Development of PCR-Multiplex Assays for Identification of the Herpotrichiellaceae Family and Agents Causing Chromoblastomycosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Strains
2.2. DNA Extraction
2.3. Molecular Identification through Sequencing
2.4. Phylogenetic Analysis
2.5. Primer Design
2.6. In Silico PCR
2.7. PCR Optmization
2.8. Assessment of Nonspecific Amplification
2.9. Evaluation of the Minimum Amplification Threshold
3. Results
3.1. Isolates Used and Phylogenetic Analysis
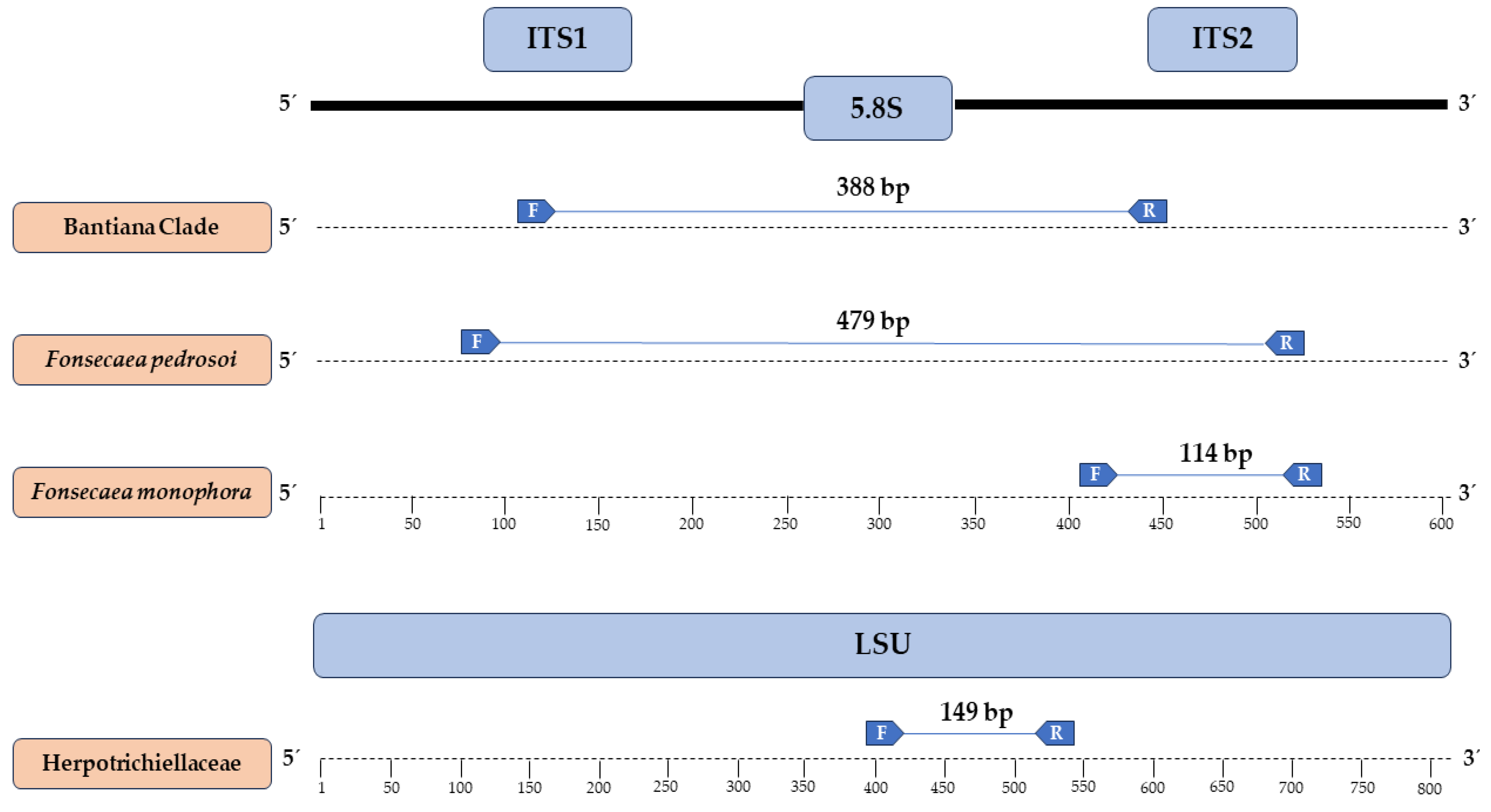
3.2. Primers Designed and In Silico Specificity
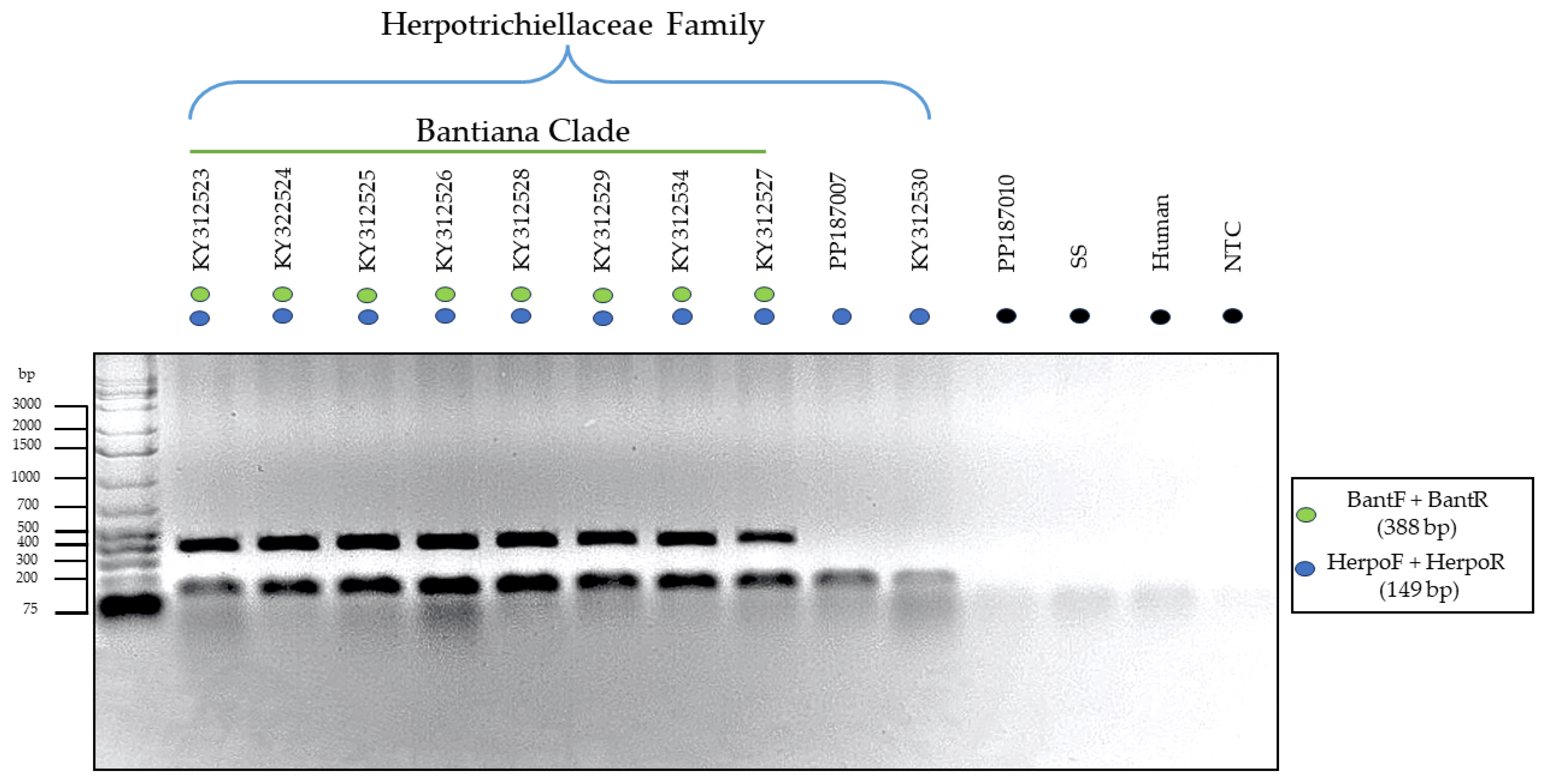
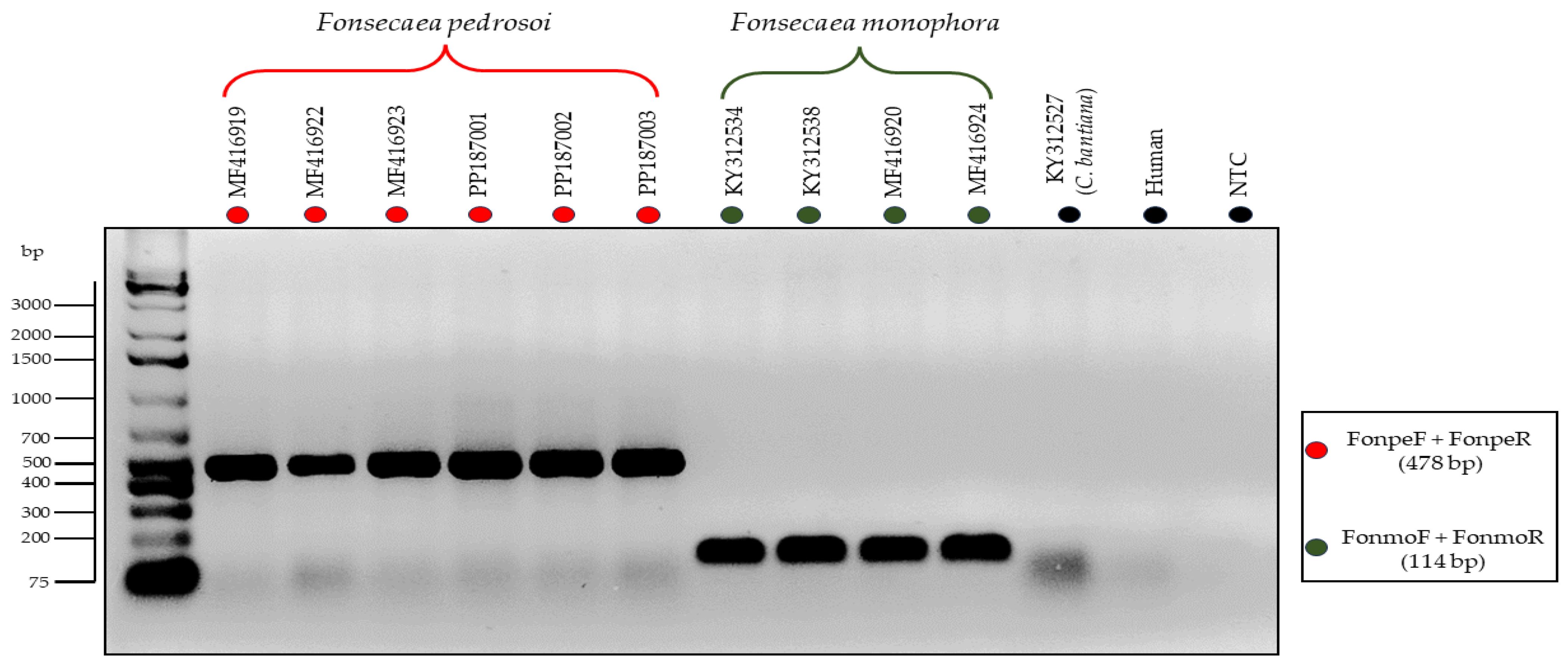
3.3. Testing of the Primers through In Vitro PCR
3.4. Detection Limit of the Primers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, R.J.; Turgeon, B.G. Fungal Sex: The Ascomycota. Microbiol. Spectr. 2016, 4, 1–28. [Google Scholar] [CrossRef]
- Tian, Q.; Chomnunti, P.; Lumyong, S.; Liu, J.K.; Hyde, K.D. Phylogenetic Relationships and Morphological Reappraisal of Chaetothyriales. Mycosphere 2021, 12, 1157–1261. [Google Scholar] [CrossRef]
- Geiser, D.M.; Gueidan, C.; Miadlikowska, J.; Lutzoni, F.; Kauff, F.; Hofstetter, V.; Fraker, E.; Schoch, C.L.; Tibell, L.; Untereiner, W.A.; et al. Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 2006, 98, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Deng, S.; Prenafeta-Boldủ, F.X.; Mayer, V.E.; Muggia, L.; Cometto, A.; Vicente, V.A.; da Silva, N.M.; Grisolia, M.E.; Song, Y.; et al. The Origin of Human Pathogenicity and Biological Interactions in Chaetothyriales. Fungal Divers. 2023, 125, 99–120. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Netea, M.G.; Mouton, J.W.; Melchers, W.J.G.; Verweij, P.E.; de Hoog, G.S. Black Yeasts and Their Filamentous Relatives: Principles of Pathogenesis and Host Defense. Clin. Microbiol. Rev. 2014, 27, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Gibani, M.M.; de Groot, T.; Borman, A.M.; Hoffman, P.; Azadian, B.S.; Mughal, N.; Moore, L.S.P.; Johnson, E.M.; Meis, J.F. A Pseudo-Outbreak of Rhinocladiella Similis in a Bronchoscopy Unit of a Tertiary Care Teaching Hospital in London, United Kingdom. Mycoses 2021, 64, 394–404. [Google Scholar] [CrossRef]
