The C2H2 Transcription Factor Con7 Regulates Vegetative Growth, Cell Wall Integrity, Oxidative Stress, Asexual Sporulation, Appressorium and Hyphopodium Formation, and Pathogenicity in Colletotrichum graminicola and Colletotrichum siamense
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Nucleic Acid Manipulations and Sequence Analysis
2.3. Target Disruption and Complementation
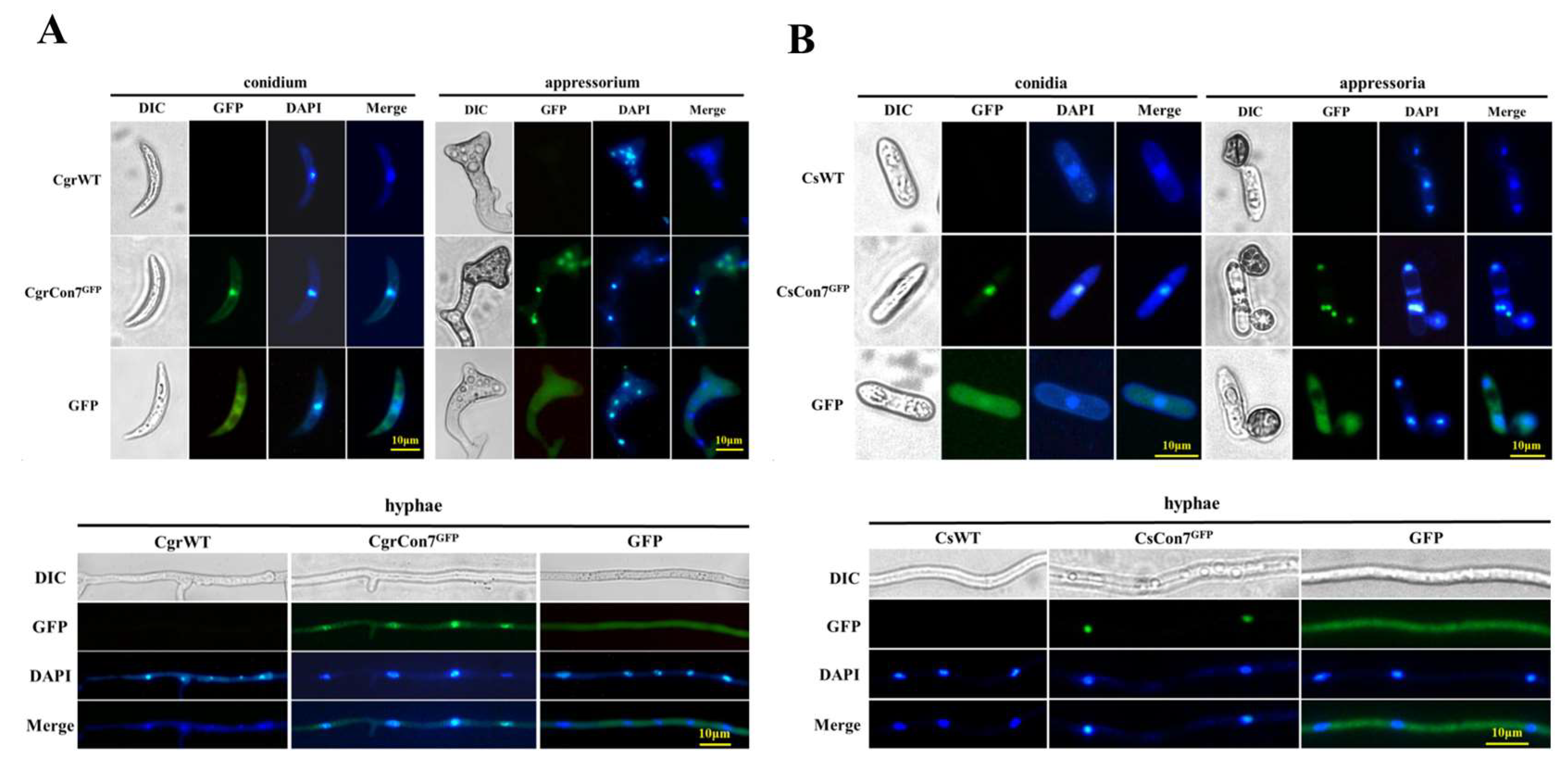
2.4. Subcellular Localization
2.5. Stress Response Assays
2.6. Conidiation, Germination, and Appressorium Formation
2.7. Virulence Assay
2.8. RNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. Characterization of CgrCon7 and CsCon7
3.2. Gene Disruption and Complementation of Cgrcon7 and Cscon7
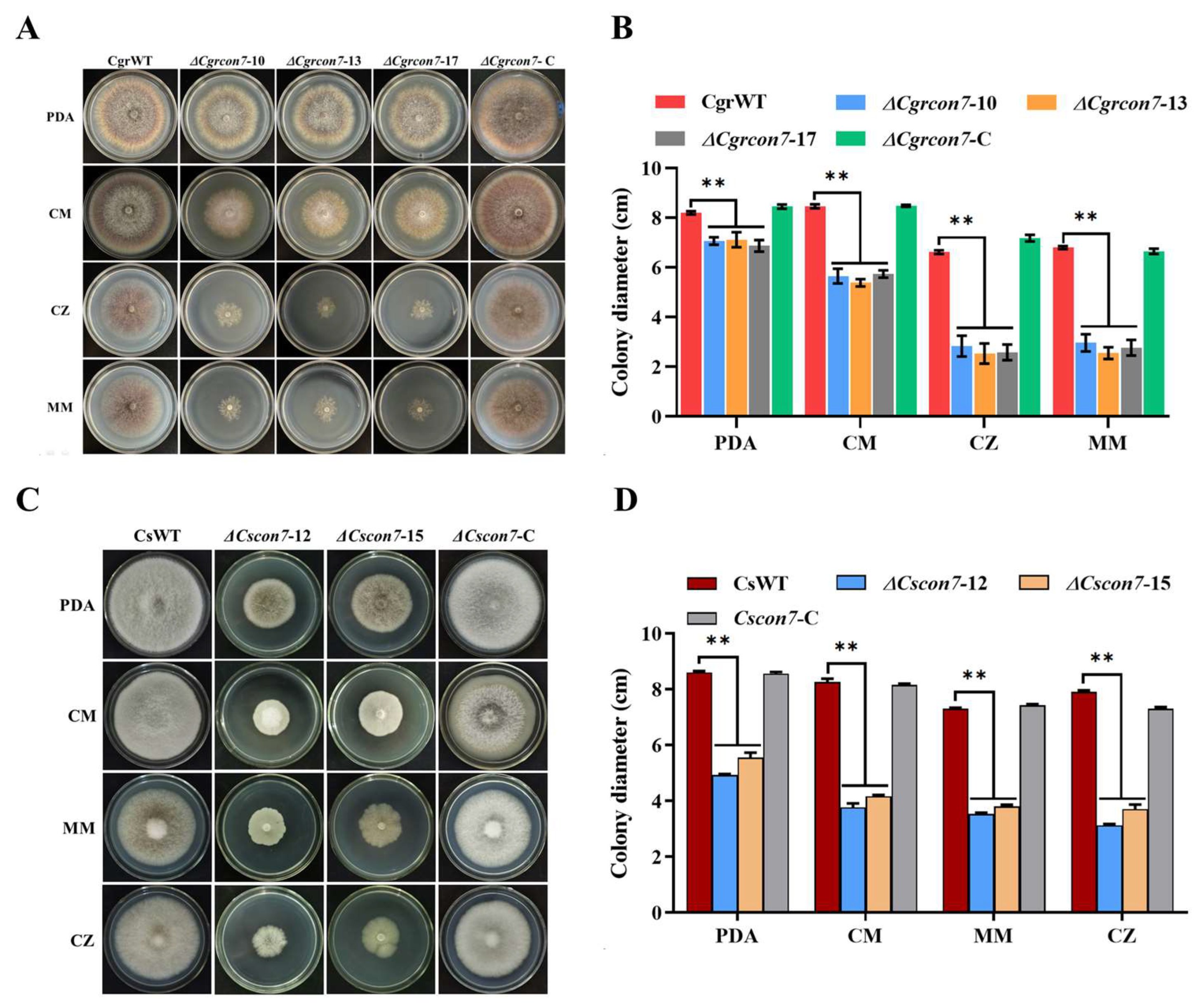
3.3. Con7 Is Required for Vegetative Growth in C. graminicola and C. siamense
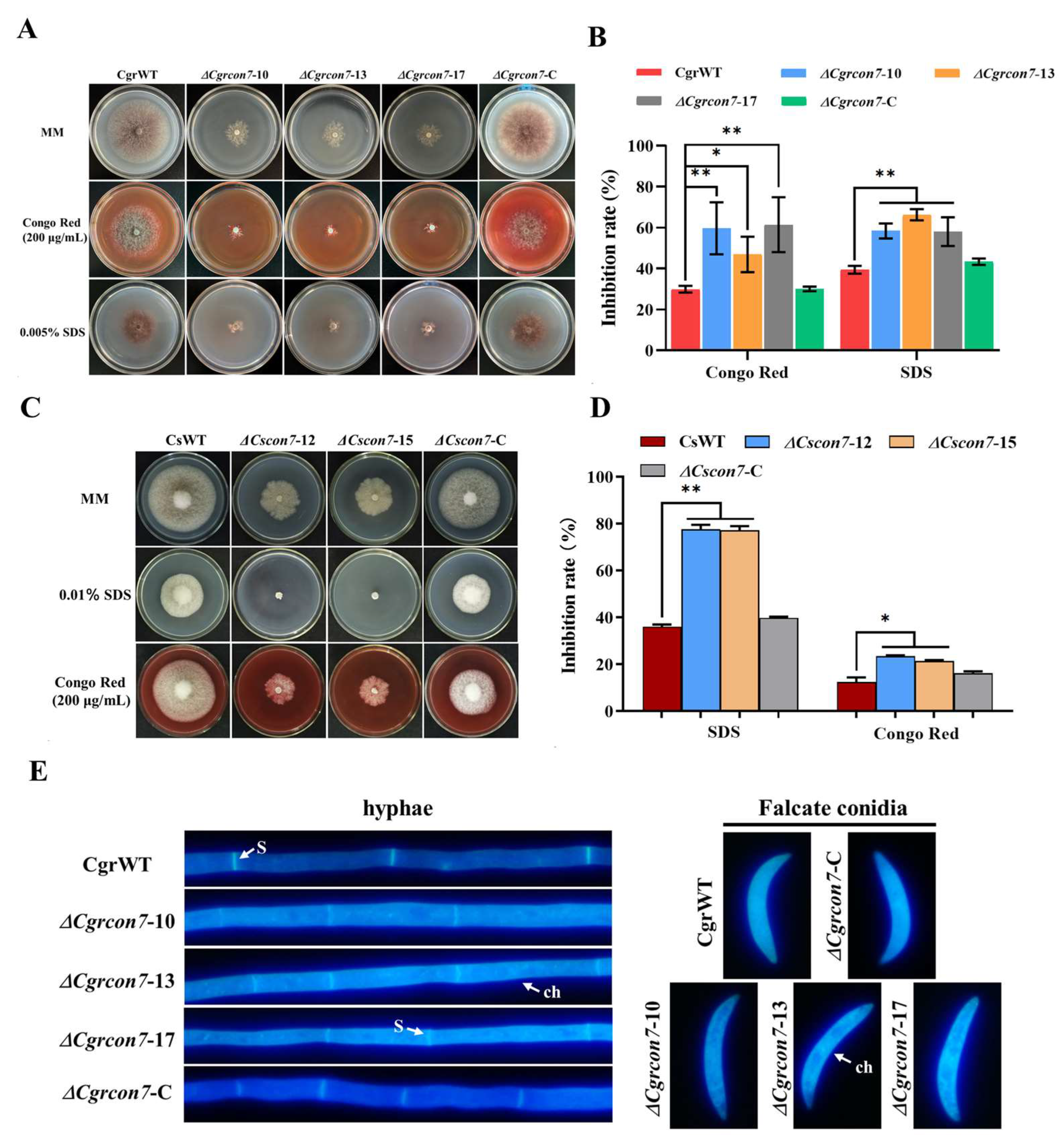
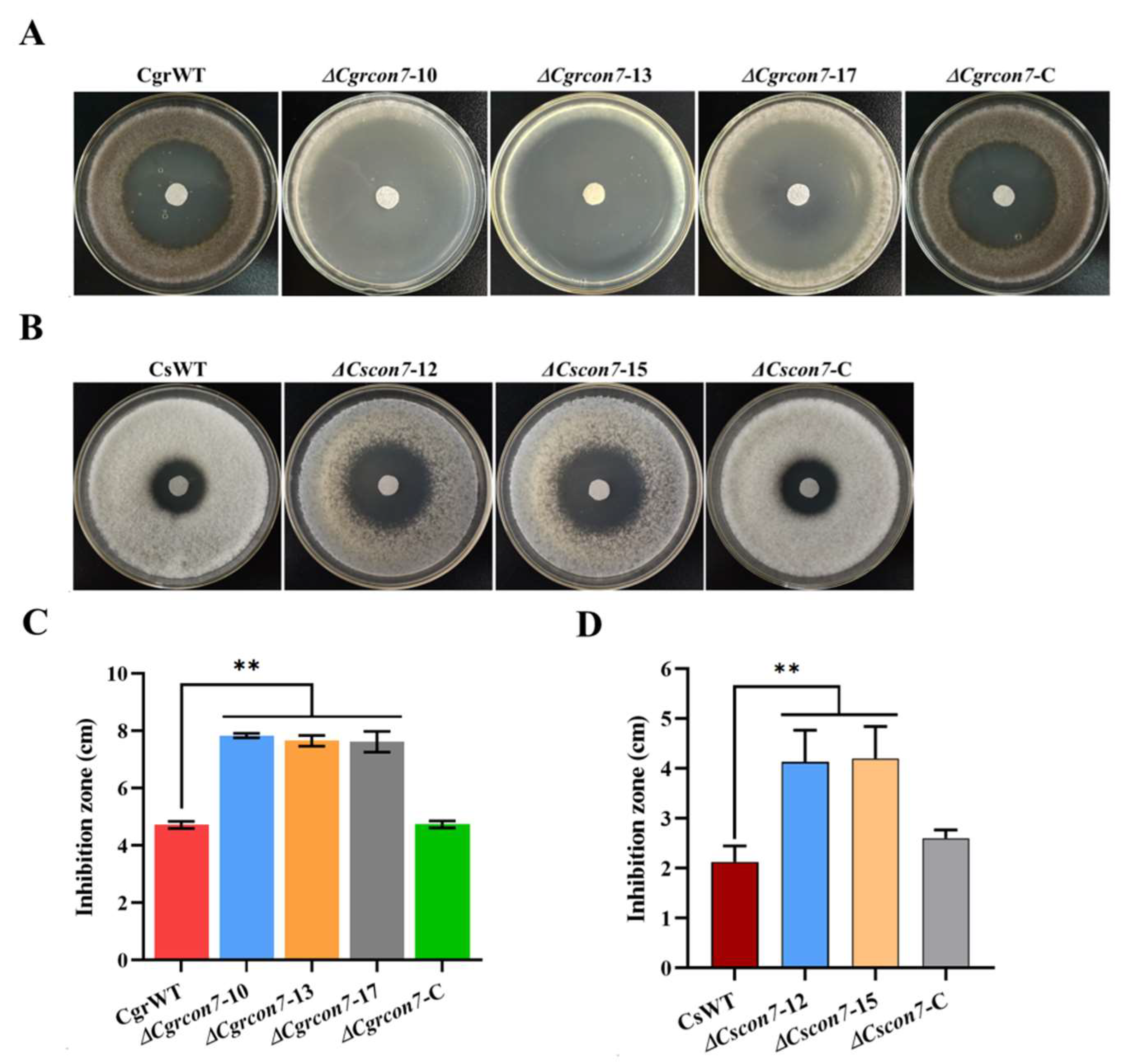
3.4. Con7 Regulates CWI and Oxidative Stress in C. graminicola and C. siamense
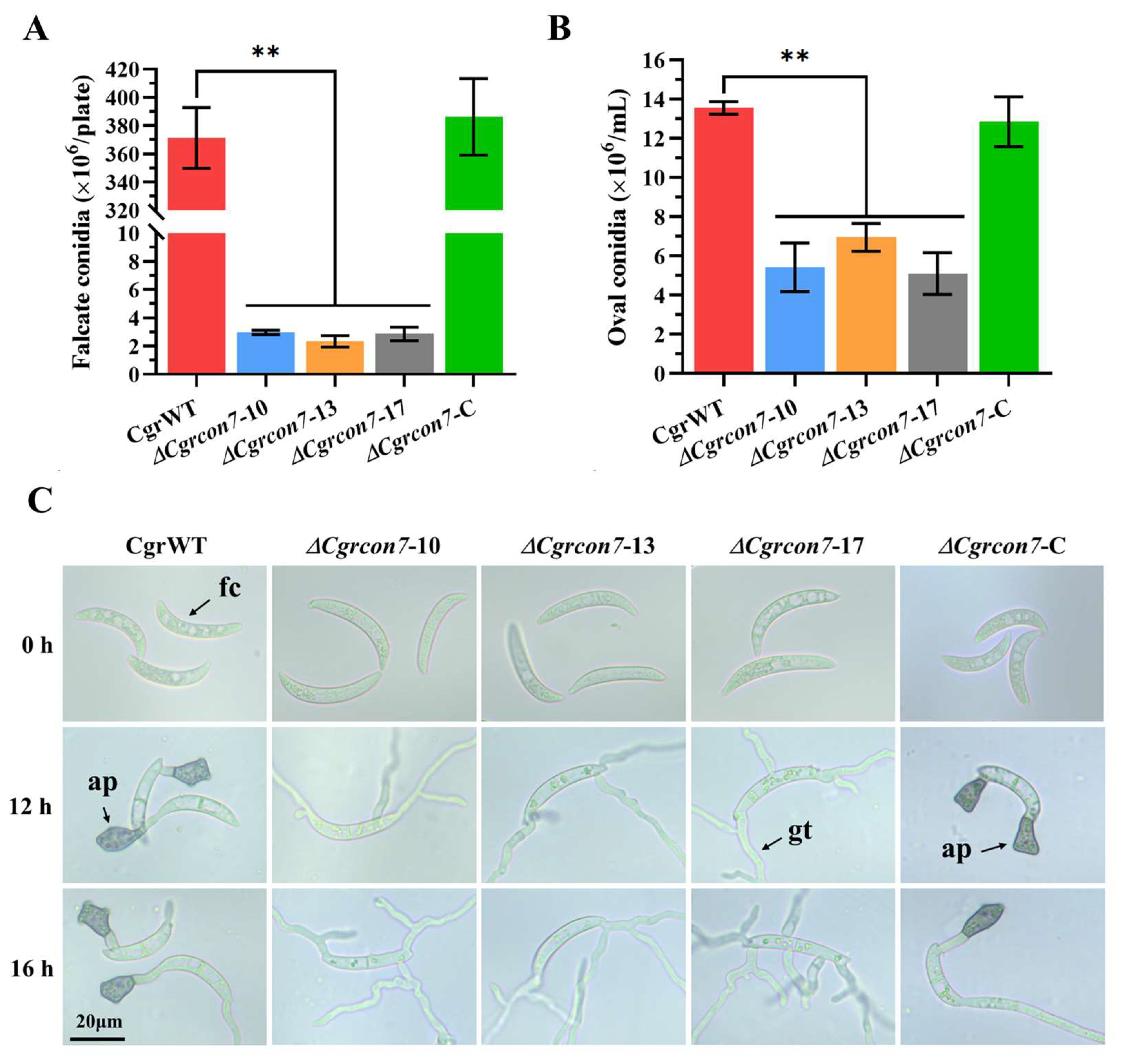
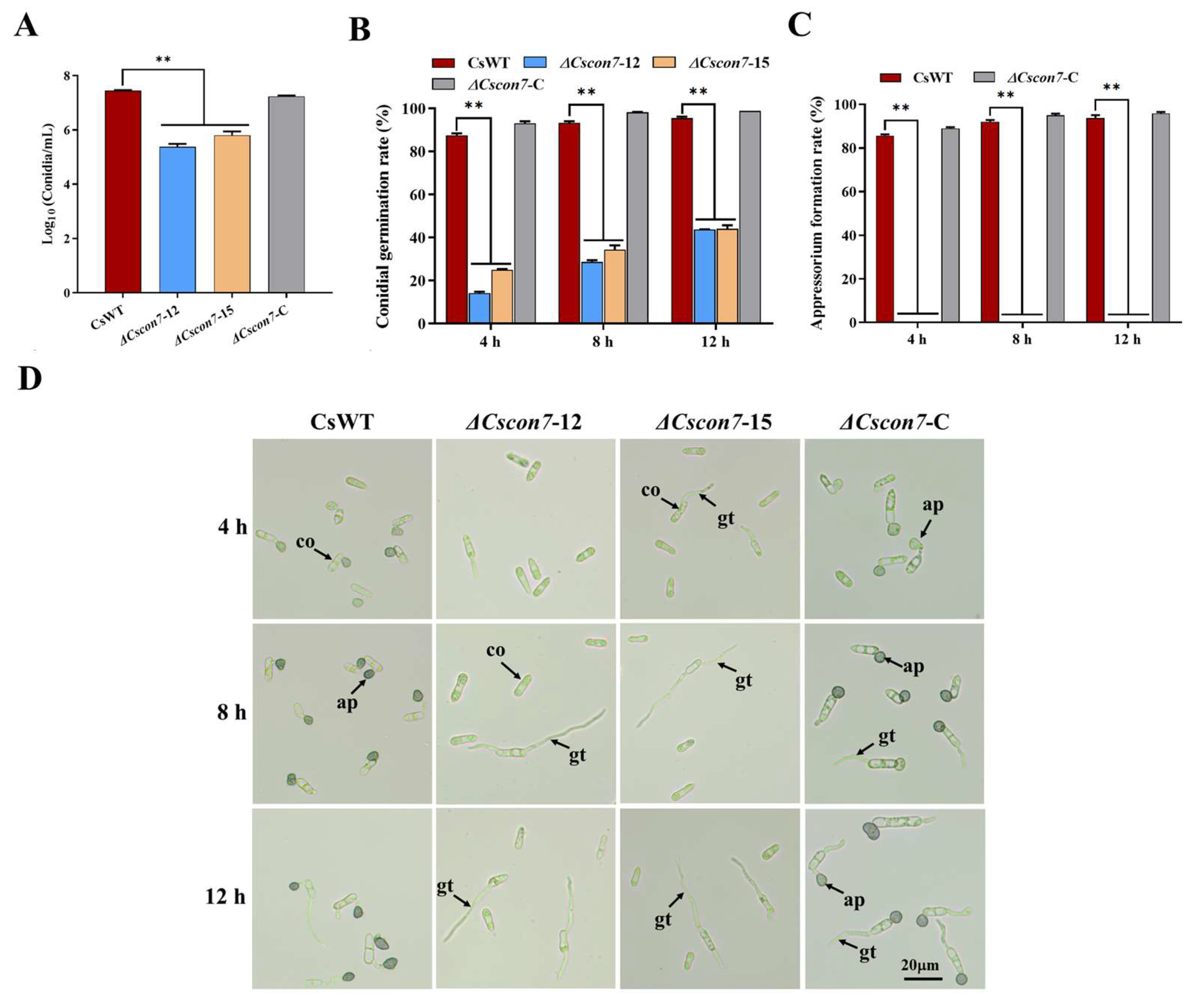
3.5. Con7 Is Involved in Conidiation, Conidial Morphology, Appressorium, and Hyphopodium Formation
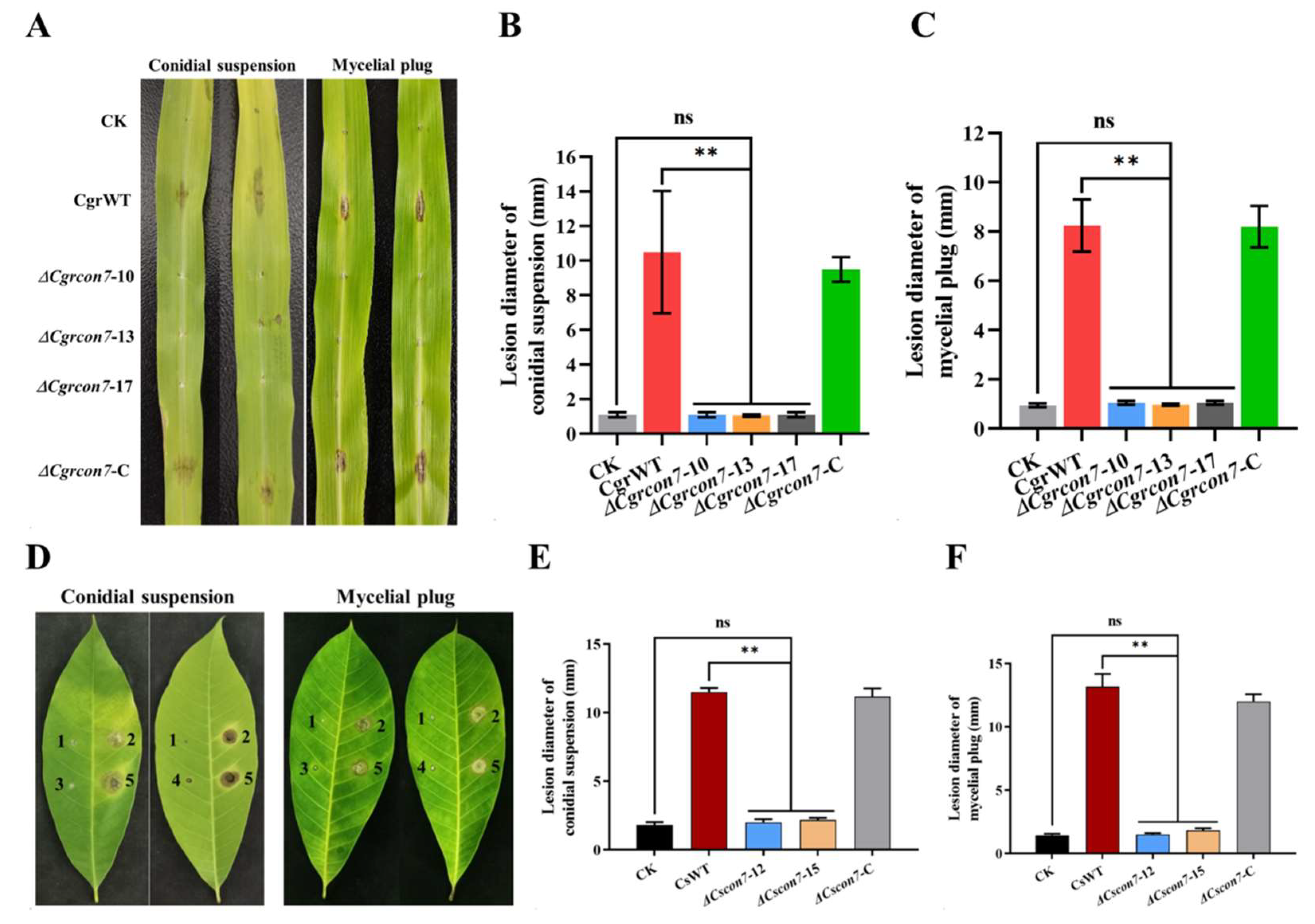
3.6. Con7 Is Required for the Virulence of C. graminicola and C. siamense
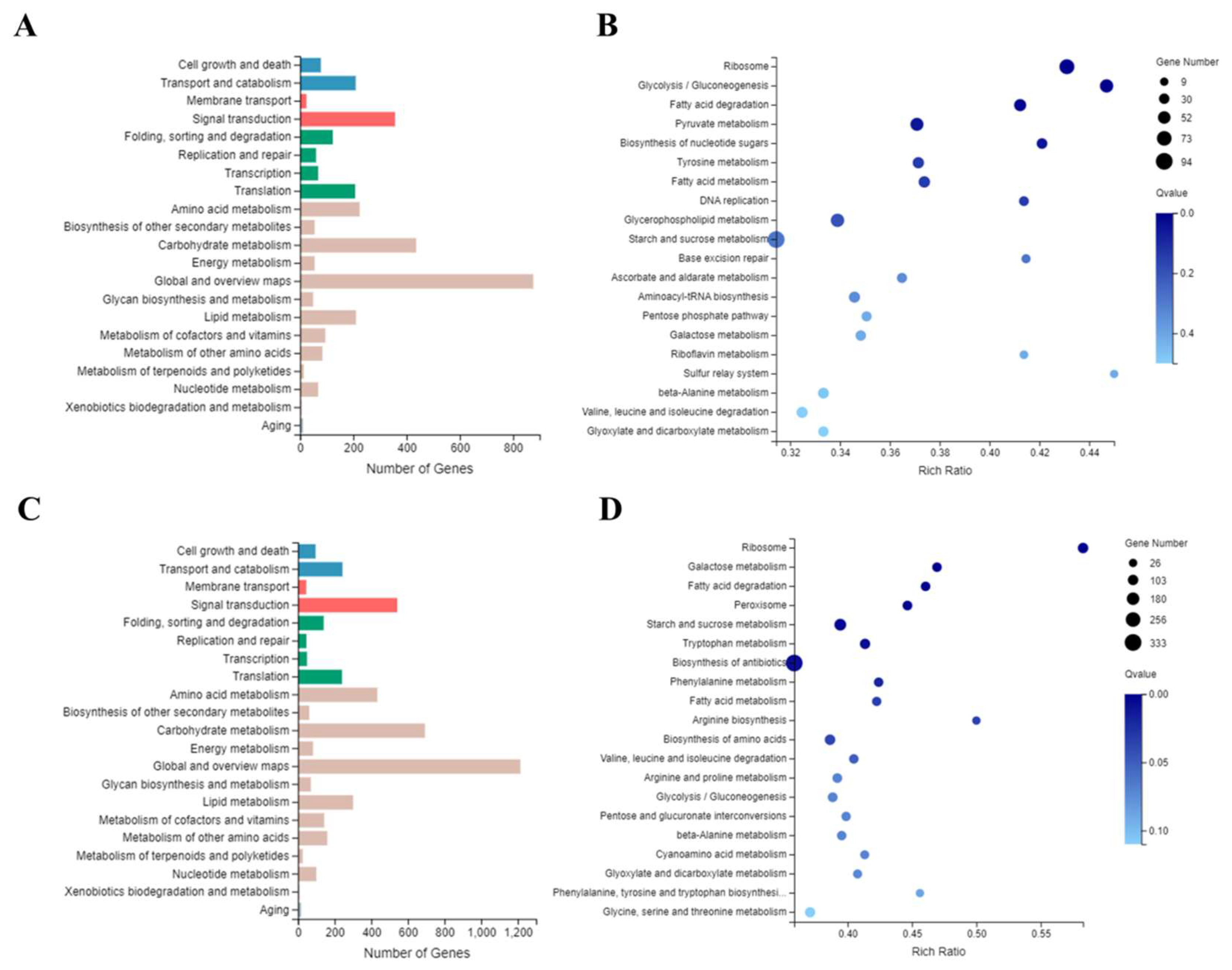
3.7. Transcriptomic Analysis of CgrCon7 and CsCon7
