to farmlands in Eastern Africa. Plant Physiol. 2021, 185, 1457–1467. [Google Scholar] [CrossRef]
- Shen, H.; Hong, L.; Ye, W.; Cao, H.; Wang, Z. The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha. J. Exp. Bot. 2007, 58, 2929–2937. [Google Scholar] [CrossRef]
- Wu, A.P.; Zhong, W.; Yuan, J.R.; Qi, L.Y.; Chen, F.L.; Liang, Y.S.; He, F.F.; Wang, Y.H. The factors affecting a native obligate parasite, Cuscuta australis, in selecting an exotic weed, Humulus scandens, as its host. Sci. Rep. 2019, 9, 511. [Google Scholar] [CrossRef]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A review on phytoconstituents and biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Rehman, K.; Hussain, I.; Farooq, T.; Ali, B.; Majeed, I.; Akash, M.S.H. Ethnopharmacological investigations of phytochemical constituents isolated from the genus Cuscuta. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 113–150. [Google Scholar] [CrossRef]
- Shimizu, K.; Aoki, K. Development of parasitic organs of a stem holoparasitic plant in genus Cuscuta. Front. Plant Sci. 2019, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Fürst, U.; Hegenauer, V.; Kaiser, B.; Körner, M.; Welz, M.; Albert, M. Parasitic Cuscuta factor(s) and the detection by tomato initiates plant defense. Commun. Integr. Biol. 2016, 9, e1244590. [Google Scholar] [CrossRef][Green Version]
- Johnsen, H.R.; Striberny, B.; Olsen, S.; Vidal-Melgosa, S.; Fangel, J.U.; Willats, W.G.T.; Rose, J.K.C.; Krause, K. Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: A priori differences and induced changes. New Phytol. 2015, 207, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qu, F.; Li, Z.; Doohan, D. Inter-species protein trafficking endows dodder (Cuscuta pentagona) with a host-specific herbicide-tolerant trait. New Phytol. 2013, 198, 1017–1022. [Google Scholar] [CrossRef]
- Moreno-Robles, A.; Cala Peralta, A.; Zorrilla, J.G.; Soriano, G.; Masi, M.; Vilariño-Rodríguez, S.; Cimmino, A.; Fernández-Aparicio, M. Identification of structural features of hydrocinnamic acid related to its allelopathic activity against the parasitic weed Cuscuta campestris. Plants 2022, 11, 2846. [Google Scholar] [CrossRef]
- Chaudhury, F.A.; Khan, I.H.; Javaid, A. Effect of quinoa biomass and biocontrol fungi on expression of IPER gene in mung bean in Macrophomina phaseolina contaminated soil. Adv. Life Sci. 2023, 10, 193–199. [Google Scholar]
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014; pp. 245–266. [Google Scholar]
- Liu, Y.; He, P.; He, P.; Munir, S.; Ahmed, A.; Wu, Y.; Yang, Y.; Lu, J.; Wang, J.; Yang, J.; et al. Potential biocontrol efficiency of Trichoderma species against oomycete pathogens. Front. Microbiol. 2022, 13, 974024. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCRProtoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Stielow, J.B.; Lévesque, C.A.; Seifert, K.A.; Meyer, W.; Irinyi, L.; Smits, D.; Renfurm, R.; Verkley, G.J.M.; Groenewald, M.; Chaduli, D.; et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia—Mol. Phylogeny Evol. Fungi 2015, 35, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenetics Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gui, T.; Ahmed, A.; Munir, S.; He, P.; He, P.; Wu, Y.; Tang, P.; Luo, Q.; He, Y. Pyricularia pennisetigena as leaf blast disease-causing pathogen in king grass (Pennisetum sinese) and its assessment of the pathogenic risk. J. Plant Pathol. 2024. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, A.; Munir, S.; He, P.; He, P.; Wu, Y.; Tang, P.; Wang, Z.; Kong, B.; He, Y. First report of Aloe root and stem rot caused by Phytophthora palmivora in Yunnan Province, China. Plant Dis. 2023, 107, 2892. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.S.; Paul, C.; Achor, D.; Brlansky, R.H. Colonization of dodder, Cuscuta indecora, by ‘Candidatus Liberibacter asiaticus’ and ‘Ca. L. americanus’. Phytopathology 2010, 100, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hettenhausen, C.; Sun, G.; Zhuang, H.; Li, J.-H.; Wu, J. The parasitic plant Cuscuta australis is highly insensitive to abscisic acid-induced suppression of hypocotyl elongation and seed germination. PLoS ONE 2015, 10, e0135197. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, J.; Hettenhausen, C.; Liu, H.; Li, S.; Shen, G.; Cao, G.; Wu, J. The host jasmonic acid pathway regulates the transcriptomic changes of dodder and host plant under the scenario of caterpillar feeding on dodder. BMC Plant Biol. 2019, 19, 540. [Google Scholar] [CrossRef] [PubMed]