- Arcobello, J.T.; Revankar, S.G. Phaeohyphomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.W.C.L.; de Maria Pedrozo e Silva de Azevedo, C.; Vicente, V.A.; Queiroz-Telles, F.; Rodrigues, A.M.; de Hoog, G.S.; Denning, D.W.; Colombo, A.L. The Global Burden of Chromoblastomycosis. PLoS Negl. Trop. Dis. 2021, 15, e0009611. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Strategic Framework for Integrated Control and Management of Skin-Related Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.C.L.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; de Maria Pedrozo e Silva Azevedo, C.; Da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2017, 30, 233–276. [Google Scholar] [CrossRef]
- Liu, S.; Zhi, H.; Shen, H.; Lv, W.; Sang, B.; Li, Q.; Zhong, Y.; Liu, Z.; Xia, X. Chromoblastomycosis: A Case Series from Eastern China. PLoS Negl. Trop. Dis. 2022, 16, e0010800. [Google Scholar] [CrossRef]
- Sendrasoa, F.A.; Razanakoto, N.H.; Rakotoarisaona, M.F.; Andrianarison, M.; Raharolahy, O.; Rasamoelina, T.; Ranaivo, I.M.; Sata, M.; Ratovonjanahary, V.; Maubon, D.; et al. Clinical Aspects of Previously Treated Chromoblastomycosis: A Case Series from Madagascar. Int. J. Infect. Dis. 2020, 101, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Singh, G.; Ghosh, A.; Verma, K.K.; Pandey, M.; Xess, I. Chromoblastomycosis in India: Review of 169 Cases. PLoS Negl. Trop. Dis. 2017, 11, e0005534. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zheng, H.L.; Mei, H.; Lv, G.X.; Liu, W.D.; Li, X.F. Phaeohyphomycosis in China. Front. Cell. Infect. Microbiol. 2022, 12, 895329. [Google Scholar] [CrossRef]