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Rogério, F.; Baroncelli, R.; Cuevas-Fernández, F.B.; Becerra, S.; Crouch, J.; Bettiol, W.; Azcárate-Peril, M.A.; Malapi-Wight, M.; Ortega, V.; Betran, J.; et al. Population genomics provide insights into the global genetic structure of Colletotrichum graminicola, the causal agent of maize anthracnose. Mbio 2023, 14, e02878-22. [Google Scholar] [CrossRef]
- Panaccione, D.G.; Vaillancourt, L.J.; Hanau, R.M. Conidial dimorphism in Colletotrichum graminicola. Mycologia 1989, 81, 876–883. [Google Scholar] [CrossRef]
- Bergstrom, G.C.; Nicholson, R.L. The biology of corn anthracnose: Knowledge to exploit for improved management. Plant Dis. 1999, 83, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, B.; Cai, J.; Zheng, X.; Feng, Y.; Huang, G. Colletotrichum species causing anthracnose of rubber trees in China. Sci. Rep. 2018, 8, 10435. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; Lichter, A. Mechanisms modulating fungal attack in post-harvest pathogen interactions and their control. Eur. J. Plant Pathol. 2008, 121, 281–289. [Google Scholar] [CrossRef]
- Sukno, S.A.; Garcia, V.M.; Shaw, B.D.; Thon, M.R. Root infection and systemic colonization of maize by Colletotrichum graminicola. Appl. Environ. Microbiol. 2008, 74, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.H.; Zhao, X.; Xie, Q.R.; Huang, Q.P.; Zhang, C.K.; Zhai, H.C.; Xu, L.P.; Lu, G.D.; Shim, W.B.; Wang, Z.H. A conserved homeobox transcription factor Htf1 is required for phialide development and conidiogenesis in Fusarium species. PLoS ONE 2012, 7, e45432. [Google Scholar] [CrossRef]