- Triolet, M.; Edel-Hermann, V.; Gautheron, N.; Mondy, S.; Reibel, C.; André, O.; Guillemin, J.-P.; Steinberg, C.; Druzhinina, I.S. Weeds harbor an impressive diversity of fungi, which offers possibilities for biocontrol. Appl. Environ. Microbiol. 2022, 88, e0217721. [Google Scholar] [CrossRef]
- Den Breeyen, A.; Lange, C.; Fowler, S.V. Plant pathogens as introduced weed biological control agents: Could antagonistic fungi be important factors determining agent success or failure? Front. Fungal Biol. 2022, 3, 959753. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Rai, M.; Zimowska, B.; Shinde, S.; Tres, M.V. Bioherbicidal potential of different species of Phoma: Opportunities and challenges. Appl. Microbiol. Biotechnol. 2021, 105, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Osunkoya, O.O.; Soni, A.; Campbell, S.; Dhileepan, K. Growth of the invasive Navua sedge (Cyperus aromaticus) under competitive interaction with pasture species and simulated grazing conditions: Implication for management. Ecol. Res. 2022, 38, 331–346. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, Y.; Guo, Q.; Xu, B. Biological weed control using Trichoderma polysporum strain HZ-31. Crop Prot. 2020, 134, 105161. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.E.F.; Patriarca, A. Alternaria species and their associated mycotoxins. In Mycotoxigenic Fungi; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1542, pp. 13–32. [Google Scholar]
- Gou, F.G.; Li, Y.H.; Deng, F.Z. Screening biological control fungi for dodders on woody hosts. Chin. J. Biol. Control 1998, 14, 159–162. (In Chinese) [Google Scholar]
- DeMers, M. Alternaria alternata as endophyte and pathogen. Microbiology 2022, 168, 001153. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, Ž.; Blagojević, J.; Jovanović, G.; Ivanović, B.; Žeželj, D. New insight in the occurrence of early blight disease on potato reveals high distribution of Alternaria solani and Alternaria protenta in Serbia. Front. Microbiol. 2022, 13, 856898. [Google Scholar] [CrossRef]
- Zhang, F.; Yue, Y.; Shen, S.; Xu, G.; Guo, J.; Zhang, Y. The control effect of Cuscuta on Mikania micrantha and their ecological index in Yunnan. Ecol. Environ. Sci. 2017, 26, 365–370. (In Chinese) [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ahmed, A.; Munir, S.; Chen, L.; He, P.; He, Y.; Tang, P.; Kong, B.; Wu, Y.; He, P. Alternaria alternata Pathogen from Cuscuta japonica Could Serve as a Potential Bioherbicide. J. Fungi 2024, 10, 494. https://doi.org/10.3390/jof10070494
Liu Y, Ahmed A, Munir S, Chen L, He P, He Y, Tang P, Kong B, Wu Y, He P. Alternaria alternata Pathogen from Cuscuta japonica Could Serve as a Potential Bioherbicide. Journal of Fungi. 2024; 10(7):494. https://doi.org/10.3390/jof10070494
Chicago/Turabian StyleLiu, Yinglong, Ayesha Ahmed, Shahzad Munir, Lei Chen, Pengfei He, Yueqiu He, Ping Tang, Baohua Kong, Yixin Wu, and Pengbo He. 2024. "Alternaria alternata Pathogen from Cuscuta japonica Could Serve as a Potential Bioherbicide" Journal of Fungi 10, no. 7: 494. https://doi.org/10.3390/jof10070494
APA StyleLiu, Y., Ahmed, A., Munir, S., Chen, L., He, P., He, Y., Tang, P., Kong, B., Wu, Y., & He, P. (2024). Alternaria alternata Pathogen from Cuscuta japonica Could Serve as a Potential Bioherbicide. Journal of Fungi, 10(7), 494. https://doi.org/10.3390/jof10070494