- Kilbourn, K.J.; Green, J.; Zacharewski, N.; Aferzon, J.; Lawlor, M.; Jaffa, M. Intracranial Fungal Cladophialophora Bantiana Infection in a Nonimmunocompromised Patient: A Case Report and Review of the Literature. Surg. Neurol. Int. 2022, 13, 165. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, S.W.; Cia, C.T.; Chen, P.L.; Shih, H.I.; Choi, P.C.; Wu, C.J.; Chan, S.H. Pneumonia and Brain Abscess Likely Due to Cladophialophora Bantiana in a Patient with Systemic Lupus Erythematosus in Taiwan. J. Microbiol. Immunol. Infect. 2024, 57, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Castillo Bejarano, J.I.; de los Santos, A.M.; Saldaña, D.C.; Zambrano Lucio, M.; Pérez Cavazos, S.; Espinosa Villaseñor, F.; de la O Cavazos, M.E.; Vaquera Aparicio, D.N. Pediatric Phaeohyphomycosis: A 44-Year Systematic Review of Reported Cases. J. Pediatr. Infect. Dis. Soc. 2022, 12, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Taneja, J.; Passi, S.; Ranjan, R.; Abbas, S.Z.; Ramesh, V. Sporotrichoid Lesions Caused by Rhinocladiella Similis. J. Med. Mycol. 2023, 33, 101336. [Google Scholar] [CrossRef] [PubMed]
- Carolina Rojas, O.; León-Cachón, R.B.R.; Pérez-Maya, A.A.; Aguirre-Garza, M.; Moreno-Treviño, M.G.; González, G.M. Phenotypic and Molecular Identification of Fonsecaea pedrosoi Strains Isolated from Chromoblastomycosis Patients in Mexico and Venezuela. Mycoses 2015, 58, 267–272. [Google Scholar] [CrossRef]
- Coelho, R.A.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; dos Santos Silva, J.V.; Gutierrez-Galhardo, M.C.; do Valle, A.C.F.; Zancopé-Oliveira, R.M.; Trilles, L.; Meyer, W.; Freitas, D.F.S.; et al. Molecular Identification and Antifungal Susceptibility Profiles of Clinical Strains of Fonsecaea Spp. Isolated from Patients with Chromoblastomycosis in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006675. [Google Scholar] [CrossRef]
- Gomes, R.R.; Vicente, V.A.; de Azevedo, C.M.P.S.; Salgado, C.G.; da Silva, M.B.; Queiroz-Telles, F.; Marques, S.G.; Santos, D.W.C.L.; de Andrade, T.S.; Takagi, E.H.; et al. Molecular Epidemiology of Agents of Human Chromoblastomycosis in Brazil with the Description of Two Novel Species. PLoS Negl. Trop. Dis. 2016, 10, e0005102. [Google Scholar] [CrossRef]