- Li, X.; Ke, Z.; Xu, S.; Tang, W.; Liu, Z. The G-protein alpha subunit CgGa1 mediates growth, sporulation, penetration and pathogenicity in Colletotrichum gloeosporioides. Microb. Pathog. 2021, 161, 105254. [Google Scholar] [CrossRef]
- Jolma, A.; Yin, Y.; Nitta, K.R.; Dave, K.; Popov, A.; Taipale, M.; Enge, M.; Kivioja, T.; Morgunova, E.; Taipale, J. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature 2015, 527, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Situ, J.; Guan, T.; Dou, Z.; Kong, G.; Jiang, Z.; Xi, P. A C2H2 zinc finger protein PlCZF1 is necessary for oospore development and virulence in Peronophythora litchii. Int. J. Mol. Sci. 2022, 23, 2733. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Leung, H. Genetic analysis of sporulation in Magnaporthe grisea by chemical and insertional mutagenesis. Mol. Plant Microbe Interact. 1995, 8, 949–959. [Google Scholar] [CrossRef]
- Shi, Z.; Christian, D.; Leung, H. Interactions between spore morphogenetic mutations affect cell types, sporulation, and pathogenesis in Magnaporthe grisea. Mol. Plant Microbe Interact. 1998, 11, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Odenbach, D.; Breth, B.; Thines, E.; Weber, R.W.; Anke, H.; Foster, A.J. The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol. Microbiol. 2007, 64, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Braus-Stromeyer, S.A.; Kusch, H.; Reusche, M.; Kaever, A.; Kuhn, A.; Valerius, O.; Landesfeind, M.; Asshauer, K.; Tech, M.; et al. Verticillium transcription activator of adhesion Vta2 suppresses microsclerotia formation and is required for systemic infection of plant roots. New Phytol. 2014, 202, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roldan, C.; Pareja-Jaime, Y.; Gonzalez-Reyes, J.A.; Roncero, M.I. The transcription factor Con7-1 is a master regulator of morphogenesis and virulence in Fusarium oxysporum. Mol. Plant Microbe Interact. 2015, 28, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.; Yang, L.; Kim, H.; Choi, G.J.; Lee, Y.W.; Kim, J.E.; Son, H. Con7 is a key transcription regulator for conidiogenesis in the plant pathogenic fungus Fusarium graminearum. Msphere 2024, 9, e00818-23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, S.; Zhang, N.; Liu, Z. Function and transcriptome analysis of an oligopeptide transporter CgOPT2 in the rubber anthracnose fungus Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 2021, 115, 101661. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Liu, Z.; Zhang, C. The function and transcriptome analysis of a bZIP transcription factor CgAP1 in Colletotrichum gloeosporioides. Microbiol. Res. 2017, 197, 39–48. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, S.; Tang, W.; Wang, J.; Liu, H.; Zhang, Y.; Wang, L.; Li, X.; Liu, Z. The velvet proteins CsVosA and CsVelB coordinate growth, cell wall integrity, sporulation, conidial viability and pathogenicity in the rubber anthracnose fungus Colletotrichum siamense. Microbiol. Res. 2022, 268, 127290. [Google Scholar] [CrossRef]
- Werner, S.; Sugui, J.A.; Steinberg, G.; Deising, H.B. A chitin synthase with a myosin-like motor domain is essential for hyphal growth, appressorium differentiation, and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola. Mol. Plant Microbe Interact. 2007, 20, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Wells, R.; Rechsteiner, M. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science 1986, 234, 364–368. [Google Scholar] [CrossRef]
- Zou, S.; Wang, H.; Li, Y.; Kong, Z.; Tang, D. The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 2018, 218, 298–309. [Google Scholar] [CrossRef]
- Wolfe, S.A.; Nekludova, L.; Pabo, C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, P.; Abubakar, Y.S.; Lu, P.; Li, Y.; Mao, X.; Zhang, C.; Zheng, W.; Wang, Z.; Lu, G.D.; et al. A feedback regulation of FgHtf1-FgCon7 loop in conidiogenesis and development of Fusarium graminearum. Int. J. Biol. Macromol. 2024, 261, 129841. [Google Scholar] [CrossRef]