- Monteiro, R.C. Epidemiologia Molecular e Caracterização Fenotípica de Fungos Demáceos Agentes de Cromoblastomicose no Estado do Pará, com Relato de Fungos Neurotrópicos na Região Amazônica. Dissertação de Mestrado, Universidade Federal do Pará, Belém, Brasil, 2017. [Google Scholar]
- Sun, J.; Najafzadeh, M.J.; Gerrits van den Ende, A.H.G.; Vicente, V.A.; Feng, P.; Xi, L.; de Hoog, G.S. Molecular Characterization of Pathogenic Members of the Genus Fonsecaea Using Multilocus Analysis. PLoS ONE 2012, 7, e41512. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular Diagnosis of Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Greer, E.M.; McGrail, C.W. The Role of Melanins in Melanotic Fungi for Pathogenesis and Environmental Survival. Appl. Microbiol. Biotechnol. 2020, 104, 4247–4257. [Google Scholar] [CrossRef]
- Passero, L.F.D.; Cavallone, I.N.; Belda, W. Reviewing the Etiologic Agents, Microbe-Host Relationship, Immune Response, Diagnosis, and Treatment in Chromoblastomycosis. J. Immunol. Res. 2021, 2021, 9472832. [Google Scholar] [CrossRef]
- Thomas, E.; Bertolotti, A.; Barreau, A.; Klisnick, J.; Tournebize, P.; Borgherini, G.; Zemali, N.; Jaubert, J.; Jouvion, G.; Bretagne, S.; et al. From Phaeohyphomycosis to Disseminated Chromoblastomycosis: A Retrospective Study of Infections Caused by Dematiaceous Fungi. Med. Mal. Infect. 2018, 48, 278–285. [Google Scholar] [CrossRef]
- De Brito, A.C.; de Jesus Semblano Bittencourt, S. Chromoblastomycosis: An Etiological, Epidemiological, Clinical, Diagnostic, and Treatment Update. An. Bras. Dermatol. 2018, 93, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.S.M.; De Oliveira, R.S.; De Souza, A.B.; Monteiro, R.C.; Santo, E.P.T.E.; Franco Filho, L.C.; Da Silva, S.H.M. Identification of Chromoblastomycosis and Phaeohyphomycosis Agents through ITS-RFLP. J. Fungi 2024, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi, 2nd ed.; Amer Society for Microbiology: Utrech, The Netherlands; Universitat Rovira i Virgili Reus: Reus, Spain, 2000; pp. 560–680. [Google Scholar]