- Pareja-Jaime, Y.; Martin-Urdiroz, M.; Roncero, M.I.G.; Gonzalez-Reyes, J.A.; Roldan, M.D.R. Chitin synthase-deficient mutant of Fusarium oxysporum elicits tomato plant defence response and protects against wild-type infection. Mol. Plant Pathol. 2010, 11, 479–493. [Google Scholar] [CrossRef]
- Silva, A.D.; Aliyeva-Schnorr, L.; Wirsel, S.G.R.; Deising, H.B. Fungal pathogenesis-related cell wall biogenesis, with emphasis on the maize anthracnose fungus Colletotrichum graminicola. Plants 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Odenbach, D.; Thines, E.; Anke, H.; Foster, A.J. The Magnaporthe grisea class VII chitin synthase is required for normal appressorial development and function. Mol. Plant Pathol. 2009, 10, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.J.; Huang, P.Y.; Zhang, L.L.; Shi, Y.K.; Sun, D.D.; Yan, Y.X.; Liu, X.H.; Dong, B.; Chen, G.Q.; Snyder, J.H.; et al. Characterization of 47 Cys(2)-His(2) zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae. New Phytol. 2016, 211, 1035–1051. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Yu, J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.Y.; Kim, K.S.; Rho, H.S.; Chi, M.H.; Choi, J.; Park, J.; Kong, S.; Park, J.; Goh, J.; et al. Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae. PLoS Genet. 2009, 5, e1000757. [Google Scholar] [CrossRef]
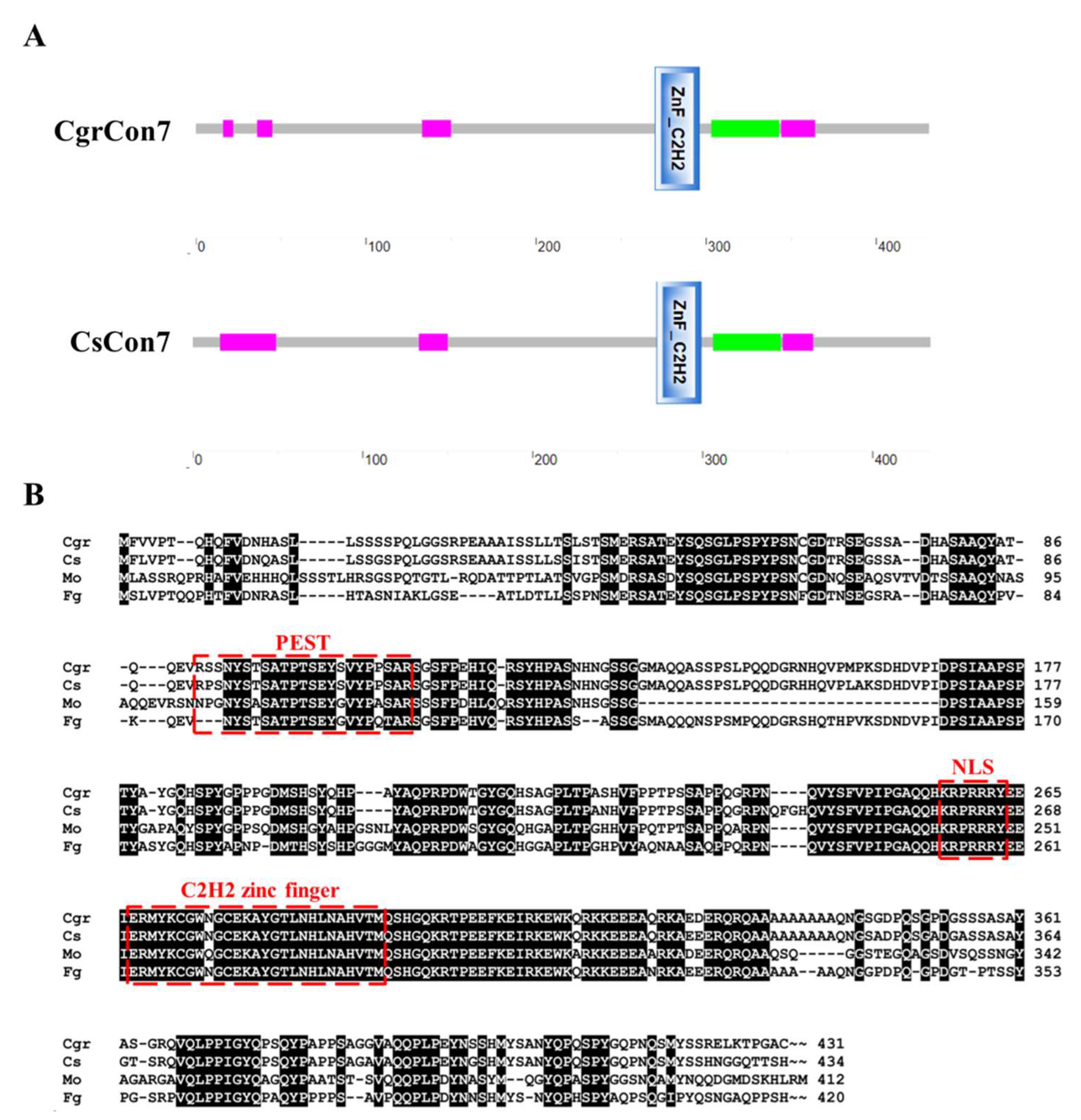
| Functional Classification | C. graminicola | C. siamense | ||||