- Usuda, D.; Higashikawa, T.; Hotchi, Y.; Usami, K.; Shimozawa, S.; Tokunaga, S.; Osugi, I.; Katou, R.; Ito, S.; Yoshizawa, T.; et al. Exophiala Dermatitidis. World J. Clin. Cases 2021, 9, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, D.; Pagani, D.M.; Koehler, A.; de Oliveira Alves, K.; Scrofernekera, M.L. Effect of Melanin Biosynthesis Inhibition on the Antifungal Susceptibility of Chromoblastomycosis Agents. Antimicrob. Agents Chemother. 2021, 65, e00546-21. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, J.; Li, M.; Cai, W.; Chen, Y.; Liu, Y.; Huang, H.; Xie, Z.; Zeng, W.; Xi, L. Molecular Characteristics of Regional Chromoblastomycosis in Guangdong, China: Epidemiological, Clinical, Antifungal Susceptibility, and Serum Cytokine Profiles of 45 Cases. Front. Cell. Infect. Microbiol. 2022, 12, 810604. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Jia, G.; Tan, J.; Yang, L.; Wang, Y.; Li, L. Analysis of the Synergistic Antifungal Activity of Everolimus and Antifungal Drugs against Dematiaceous Fungi. Front. Cell. Infect. Microbiol. 2023, 13, 1131416. [Google Scholar] [CrossRef]
- Maubon, D.; Garnaud, C.; Ramarozatovo, L.S.; Fahafahantsoa, R.R.; Cornet, M.; Rasamoelina, T. Molecular Diagnosis of Two Major Implantation Mycoses: Chromoblastomycosis and Sporotrichosis. J. Fungi 2022, 8, 382. [Google Scholar] [CrossRef]
- Xu, J. Fungal DNA Barcoding1. In Genome; Canadian Science Publishing: Ottawa, ON, Canada, 2016; pp. 913–932. [Google Scholar] [CrossRef]
- Fajarningsih, N.D. Internal Transcribed Spacer (ITS) as Dna Barcoding to Identify Fungal Species: A Review. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016, 11, 37. [Google Scholar] [CrossRef]
- Revankar, S.G.; Sutton, D.A. Melanized Fungi in Human Disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef]