|---|---|---|---|---|---|---|
| Reference Gene ID | Gene Function Annotation | Log2(ΔCgrcon7/CgrWT) | Reference Gene ID | Gene Function Annotation | Log2(ΔCscon7/CsWT) | |
| ROS detoxification | GLRG_01010 | Iron/manganese superoxide dismutase | −4.4 | CGCS363_v014870 | Catalase | −6.1 |
| GLRG_09508 | Peroxidase | −3.6 | CGCS363_v004531 | Catalase-peroxidase | −5.5 | |
| GLRG_11144 | Peroxidase | −1.5 | CGCS363_v006076 | Catalase-1 | −2.4 | |
| GLRG_04508 | Peroxidase | −1.2 | CGCS363_v002325 | CgAP1 | −1.4 | |
| GLRG_00895 | Catalase | −1.6 | ||||
| Chitin synthesis | GLRG_05787 | Chitin synthase | 1.2 | CGCS363_v013013 | Chitin synthase 1 | 2.8 |
| GLRG_03399 | Chitin synthase | −1.2 | CGCS363_v013350 | Chitin synthase 3 | 1.7 | |
| CGCS363_v008225 | Chitin synthase 8 | 1.1 | ||||
| Cell wall degradation | GLRG_02128 | Cutinase | −4.1 | CGCS363_v008243 | Cutinase | −1.6 |
| GLRG_06347 | Pectin lyase | −4.4 | CGCS363_v013515 | Pectinesterase | −8.1 | |
| GLRG_11038 | Glucanase | −1.4 | CGCS363_v014756 | Pectin lyase F-1 | −2.5 | |
| GLRG_04872 | β-glucosidase | −1.1 | CGCS363_v002605 | Endo-beta-1,4-glucanase D | −10.5 | |
| CGCS363_v013283 | Endo-1,3(4)-beta-glucanase | −4.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Liu, S.; Guo, C.; Wei, H.; He, Z.; Liu, Z.; Li, X. The C2H2 Transcription Factor Con7 Regulates Vegetative Growth, Cell Wall Integrity, Oxidative Stress, Asexual Sporulation, Appressorium and Hyphopodium Formation, and Pathogenicity in Colletotrichum graminicola and Colletotrichum siamense. J. Fungi 2024, 10, 495. https://doi.org/10.3390/jof10070495
Zhou S, Liu S, Guo C, Wei H, He Z, Liu Z, Li X. The C2H2 Transcription Factor Con7 Regulates Vegetative Growth, Cell Wall Integrity, Oxidative Stress, Asexual Sporulation, Appressorium and Hyphopodium Formation, and Pathogenicity in Colletotrichum graminicola and Colletotrichum siamense. Journal of Fungi. 2024; 10(7):495. https://doi.org/10.3390/jof10070495
Chicago/Turabian StyleZhou, Shuangzhen, Shayu Liu, Chenchen Guo, Hanwen Wei, Zhihui He, Zhiqiang Liu, and Xiaoyu Li. 2024. "The C2H2 Transcription Factor Con7 Regulates Vegetative Growth, Cell Wall Integrity, Oxidative Stress, Asexual Sporulation, Appressorium and Hyphopodium Formation, and Pathogenicity in Colletotrichum graminicola and Colletotrichum siamense" Journal of Fungi 10, no. 7: 495. https://doi.org/10.3390/jof10070495
APA StyleZhou, S., Liu, S., Guo, C., Wei, H., He, Z., Liu, Z., & Li, X. (2024). The C2H2 Transcription Factor Con7 Regulates Vegetative Growth, Cell Wall Integrity, Oxidative Stress, Asexual Sporulation, Appressorium and Hyphopodium Formation, and Pathogenicity in Colletotrichum graminicola and Colletotrichum siamense. Journal of Fungi, 10(7), 495. https://doi.org/10.3390/jof10070495