- Assunção, C.B.; de Aguiar, E.L.; Al-Hatmi, A.M.S.; Silva Vieira, V.C.; Machado, A.S.; Junta, C.; de Hoog, S.; Caligiorne, R.B. New Molecular Marker for Phylogenetic Reconstruction of Black Yeast-like Fungi (Chaetothyriales) with Hypothetical EIF2AK2 Kinase Gene. Fungal Biol. 2020, 124, 1032–1038. [Google Scholar] [CrossRef]
- Firacative, C.; Meyer, W.; Castañeda, E. Cryptococcus neoformans and Cryptococcus gattii Species Complexes in Latin America: A Map of Molecular Types, Genotypic Diversity, and Antifungal Susceptibility as Reported by the Latin American Cryptococcal Study Group. J. Fungi 2021, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Moreno, L.F.; Stielow, B.J.; Muszewska, A.; Hainaut, M.; Gonzaga, L.; Abouelleil, A.; Patané, J.S.L.; Priest, M.; Souza, R.; et al. Exploring the Genomic Diversity of Black Yeasts and Relatives (Chaetothyriales, Ascomycota). Stud. Mycol. 2017, 86, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.W.C.L.; Vicente, V.A.; Weiss, V.A.; Sybren de Hoog, G.; Gomes, R.R.; Batista, E.M.M.; Marques, S.G.; de Queiroz-Telles, F.; Colombo, A.L.; Azevedo, C.d.M.P.e.S.d. Chromoblastomycosis in an Endemic Area of Brazil: A Clinical-Epidemiological Analysis and a Worldwide Haplotype Network. J. Fungi 2020, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Guevara, A.; Siqueira, N.P.; Nery, A.F.; Cavalcante, L.R.D.S.; Hagen, F.; Hahn, R.C. Chromoblastomycosis in Latin America and the Caribbean: Epidemiology over the Past 50 Years. In Medical Mycology; Oxford University Press: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- Borman, A.M.; Fraser, M.; Patterson, Z.; Linton, C.J.; Palmer, M.; Johnson, E.M. Fungal Infections of Implantation: More Than Five Years of Cases of Subcutaneous Fungal Infections Seen at the UK Mycology Reference Laboratory. J. Fungi 2022, 8, 343. [Google Scholar] [CrossRef] [PubMed]
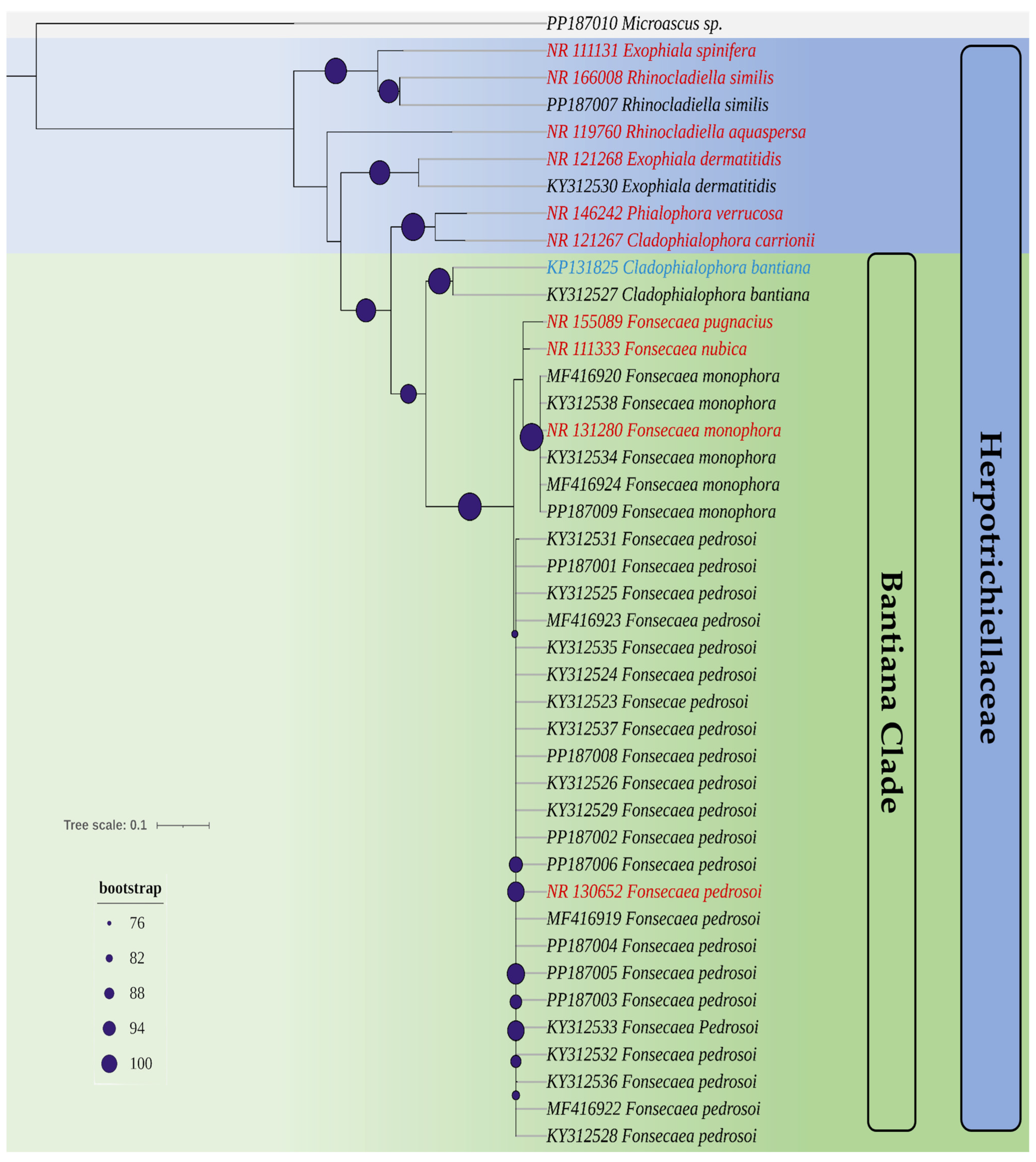

| Isolate | Genbank | Species | Anatomical Site | Host | Geographic Origin |
|---|---|---|---|---|---|
| IEC-CBM02 | KY312523 | F. pedrosoi | Thigh | Human | Pará/Brazil |
| IEC-CBM03 | KY312524 | F. pedrosoi | Foot | Human | Pará/Brazil |
| IEC-CBM04 | KY312525 | F. pedrosoi | Leg | Human | Pará/Brazil |
| IEC-CBM05 | KY312526 | F. pedrosoi | Leg | Human | Pará/Brazil |
| IEC-CBM06 | KY312527 | C. bantiana | CNS † | Human | Pará/Brazil |
| IEC-CBM07 | KY312528 | F. pedrosoi | Hand | Human | Pará/Brazil |
| IEC-CBM08 | KY312529 | F. pedrosoi | Leg | Human | Pará/Brazil |
| IEC-CBM09 | KY312530 | E. dermatitidis | * | Human | Pará/Brazil |
| IEC-CBM10 | KY312531 | F. pedrosoi | Arm | Human | Pará/Brazil |
| IEC-CBM11 | KY312532 | F. pedrosoi | Leg | Human | Pará/Brazil |
| IEC-CBM12 | KY312533 | F. pedrosoi | Foot | Human | Pará/Brazil |
| IEC-CBM13 | KY312534 | F. monophora | Leg | Human | Pará/Brazil |
| IEC-CBM14 | KY312535 | F. pedrosoi | * | Human | Pará/Brazil |
| IEC-CBM15 | KY312536 | F. pedrosoi | * | Human | Pará/Brazil |
| IEC-CBM16 | KY312537 | F. pedrosoi | Thigh | Human | Pará/Brazil |
| IEC-CBM17 | KY312538 | F. monophora | Leg | Human | Pará/Brazil |
| IEC-CBM18 | MF416919 | F. pedrosoi | Thigh | Human | Pará/Brazil |
| IEC-CBM19 | MF416920 | F. monophora | Forearm | Human | Pará/Brazil |
| IEC-CBM21 | MF416922 | F. pedrosoi | Fist | Human | Pará/Brazil |
| IEC-CBM22 | MF416923 | F. pedrosoi | Ankle | Human | Pará/Brazil |
| IEC-CBM23 | MF416924 | F. monophora | * | Human | Pará/Brazil |
| IEC-CBM5804 | PP187001 | F. pedrosoi | Forearm | Human | Pará/Brazil |
| IEC-CBM5805 | PP187002 | F. pedrosoi | Hand | Human | Pará/Brazil |
| IEC-CBM6064 | PP187003 | F. pedrosoi | Leg | Human | Pará/Brazil |
| IEC-CBM6504 | PP187004 | F.pedrosoi | Arm | Human | Pará/Brazil |
| IEC-CBM6512 | PP187005 | F. pedrosoi | Foot | Human | Pará/Brazil |
| IEC-CBM6568 | PP187006 | F. pedrosoi | Arm | Human | Pará/Brazil |
| IEC-CBM6577 | PP187007 | R. similis | Foot | Human | Pará/Brazil |
| IEC-CBM6610 | PP187008 | F. pedrosoi | Leg | Human | Pará/Brazil |
| IEC-CBM6938 | PP187009 | F. monophora | * | Human | Pará/Brazil |
| IEC-CBM6563 | PP187010 | Microascus sp. | Foot | Human | Pará/Brazil |
| Target Species | Primer | Primer Sequence (5′–3′) |
|---|---|---|
| Herpotrichiellaceae Family | HerpoF | CTT GCA ACC AGA CTT GAG CGC G |
| HerpoR | CGC ATG ACA CCC TGG TCT ATA AGT C | |
| Bantiana clade | BantF | GGC AGG CCC GTC TTA ATC TGA CC |
| BantR | GCC GTC ATT GTC TTT AGG AGG GGT G | |
| Fonsecaea pedrosoi | FonpeF | CCA ACC CTT TGC TTA CTA GAC CTC |
| FonpeR | CCC TTC ATC CGA TAC GTG CTC AA | |
| Fonsecaea monophora | FonmoF | GGA CGG CTT GGT GGA GTA AG |
| FonmoR | GCC CTT CAT CCG ATA CGT GCT CAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, G.S.M.; De Oliveira, R.S.; Souza, A.B.; Monteiro, R.C.; Santo, E.P.T.E.; Franco Filho, L.C.; Moraes, D.L.O.; De Sá, S.R.; Da Silva, S.H.M. Development of PCR-Multiplex Assays for Identification of the Herpotrichiellaceae Family and Agents Causing Chromoblastomycosis. J. Fungi 2024, 10, 548. https://doi.org/10.3390/jof10080548
Sousa GSM, De Oliveira RS, Souza AB, Monteiro RC, Santo EPTE, Franco Filho LC, Moraes DLO, De Sá SR, Da Silva SHM. Development of PCR-Multiplex Assays for Identification of the Herpotrichiellaceae Family and Agents Causing Chromoblastomycosis. Journal of Fungi. 2024; 10(8):548. https://doi.org/10.3390/jof10080548
Chicago/Turabian StyleSousa, Gabriel S. M., Rodrigo S. De Oliveira, Alex B. Souza, Ruan C. Monteiro, Elaine P. T. E. Santo, Luciano C. Franco Filho, Denison L. O. Moraes, Sarah R. De Sá, and Silvia H. M. Da Silva. 2024. "Development of PCR-Multiplex Assays for Identification of the Herpotrichiellaceae Family and Agents Causing Chromoblastomycosis" Journal of Fungi 10, no. 8: 548. https://doi.org/10.3390/jof10080548
APA StyleSousa, G. S. M., De Oliveira, R. S., Souza, A. B., Monteiro, R. C., Santo, E. P. T. E., Franco Filho, L. C., Moraes, D. L. O., De Sá, S. R., & Da Silva, S. H. M. (2024). Development of PCR-Multiplex Assays for Identification of the Herpotrichiellaceae Family and Agents Causing Chromoblastomycosis. Journal of Fungi, 10(8), 548. https://doi.org/10.3390/jof10080548







